Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
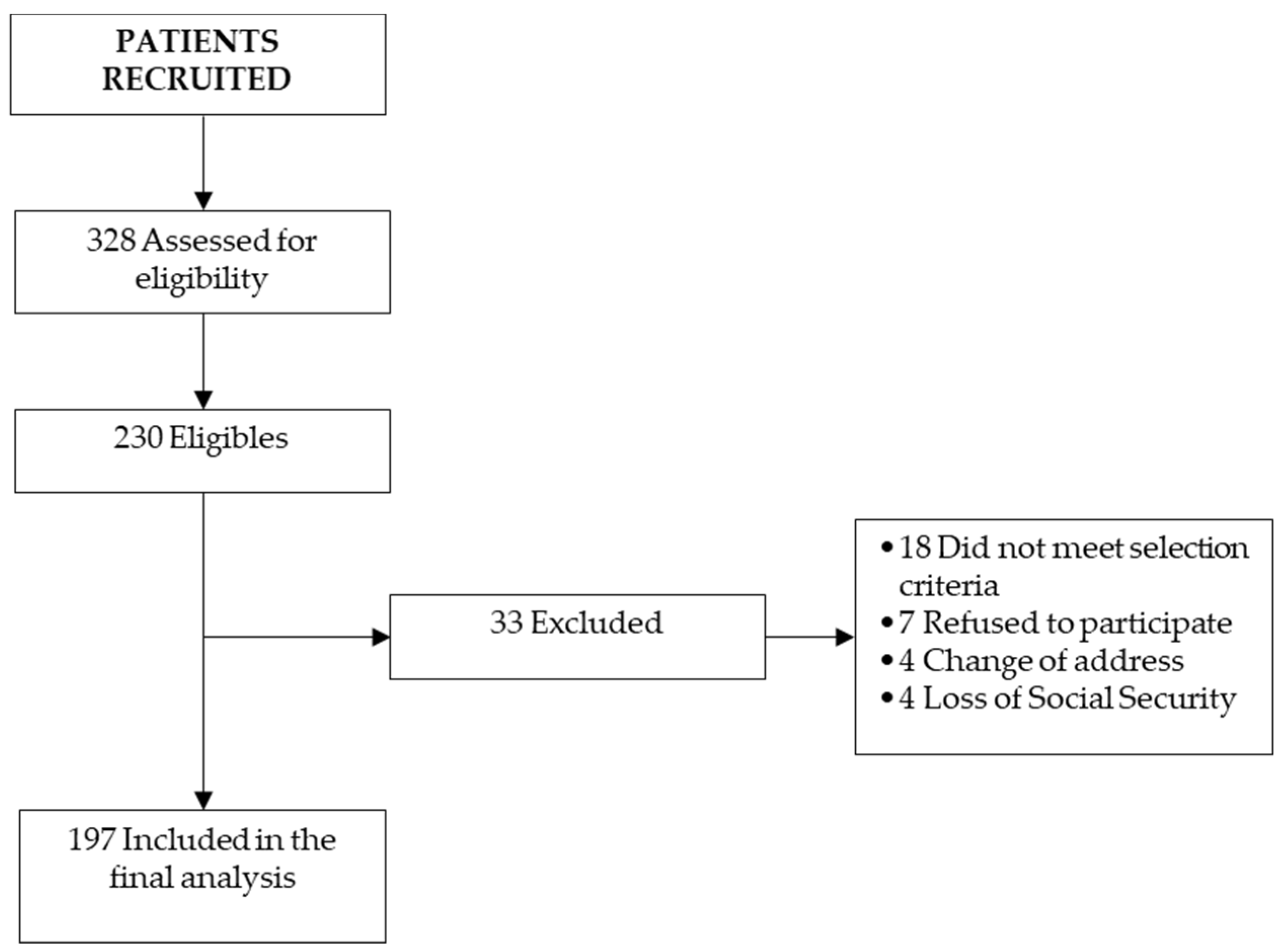
2.2. Patient Selection
2.3. Dialysis Schedule
2.4. Data Collection
2.5. Biochemical Assessments
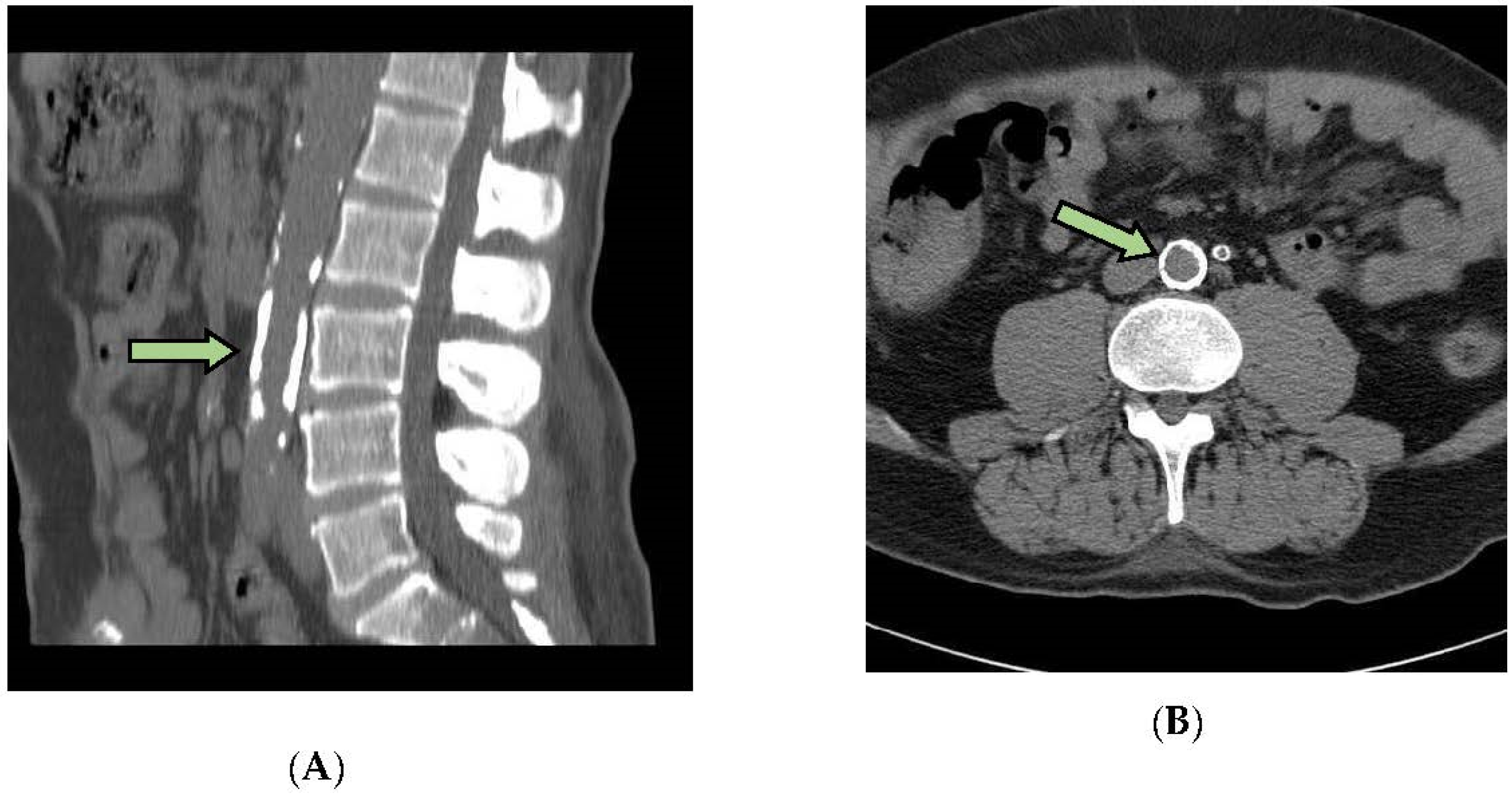
2.6. Measurement of Arterial Calcifications
2.7. Statistical Analysis
3. Results
3.1. Baseline Biochemical Data
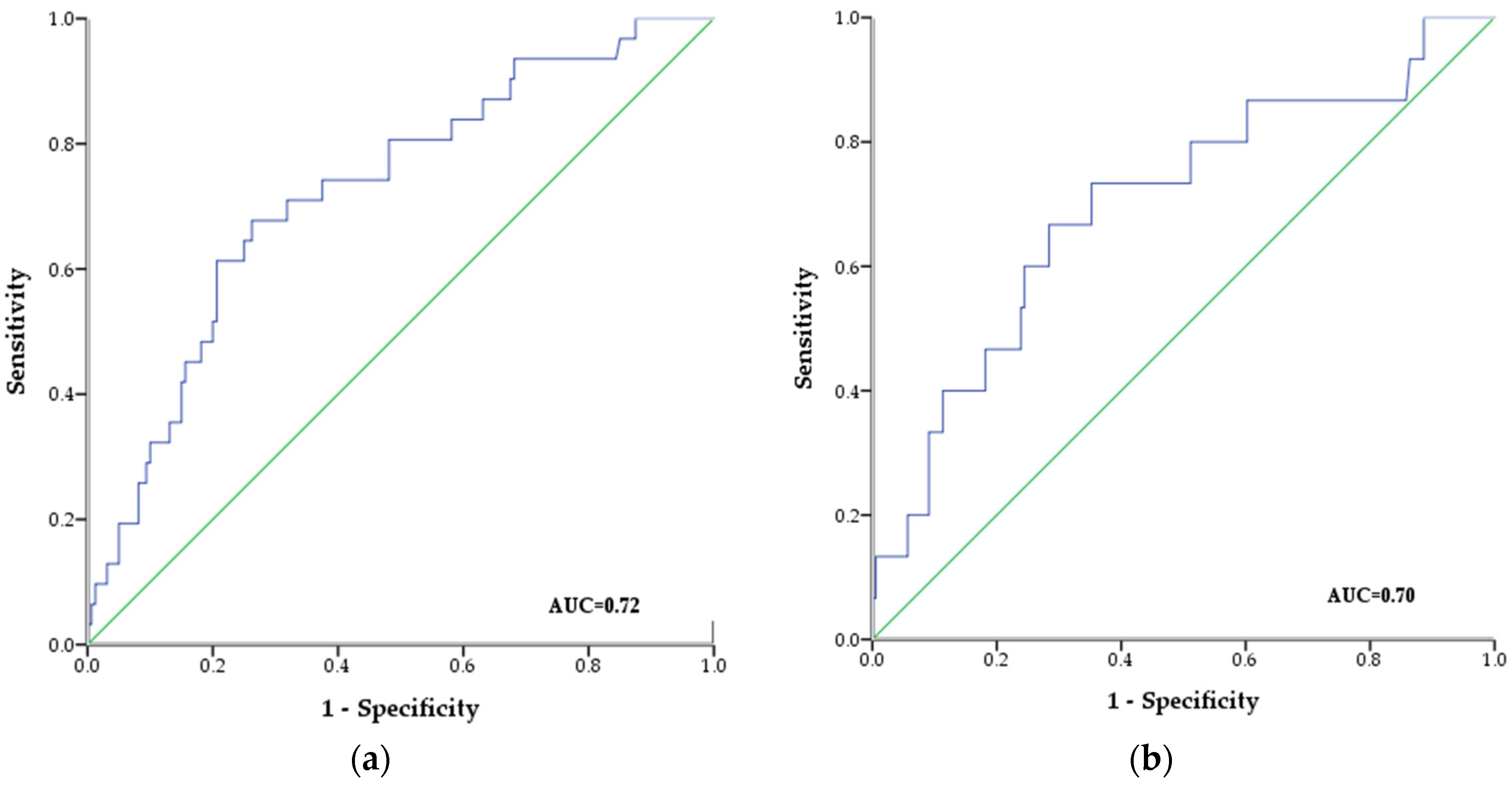
3.2. Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, R.N.; Murray, A.M.; Li, S.; Herzog, C.A.; McBean, A.M.; Eggers, P.W.; Collins, A.J. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. JASN 2005, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Arteriosclerosis and arterial calcifications in chronic kidney insufficiency. Nephrol. Ther. 2005, 1 (Suppl. 4), S351–S354. [Google Scholar] [PubMed]
- Gorriz, J.L.; Molina, P.; Cerveron, M.J.; Vila, R.; Bover, J.; Nieto, J.; Barril, G.; Martinez-Castelao, A.; Fernandez, E.; Escudero, V.; et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 654–666. [Google Scholar] [CrossRef]
- Raggi, P.; Boulay, A.; Chasan-Taber, S.; Amin, N.; Dillon, M.; Burke, S.K.; Chertow, G.M. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002, 39, 695–701. [Google Scholar] [CrossRef]
- Okuno, S.; Ishimura, E.; Kitatani, K.; Fujino, Y.; Kohno, K.; Maeno, Y.; Maekawa, K.; Yamakawa, T.; Imanishi, Y.; Inaba, M.; et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; London, G. Con: Vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2015, 30, 352–357. [Google Scholar] [CrossRef]
- Raggi, P.; Chertow, G.M.; Torres, P.U.; Csiky, B.; Naso, A.; Nossuli, K.; Moustafa, M.; Goodman, W.G.; Lopez, N.; Downey, G.; et al. The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2011, 26, 1327–1339. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Bernardi, S.; Voltan, R.; Rimondi, E.; Melloni, E.; Milani, D.; Cervellati, C.; Gemmati, D.; Celeghini, C.; Secchiero, P.; Zauli, G.; et al. TRAIL, OPG, and TWEAK in kidney disease: Biomarkers or therapeutic targets? Clin. Sci. 2019, 133, 1145–1166. [Google Scholar] [CrossRef]
- Kurz, K.; Herold, M.; Russe, E.; Klotz, W.; Weiss, G.; Fuchs, D. Effects of Antitumor Necrosis Factor Therapy on Osteoprotegerin, Neopterin, and sRANKL Concentrations in Patients with Rheumatoid Arthritis. Dis. Markers 2015, 2015, 276969. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321–329. [Google Scholar] [CrossRef]
- Janda, K.; Krzanowski, M.; Chowaniec, E.; Kusnierz-Cabala, B.; Dumnicka, P.; Krasniak, A.; Podolec, P.; Sulowicz, W. Osteoprotegerin as a marker of cardiovascular risk in patients on peritoneal dialysis. Pol. Arch. Med. Wewn. 2013, 123, 149–155. [Google Scholar] [CrossRef]
- Winther, S.; Christensen, J.H.; Flyvbjerg, A.; Schmidt, E.B.; Jorgensen, K.A.; Skou-Jorgensen, H.; Svensson, M. Osteoprotegerin and mortality in hemodialysis patients with cardiovascular disease. Clin. Nephrol. 2013, 80, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Avignon, A.; Sultan, A.; Piot, C.; Mariano-Goulart, D.; Thuan Dit Dieudonne, J.F.; Cristol, J.P.; Dupuy, A.M. Osteoprotegerin: A novel independent marker for silent myocardial ischemia in asymptomatic diabetic patients. Diabetes Care 2007, 30, 2934–2939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pedersen, E.R.; Ueland, T.; Seifert, R.; Aukrust, P.; Schartum-Hansen, H.; Ebbing, M.; Bleie, O.; Igland, J.; Svingen, G.; Nordrehaug, J.E.; et al. Serum osteoprotegerin levels and long-term prognosis in patients with stable angina pectoris. Atherosclerosis 2010, 212, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Gavriatopoulou, M.; Dimopoulos, M.A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Roussou, M.; Pamboucas, C.; Toumanidis, S.T.; Terpos, E. Osteoprotegerin is a significant prognostic factor for overall survival in patients with primary systemic amyloidosis independent of the Mayo staging. Blood Cancer J. 2015, 5, e319. [Google Scholar] [CrossRef][Green Version]
- Vik, A.; Mathiesen, E.B.; Brox, J.; Wilsgaard, T.; Njolstad, I.; Jorgensen, L.; Hansen, J.B. Serum osteoprotegerin is a predictor for incident cardiovascular disease and mortality in a general population: The Tromso Study. J. Thromb. Haemost. JTH 2011, 9, 638–644. [Google Scholar] [CrossRef]
- Lo, W.K.; Bargman, J.M.; Burkart, J.; Krediet, R.T.; Pollock, C.; Kawanishi, H.; Blake, P.G.; ISPD Adequacy of Peritoneal Dialysis Working Group. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit. Dial. Int. 2006, 26, 520–522. [Google Scholar]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Scialla, J.J.; Kao, W.H.; Crainiceanu, C.; Sozio, S.M.; Oberai, P.C.; Shafi, T.; Coresh, J.; Powe, N.R.; Plantinga, L.C.; Jaar, B.G.; et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Avila-Diaz, M.; Mora-Villalpando, C.; Prado-Uribe Mdel, C.; Orihuela-Rodriguez, O.; Villegas-Antelo, E.; Gomez-Noriega, A.M.; Villanueva-Noches, D.; Hinojosa-Heredia, H.; Serrato-Avila, J.; Ilabaca, B.; et al. De novo development of heart valve calcification in incident peritoneal dialysis patients. Arch. Med. Res. 2013, 44, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Mora, C.; Prado, M.D.C.; Zavala, M.; Paniagua, R.; Mexican Collaborative, G. Osteoprotegerin Is the Strongest Predictor for Progression of Arterial Calcification in Peritoneal Dialysis Patients. Am. J. Nephrol. 2017, 46, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Collado, S.; Coll, E.; Nicolau, C.; Azqueta, M.; Pons, M.; Cruzado, J.M.; de la Torre, B.; Deulofeu, R.; Mojal, S.; Pascual, J.; et al. Serum osteoprotegerin in prevalent hemodialysis patients: Associations with mortality, atherosclerosis and cardiac function. BMC Nephrol. 2017, 18, 290. [Google Scholar] [CrossRef] [PubMed]
- Kuzniewski, M.; Fedak, D.; Dumnicka, P.; Stepien, E.; Kusnierz-Cabala, B.; Cwynar, M.; Sulowicz, W. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 2016, 61, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.K.; Levin, A.; Er, L.; McIntyre, C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2009, 24, 3157–3162. [Google Scholar] [CrossRef][Green Version]
- Marques, G.L.; Hayashi, S.; Bjallmark, A.; Larsson, M.; Riella, M.; Olandoski, M.; Lindholm, B.; Nascimento, M.M. Osteoprotegerin is a marker of cardiovascular mortality in patients with chronic kidney disease stages 3–5. Sci. Rep. 2021, 11, 2473. [Google Scholar] [CrossRef]
- Huang, Q.X.; Li, J.B.; Huang, N.; Huang, X.W.; Li, Y.L.; Huang, F.X. Elevated Osteoprotegerin Concentration Predicts Increased Risk of Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2020, 45, 565–575. [Google Scholar] [CrossRef]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef]
- Sato, K.; Niessner, A.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 2006, 203, 239–250. [Google Scholar] [CrossRef]
- McGonigle, J.S.; Giachelli, C.M.; Scatena, M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis 2009, 12, 35–46. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Q.; Liu, S.Q.; Zhang, Y.; Wang, Y.; Chu, C.; Wang, D.; Pan, S.; Wang, J.K.; Yu, Q.; Mu, J.J. Effects of Salt Loading on Plasma Osteoprotegerin Levels and Protective Role of Potassium Supplement in Normotensive Subjects. Circ. J. 2016, 81, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Avila-Diaz, M.; Ventura, M.D.; Valle, D.; Vicente-Martinez, M.; Garcia-Gonzalez, Z.; Cisneros, A.; Furlong, M.D.; Gomez, A.M.; Prado-Uribe, M.D.; Amato, D.; et al. Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2006, 26, 574–580. [Google Scholar] [CrossRef]
- Matsubara, K.; Stenvinkel, P.; Qureshi, A.R.; Carrero, J.J.; Axelsson, J.; Heimburger, O.; Barany, P.; Alvestrand, A.; Lindholm, B.; Suliman, M.E. Inflammation modifies the association of osteoprotegerin with mortality in chronic kidney disease. J. Nephrol. 2009, 22, 774–782. [Google Scholar]
- Baud’huin, M.; Duplomb, L.; Teletchea, S.; Lamoureux, F.; Ruiz-Velasco, C.; Maillasson, M.; Redini, F.; Heymann, M.F.; Heymann, D. Osteoprotegerin: Multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013, 24, 401–409. [Google Scholar] [CrossRef]
- Collin-Osdoby, P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ. Res. 2004, 95, 1046–1057. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Gregg, L.P.; Adams-Huet, B.; Li, X.; Colbert, G.; Jain, N.; de Lemos, J.A.; Hedayati, S.S. Effect Modification of Chronic Kidney Disease on the Association of Circulating and Imaging Cardiac Biomarkers With Outcomes. J. Am. Heart Assoc. 2017, 6, e005235. [Google Scholar] [CrossRef]
- Noordzij, M.; Korevaar, J.C.; Bos, W.J.; Boeschoten, E.W.; Dekker, F.W.; Bossuyt, P.M.; Krediet, R.T. Mineral metabolism and cardiovascular morbidity and mortality risk: Peritoneal dialysis patients compared with haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2006, 21, 2513–2520. [Google Scholar] [CrossRef]
- Paniagua, R.; Ventura, M.D.; Avila-Diaz, M.; Hinojosa-Heredia, H.; Mendez-Duran, A.; Cisneros, A.; Gomez, A.M.; Cueto-Manzano, A.; Trinidad, P.; Obrador, G.T.; et al. Reaching targets for mineral metabolism clinical practice guidelines and its impact on outcomes among Mexican chronic dialysis patients. Arch. Med. Res. 2013, 44, 229–234. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]

| Variables | Mean ± SD |
|---|---|
| Number of patients. | 197 |
| Age (year) | 43.97 ± 12.92 |
| Sex: male (%) | 64% |
| Diabetes (%) | 53% |
| CAPD/APA (%) | 73/27% |
| Systolic blood pressure (mmHg) | 136.41 ± 26.44 |
| Diastolic blood pressure (mmHg) | 84.20 ± 15.71 |
| Total Kt/V, median (IQR) | 1.46(1.07–2.3) |
| Patients prescribed with Ca CO3 n (%) | 125(63.5) |
| Patients prescribed with Calcitriol n (%) | 105 (53.3) |
| Body mass index (kg/m2) | 24.83 ± 4.28 |
| Hemoglobin Hg (g/dL) | 10.15 ± 2.44 |
| Glucose (mg/dL) | 97.9 (87.6–138.1) |
| Creatinine (mg/dL) | 8.66 ± 3.22 |
| Total cholesterol (mg/dL) | 192.12 ± 44.26 |
| Triglycerides, (mg/dL), median(IQR) | 155 (119.1–226.3) |
| Albumin (g/dL) | 3.41 ± 0.51 |
| cCa(mg/dL) | 9.1 ± 1.38 |
| Phosphorus, mg/dL, median (IQR) | 4.5 (1.6–9.8) |
| Intact parathormone pg/mL, median(IQR) | 104.8 (50.4–199.7) |
| Osteocalcin, ng/mL, median (IQR) | 186.2 (105.4–300) |
| Ln C-Reactive protein, mg/L, median (IQR) | 1.8 (0.6–5.0) |
| Alkaline phosphatase, U/L, median (IQR) | 99.4 (77.2–138.2) |
| Osteoprotegerin, pmol/L median (IQR) | 11.28 (7.6–17.1) |
| Vascular calcification, CaSc, median (IQR) | 424 (101–886) |
| Variables | Age | SBP | cCa | PO4 | iPTH | OPG | Alb | CRP | |
|---|---|---|---|---|---|---|---|---|---|
| SBP | r | 0.142 0.055 | 10.000 | ||||||
| p | |||||||||
| cCa | r | 0.091 0.222 | −0.034 0.643 | 10.000 | |||||
| p | |||||||||
| P | r | −0.197 0.008 b | −0.055 0.459 | 0.094 0.206 | 10.000 | ||||
| p | |||||||||
| iPTH | r | −0.212 0.004 b | 0.123 0.098 | −0.323 0.001a | 0.092 0.215 | 10.000 | |||
| p | |||||||||
| OPG | r | 0.333 0.001 a | 0.134 0.070 | 0.161 0.030 c | −0.023 0.754 | −0.087 0.242 | 10.000 | ||
| p | |||||||||
| Alb | r | −0.045 0.545 | −0.108 0.147 | −0.090 0.227 | 0.149 0.044 c | 0.078 0.292 | −0.180 0.015 c | 10.000 | |
| p | |||||||||
| CRP | r | −0.029 0.694 | −0.069 0.351 | −0.044 0.552 | 0.041 0.585 | 0.001 0.986 | −0.029 0.694 | −0.153 0.039 c | 10.000 |
| p | |||||||||
| VC | r | 0.113 0.127 | 0.064 0.393 | 0.066 0.376 | 0.038 0.606 | 0.002 0.982 | 0.200 0.007 b | 0.015 0.836 | 0.015 0.839 |
| P |
| Variables | Survivors (n = 163) | Non-Survivors (n = 34) | p-Value |
|---|---|---|---|
| Age (year) | 42.25 ± 12.98 | 51.06 ± 9.91 | 0.001 a |
| Sex, male, (%) | 50.3% | 40% | 0.840 |
| Diabetes n (%) | 76 (46.6%) | 29 (85.3%) | 0.001 a |
| CAPD/APD (%) | (51.5%)/(48.5%) | (43.2%)/(56.8%) | 0.400 |
| Urine volume (mL/24 h) | 573.78 ± 516.71 | 585.7 ± 547.97 | 0.904 |
| Systolic blood pressure (mmHg) | 134.22 ± 26.34 | 146.79 ± 24.72 | 0.011 b |
| Dyastolic blood pressure (mmHg) | 84.61 ± 16.02 | 82.26 ± 14.25 | 0.431 |
| total Kt/V | 1.81 ± 1.1 | 1.85 ± 1.2 | 0.809 |
| Body mass index (kg/m2) | 24.57 ± 4.32 | 26.05 ± 3.94 | 0.067 |
| Hemoglobin (g/dL) | 10.03 ± 2.42 | 10.69 ± 3.01 | 0.171 |
| Glucose (mg/dL) | 95.7 (86.7–129.4) | 115.2 (95.9–185.9) | 0.004 a |
| Creatinine (mg/dL) | 8.81 ± 3.28 | 7.98 ± 2.89 | 0.173 |
| BUN (mg/dL) | 56.23 ± 19.79 | 57.07 ± 21.19 | 0.824 |
| Total Cholesterol (mg/dL) | 193.16 ± 44.04 | 187.14 ± 45.61 | 0.472 |
| Triglycerides (mg/dL) | 163.2 (112.6–232.1) | 140.6 (104.7–209.9) | 0.175 |
| Albumin (g/dL) | 3.48 ± 0.51 | 3.10 ± 0.44 | 0.001 a |
| Calcium (mg/dL) | 8.69 ± 1.17 | 8.76 ± 2.07 | 0.798 |
| cCa (mg/dL) | 9.09 ± 1.21 | 9.47 ± 2.07 | 0.168 |
| Phosphorus (mg/dL) | 4.45 (3.7–5.8) | 4.7 (3.5–6.0) | 0.663 |
| iPTH (pg/mL) | 107.15 (55.3–199.5) | 97.8 (22.2–216.7) | 0.922 |
| Osteocalcin (ng/mL) | 195.8 (104–300) | 111.5 (109–285) | 0.606 |
| Ln C-reactive protein (mg/L) | 0.53 (−0.5–1.6) | 0.69 (−0.5–1.9) | 0.519 |
| Alkaline phosphatase (U/L) | 95.5 (76.8–130.7) | 111.5 (86.4–151.3) | 0.249 |
| Osteoprotegerin (pmol/L) | 10.33 (7.23–15.34) | 17.61 (10.48–23) | 0.005 |
| Vascular calcification (Ca Sc) | 306.83 ± 898.91 | 960.16 ± 1888.08 | 0.014 a |
| Univariate Cox Regression Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | |||||||
| Variable | p-Value | HR | CI 95% | p-Value | HR | 95% CI | ||
| Low | Upper | p | Low | Upper | ||||
| Diabetes (non) | 0.001 a | 0.182 | 0.070 | 0.472 | 0.013 b | 0.15 | 0.03 | 0.673 |
| Age (y) | 0.001 a | 1.018 | 1.004 | 1.031 | 0.014 b | 1.08 | 1.02 | 1.15 |
| SBP (mmHg) | 0.009 b | 1.02 | 1.00 | 1.03 | 0.107 | 1.02 | 0.99 | 1.04 |
| OPG(pmol/L) | 0.013 b | 1.09 | 1.01 | 1.16 | 0.001 a | 1.10 | 1.04 | 1.17 |
| sAlb (g/dL) | 0.001 a | 0.12 | 0.06 | 0.23 | 0.016 b | 0.30 | 0.11 | 0.79 |
| cCa (mg/dL) | 0.119 | 1.29 | 0.88 | 1.91 | 0.001 a | 1.41 | 1.15 | 1.73 |
| VC (CaSc) | 0.019 b | 1.00 | 1.00 | 1.00 | 0.003 a | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.723 | 0.96 | 0.76 | 1.21 | 0.885 | 0.90 | 0.69 | 1.37 |
| iPTH (pg/mL) | 0.530 | 1.00 | 1.00 | 1.01 | 0.965 | 1.00 | 0.99 | 1.01 |
| LnCRP (mg/L) | 0.057 | 1.28 | 0.99 | 1.65 | 0.147 | 1.32 | 0.91 | 1.92 |
| Multivariate Cox Regression Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | |||||||
| Variable: | p | HR | 95% CI | p | HR | 95% CI | ||
| Model 1: OPG (pmol/L) | 0.002 a | 1.08 | 1.03 | 1.14 | 0.013 b | 1.09 | 1.02 | 1.66 |
| Alb (g/dL) | 0.012 b | 0.35 | 0.15 | 0.79 | 0.194 | 0.45 | 0.13 | 1.50 |
| cCa (mg/dL) | 0.353 | 1.13 | 0.87 | 1.46 | 0.001 a | 1.53 | 1.19 | 1.98 |
| VC (CaSc) | 0.616 | 1.00 | 1.00 | 1.00 | 0.163 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.445 | 1.11 | 0.85 | 1.44 | 0.691 | 0.91 | 0.59 | 1.40 |
| PTH (pg/mL) | 0.297 | 1.00 | 0.99 | 1.01 | 0.030 b | 1.00 | 1.00 | 1.01 |
| LnCRP(mg/L) | 0.090 | 1.29 | 0.96 | 1.74 | 0.092 | 1.45 | 0.94 | 2.25 |
| Model 2: Model 1 + DM | ||||||||
| OPG (pmol/L) | 0.040 | 1.06 | 1.00 | 1.12 | 0.279 | 1.05 | 0.96 | 1.13 |
| Alb (g/dL) | 0.054 | 0.43 | 0.182 | 1.05 | 0.385 | 0.58 | 0.17 | 1.98 |
| cCa (mg/dL) | 0.231 | 1.17 | 0.90 | 1.53 | 0.001 a | 1.65 | 1.25 | 2.17 |
| VC (CaSc) | 0.791 | 1.00 | 1.00 | 1.00 | 2.242 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.353 | 1.41 | 0.86 | 1.50 | 0.843 | 0.96 | 0.62 | 1.48 |
| iPTH (pg/mL) | 0.154 | 1.002 | 0.99 | 1.00 | 0.008 b | 1.00 | 1.00 | 1.01 |
| Ln CRP (mg/L) | 0.069 | 1.32 | 0.97 | 1.79 | 0.060 | 1.54 | 0.982 | 2.415 |
| DM (yes) | 0.087 | 0.343 | 0.101 | 1.16 | 0.054 | 7.14 | 0.966 | 52.786 |
| Model 3: Model 2 + Age | ||||||||
| OPG (pmol/L) | 0.127 | 1.048 | 0.987 | 1.112 | 0.409 | 1.03 | 0.954 | 1.124 |
| Alb (g/dL) | 0.042 b | 0.411 | 0.174 | 1.53 | 0.155 | 0.420 | 0.127 | 1.389 |
| cCa (mg/dL) | 0.225 | 1.181 | 0.903 | 1.544 | 0.001 a | 1.67 | 1.25 | 2.219 |
| VC (CaSc) | 0.819 | 1.00 | 1.00 | 1.00 | 0.190 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.252 | 1.18 | 0.89 | 1.56 | 0.891 | 0.970 | 0.624 | 1.508 |
| iPTH (pg/mL) | 0.225 | 1.18 | 0.90 | 1.54 | 0.012 b | 1.01 | 1.00 | 1.009 |
| lnCRP (mg/L) | 0.074 | 1.32 | 0.97 | 1.79 | 0.079 | 1.51 | 0.954 | 2.393 |
| DM (yes) | 0.321 | 0.51 | 0.13 | 1.93 | 0.300 | 3.14 | 0.361 | 27.21 |
| Age (y) | 0.227 | 1.03 | 0.98 | 1.08 | 0.030 b | 1.08 | 1.007 | 1.159 |
| Variable | All-Cause Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| Score | p-Value | Score | p-Value | |
| OPG (pmol/L) | 18.77 | 0.001 a | 11.90 | 0.001 a |
| sAlb (g/dL) | 13.84 | 0.001 a | 5.99 | 0.014 b |
| cCa (mg/dL) | 2.24 | 0.134 b | 11.07 | 0.001 a |
| VC (CaSc) | 5.59 | 0.018 | 10.38 | 0.001 a |
| P (mg/dL) | 0.03 | 0.865 | 0.08 | 0.775 |
| iPTH (pg/mL) | 0.16 | 0.690 | 0.04 | 0.838 |
| lnCRP (mg/L) | 3.09 | 0.078 | 2.38 | 0.122 |
| DM | 13.67 | 0.001 a | 8.70 | 0.003 a |
| Age (y) | 10.56 | 0.006 b | 7.55 | 0.006 b |
| SBP (mmHg) | 5.24 | 0.022 b | 2.54 | 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, M.; Prado, M.d.C.; Romero, R.; Córdova, R.; Rigo, M.d.C.; Trejo, M.; Mora, C.; Paniagua, R.; for the Mexican Nephrology Collaborative Study Group. Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis. Biomolecules 2022, 12, 551. https://doi.org/10.3390/biom12040551
Ávila M, Prado MdC, Romero R, Córdova R, Rigo MdC, Trejo M, Mora C, Paniagua R, for the Mexican Nephrology Collaborative Study Group. Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis. Biomolecules. 2022; 12(4):551. https://doi.org/10.3390/biom12040551
Chicago/Turabian StyleÁvila, Marcela, Ma. del Carmen Prado, Renata Romero, Ricardo Córdova, Ma. del Carmen Rigo, Miguel Trejo, Carmen Mora, Ramón Paniagua, and for the Mexican Nephrology Collaborative Study Group. 2022. "Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis" Biomolecules 12, no. 4: 551. https://doi.org/10.3390/biom12040551
APA StyleÁvila, M., Prado, M. d. C., Romero, R., Córdova, R., Rigo, M. d. C., Trejo, M., Mora, C., Paniagua, R., & for the Mexican Nephrology Collaborative Study Group. (2022). Osteoprotegerin Is a Better Predictor for Cardiovascular and All-Cause Mortality than Vascular Calcifications in a Multicenter Cohort of Patients on Peritoneal Dialysis. Biomolecules, 12(4), 551. https://doi.org/10.3390/biom12040551






