CAST as a Potential Oncogene, Identified by Machine Search, in Gastric Cancer Infiltrated with Macrophages and Associated with Lgr5
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Cancer Genome Atlas (TCGA) Program Analysis Using Machine Searching
2.2. Protein–Protein Interaction (PPI) Network from STRING
2.3. CAST Bioinformatics Analysis Using Gene Expression Profiling Interactive Analysis 2 (GEPIA2) Datasets
2.4. Using Human Protein Atlas (HPA) for Further Validation of CAST in Different Human Tissues
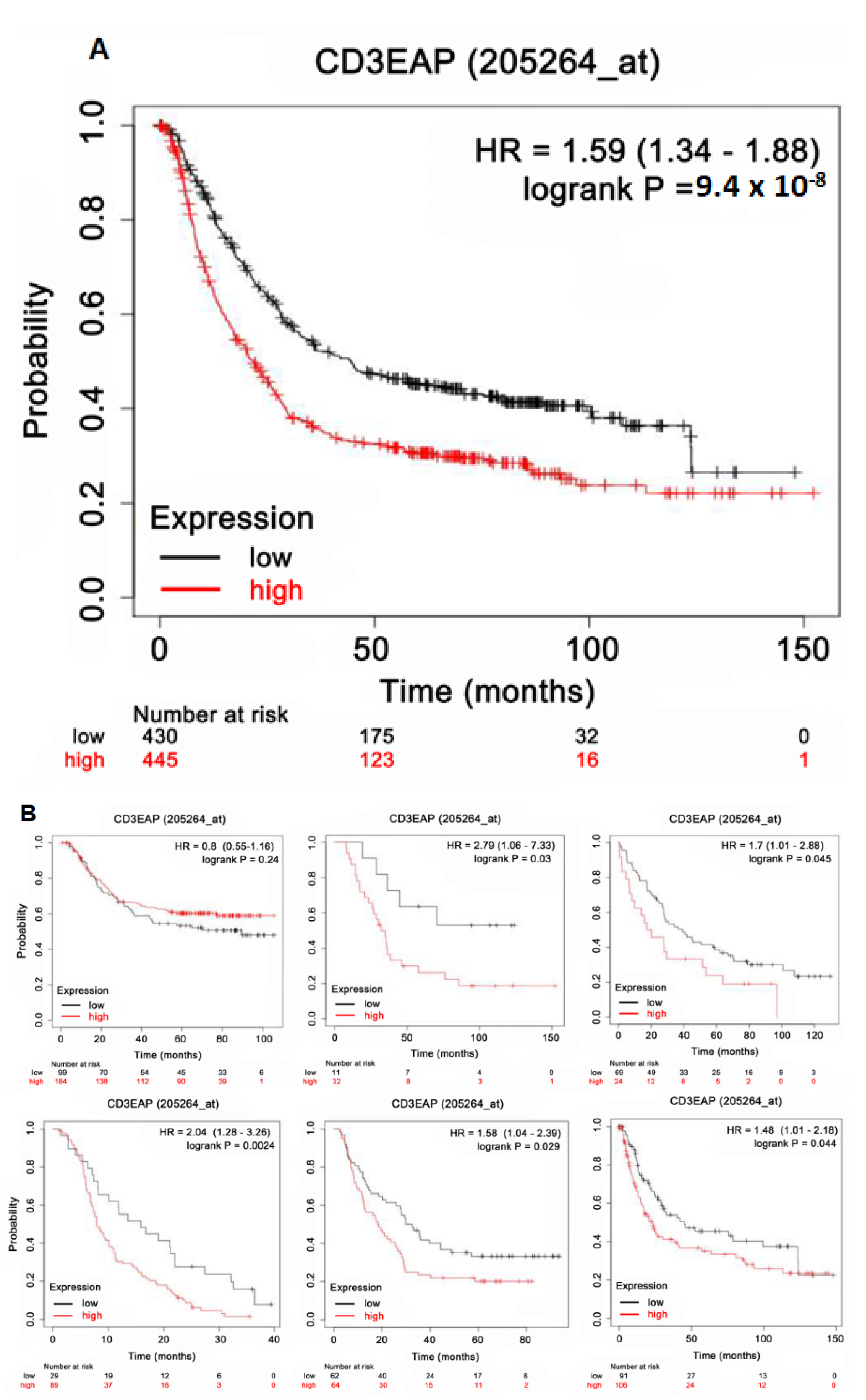
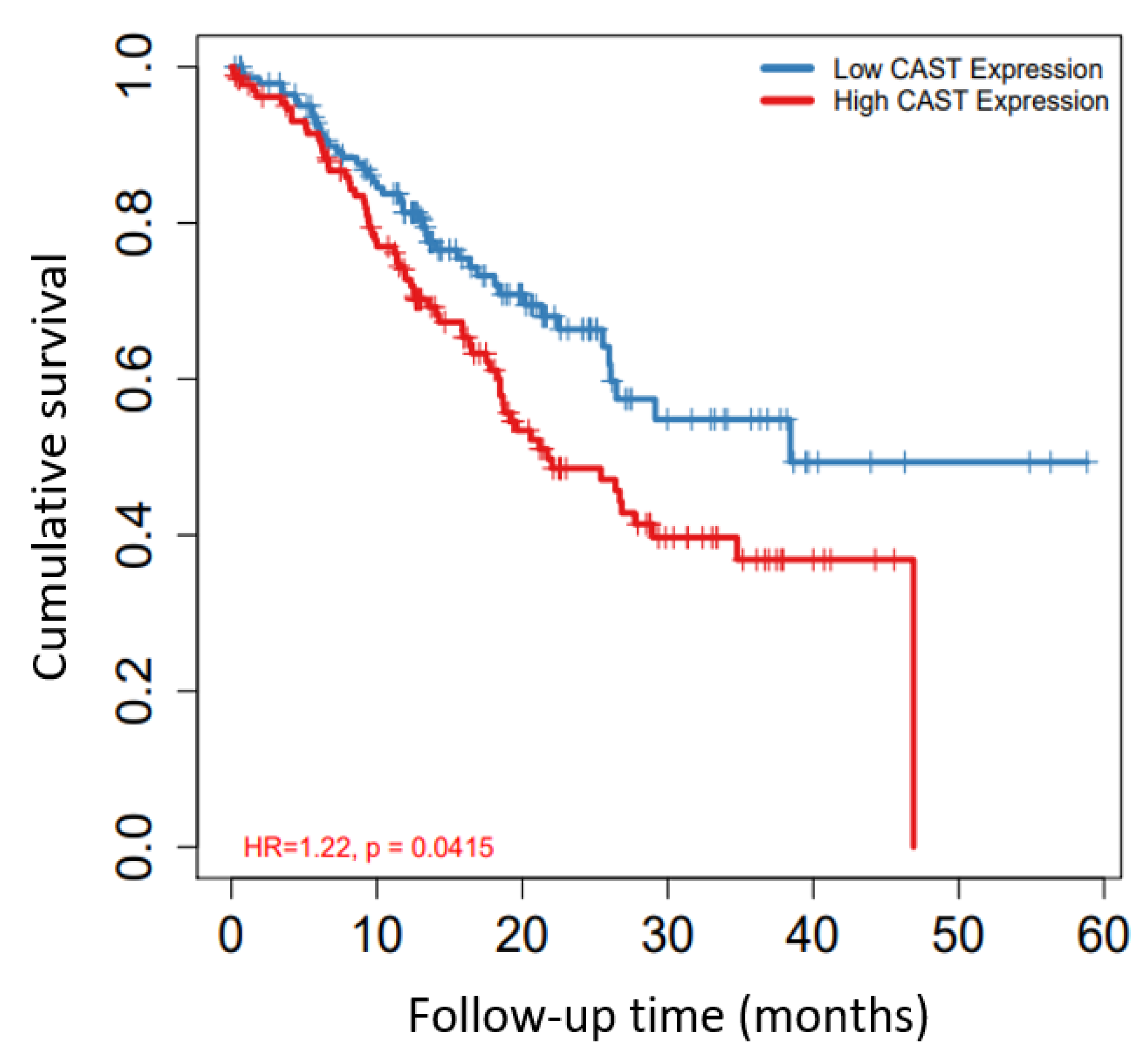
2.5. Survival Analysis Using Kaplan–Meier (KM) Plotter
2.6. TIMER 2.0 Database for Genes and Infiltrating Immune Cells
2.7. Gene and Protein Networks Analysis
2.8. Statistical Analysis
3. Results
3.1. CAST-Centered Network Interaction and Clustering Analysis
3.2. CAST Expression in Different Tissues
3.3. Validation of CAST Expression in GC
3.4. CAST Associated with Survival in GC
3.5. External Validation for KM Plotter Using TIMER 2.0 Datasets
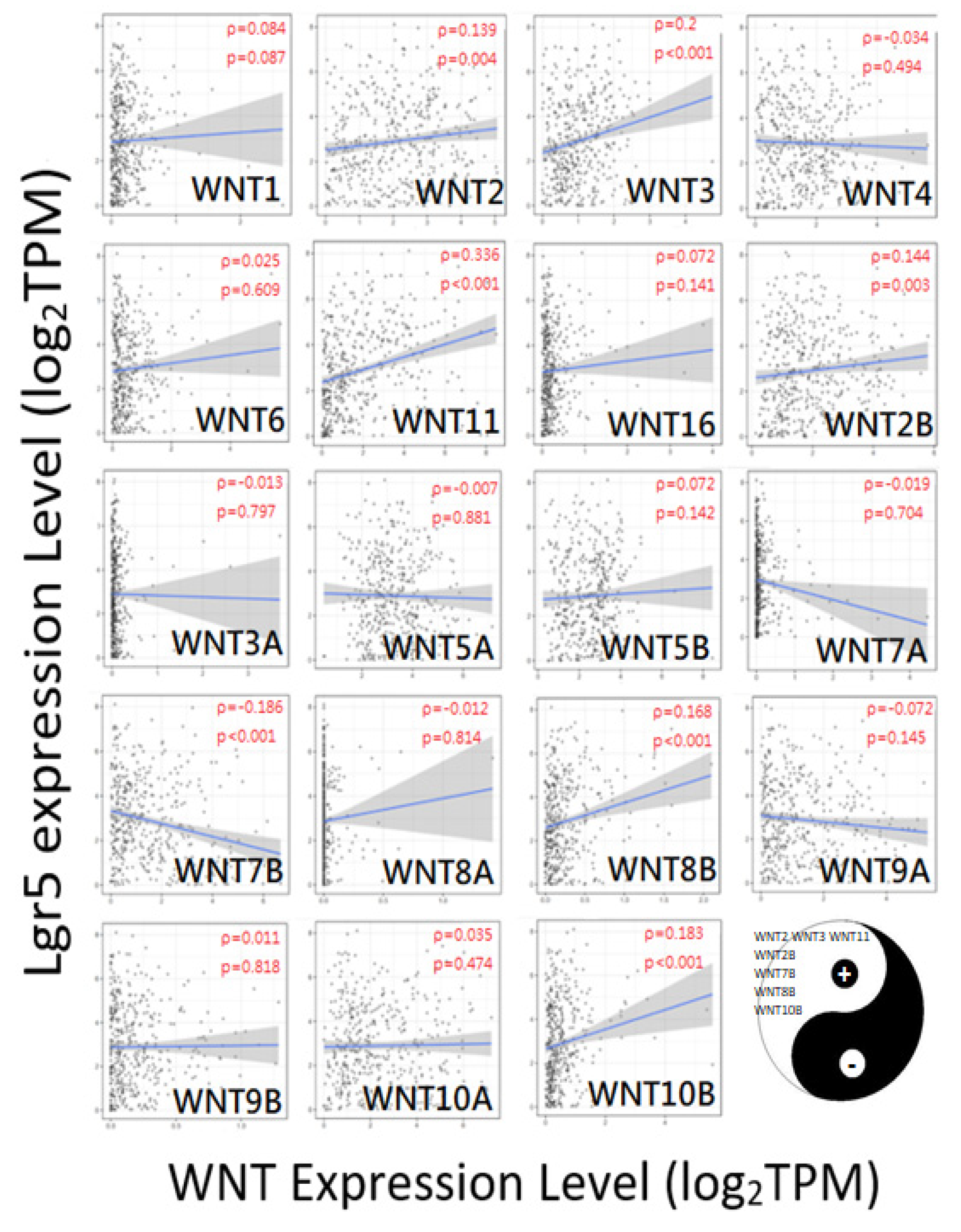
3.6. CAST/WNT/Lgr5 Co-Expressions in GC
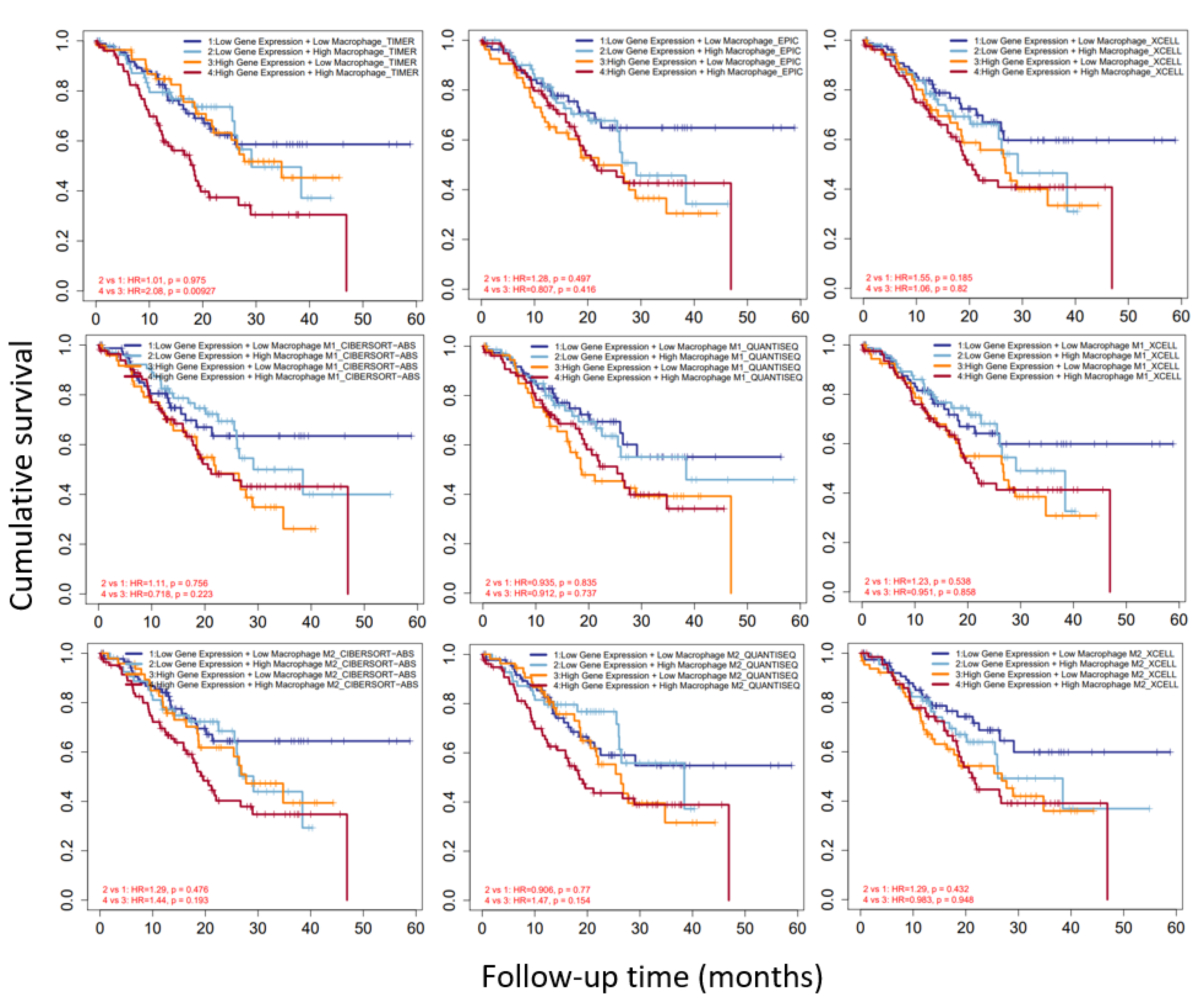
3.7. CAST and Macrophages in GC
3.8. CAST–WNT2/WNT2B–Lgr5 Linkages Associated with Gastric Carcinogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| OS | Overall survival |
| TPM | Transcripts per million |
| FPKM | Fragments per kilobase per million |
| FDR | False-discovery rate |
| ACC | Adrenocortical carcinoma |
| BLCA | Bladder urothelial carcinoma |
| BRCA | Breast invasive carcinoma |
| CESC | Cervical and endocervical cancer |
| CHOL | Cholangiocarcinoma |
| COAD | Colon adenocarcinoma |
| DLBC | Diffuse large B-cell lymphoma |
| ESCA | Esophageal carcinoma |
| GBM | Glioblastoma multiforme |
| HNSC | Head and neck cancer |
| KICH | Kidney chromophobe |
| KIRC | Kidney renal clear-cell carcinoma |
| KIRP | Kidney renal papillary-cell carcinoma |
| LAML | Acute myeloid leukemia |
| LGG | Lower-grade glioma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| MESO | Mesothelioma |
| OV | Ovarian serous cystadenocarcinoma |
| PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| PRAD | Prostate adenocarcinoma |
| READ | Rectum adenocarcinoma |
| SARC | Sarcoma |
| SKCM | Skin cutaneous melanoma |
| STAD | Stomach adenocarcinoma |
| TGCT | Testicular-germ-cell tumors |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| UCEC | Uterine corpus endometrial carcinoma |
| UCS | Uterine carcinosarcoma |
| UVM | Uveal melanoma |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1684–1735. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, Y.; Luan, F.; Yu, Z.; Feng, H.; Chen, B.; Chen, W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021, 10, 3461–3473. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. Familial gastric cancer: Genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015, 16, e60–e70. [Google Scholar] [CrossRef]
- Huntsman, D.G.; Carneiro, F.; Lewis, F.R.; MacLeod, P.M.; Hayashi, A.; Monaghan, K.G.; Maung, R.; Seruca, R.; Jackson, C.E.; Caldas, C. Early Gastric Cancer in Young, Asymptomatic Carriers of Germ-Line E-Cadherin Mutations. N. Engl. J. Med. 2001, 344, 1904–1909. [Google Scholar] [CrossRef]
- Worthley, D.L.; Phillips, K.D.; Wayte, N.; Schrader, K.; Healey, S.; Kaurah, P.; Shulkes, A.; Grimpen, F.; Clouston, A.; Moore, D.; et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut 2012, 61, 774–779. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, Y.; Dong, Y.; Wang, Y.; Kassab, M.A.; Fan, W.; Yu, X.; Wu, C. LGR5 regulates gastric adenocarcinoma cell proliferation and invasion via activating Wnt signaling pathway. Oncogenesis 2018, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Goidts, V.; Nakata, S.; Phillips, E. Emerging role for leucine-rich repeat-containing G-protein-coupled receptors LGR5 and LGR4 in cancer stem cells. Cancer Manag. Res. 2014, 6, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Xie, Y.; Gao, F.; Wang, Y.; Guo, Y.; Tian, H.; Li, Y.; Fan, W. Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene 2013, 525, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.M.; Burchett, R.; Brun, M.; Monckton, E.A.; Poon, H.-Y.; Godbout, R. Effects of nuclear factor I phosphorylation on calpastatin (CAST) gene variant expression and subcellular distribution in malignant glioma cells. J. Biol. Chem. 2019, 294, 1173–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetti, R.; Copetti, T.; Dell’Orso, S.; Melloni, E.; Brancolini, C.; Monte, M.; Schneider, C. The Calpain System Is Involved in the Constitutive Regulation of β-Catenin Signaling Functions. J. Biol. Chem. 2005, 280, 22070–22080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, Y.; Lu, D.; Liu, Y.; Zhang, S.-Q.; Xu, Y.; Li, W.; Gu, X. Comparison of the protein expression of calpain-1, calpain-2, calpastatin and calmodulin between gastric cancer and normal gastric mucosa. Oncol. Lett. 2017, 14, 3705–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J.; Rosengren, R.J.; Sahebkar, A. Tumor-associated macrophages: Role in cancer development and therapeutic implications. Cell. Oncol. 2019, 42, 591–608. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Xiong, D.-L.; Li, Q.; Wang, H.; Jin, W.-L.; Fan, X.-M.; Ma, Y.-Y. High expression of PPM1G is associated with the progression and poor prognosis of hepatocellular carcinoma. Cancer Biomark. 2022, 34, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szász, A.M.; Lánczky, A.; Nagy, A.; Förster, S.; Hark, K.; Green, J.E.; Boussioutas, A.; Busuttil, R.; Szabó, A.; Győrffy, B. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1065 patients. Oncotarget 2016, 7, 49322–49333. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Severson, E.; Pignon, J.-C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [Green Version]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [Green Version]
- Lakshmikuttyamma, A.; Selvakumar, P.; Kanthan, R.; Kanthan, S.C.; Sharma, R.K. Overexpression of m-calpain in human colorectal adenocarcinomas. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1604–1609. [Google Scholar]
- Rios-Doria, J.; Day, K.C.; Kuefer, R.; Rashid, M.G.; Chinnaiyan, A.M.; Rubin, M.A.; Day, M.L. The Role of Calpain in the Proteolytic Cleavage of E-cadherin in Prostate and Mammary Epithelial Cells. J. Biol. Chem. 2003, 278, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Panis, C.; Pizzatti, L.; Corrêa, S.; Binato, R.; Lemos, G.F.; Herrera, A.C.; Seixas, T.F.; Cecchini, R.; Abdelhay, E. The positive is inside the negative: HER2-negative tumors can express the HER2 intracellular domain and present a HER2-positive phenotype. Cancer Lett. 2015, 357, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tian, H.; Lange, A.R.; Yearsley, K.; Robertson, F.M.; Barsky, S.H. The genesis and unique properties of the lymphovascular tumor embolus are because of calpain-regulated proteolysis of E-cadherin. Oncogene 2013, 32, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mao, Y.-H.; Xiao, C.; Li, K.; Liu, W.; Li, L.-Y.; Pang, J. Calpain-2 triggers prostate cancer metastasis via enhancing CRMP4 promoter methylation through NF-κB/DNMT1 signaling pathway. Prostate 2018, 78, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Chiurillo, M.A. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J. Exp. Med. 2015, 5, 84–102. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; Van De Wetering, M.; Snippert, H.J.; Van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Oshima, H.; Oshima, M.; Kai, K.; Torii, R.; Masuko, T.; Baba, H.; Saya, H.; Nagano, O. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010, 101, 673–678. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol. Med. Rep. 2012, 5, 1191–1196. [Google Scholar]
- Mao, J.; Fan, S.; Ma, W.; Fan, P.; Wang, B.; Zhang, J.; Wang, H.; Tang, B.; Zhang, Q.; Yu, X.; et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014, 5, e1039. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-S.; Park, J.-I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.-Y.; Jun, S.; Lee, M.; Kim, H.-C.; Wang, X.; Ji, H.; McCrea, P.D.; Park, J.-I. PAF and EZH2 Induce Wnt/β-Catenin Signaling Hyperactivation. Mol. Cell 2013, 52, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; Van De Wetering, M.; Van Es, J.H.; Mohammed, S.; Heck, A.J.R.; Maurice, M.M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Wang, W.; Jun, S.; Zhang, J.; Srivastava, M.; Kim, M.J.; Lien, E.M.; Shang, J.; Chen, J.; McCrea, P.D.; et al. Deregulation of CRAD-controlled cytoskeleton initiates mucinous colorectal cancer via β-catenin. Nat. Cell Biol. 2018, 20, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Lee, W.-J.; Hua, K.-T.; Kuo, M.-L.; Lin, M.-T. Macrophage Infiltration Induces Gastric Cancer Invasiveness by Activating the β-Catenin Pathway. PLoS ONE 2015, 10, e0134122. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-K.; Wang, M.; Sun, Y.; Di Costanzo, N.; Mitchell, C.; Achuthan, A.; Hamilton, J.A.; Busuttil, R.A.; Boussioutas, A. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat. Commun. 2019, 10, 3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
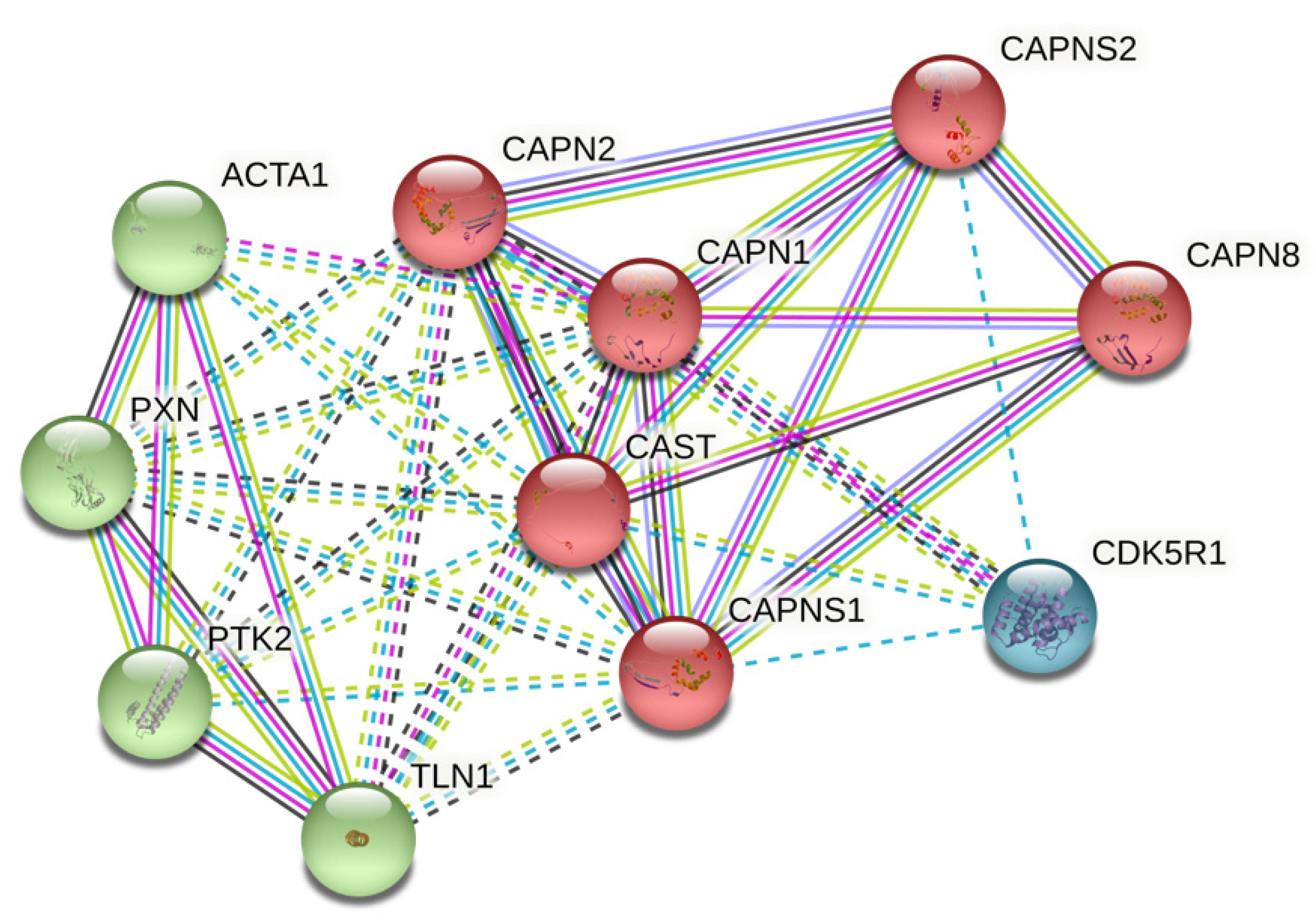




| GO-Term | Description | Count in Network | Strength | FDR |
|---|---|---|---|---|
| Biological Process | ||||
| GO:0022617 | Extracellular matrix disassembly | 4 of 66 | 2.03 | 0.00062 |
| GO:0030198 | Extracellular matrix organization | 5 of 338 | 1.42 | 0.0044 |
| GO:0007172 | Signal complex assembly | 2 of 8 | 2.65 | 0.0414 |
| GO:1901699 | Cellular response to nitrogen compound | 5 of 645 | 1.14 | 0.0414 |
| GO:0090130 | Tissue migration | 3 of 95 | 1.75 | 0.0417 |
| GO:0016043 | Cellular component organization | 10 of 5447 | 0.51 | 0.0425 |
| GO:0010506 | Regulation of autophagy | 4 of 340 | 1.32 | 0.0450 |
| Molecular Function | ||||
| GO:0004198 | Calcium-dependent cysteine-type endopeptidase activity | 4 of 15 | 2.68 | 6.74 × 10−7 |
| GO:0005509 | Calcium-ion binding | 6 of 703 | 1.18 | 0.00094 |
| GO:0050839 | Cell-adhesion-molecule binding | 5 of 538 | 1.22 | 0.0037 |
| GO:0008092 | Cytoskeletal-protein binding | 6 of 973 | 1.04 | 0.0037 |
| GO:0017166 | Vinculin binding | 2 of 11 | 2.51 | 0.0103 |
| GO:0005178 | Integrin binding | 3 of 147 | 1.56 | 0.0254 |
| GO:0043167 | Ion binding | 10 of 6188 | 0.46 | 0.0255 |
| Cellular Component | ||||
| GO:0005925 | Focal adhesion | 5 of 405 | 1.34 | 0.0028 |
| GO:0001725 | Stress fiber | 3 of 65 | 1.91 | 0.0037 |
| GO:0005829 | Cytosol | 10 of 5193 | 0.53 | 0.0037 |
| GO:0031252 | Cell leading edge | 4 of 425 | 1.22 | 0.0125 |
| GO:0015629 | Actin cytoskeleton | 4 of 477 | 1.17 | 0.0176 |
| GO:0030027 | Lamellipodium | 3 of 202 | 1.42 | 0.0271 |
| Site | Tumor (TPM) | Normal (TPM) |
|---|---|---|
| Brain | 51.76 | 20.16 |
| Esophagus | 131.69 | 253.86 |
| Thyroid | 99.97 | 83.24 |
| Thymus | 26.31 | 39.02 |
| Blood | 61.86 | 65.73 |
| Lung | 104.7 | 116.04 |
| Breast | 101.62 | 113.17 |
| Liver | 42.91 | 34.63 |
| Biliary tract | 86.27 | 35.47 |
| Pancreas | 112.23 | 21.35 |
| Stomach | 89.83 | 44.3 |
| Adrenal gland | 67.92 | 61.8 |
| Kidney | 109.01 | 85.14 |
| Colon | 124.76 | 102.46 |
| Bladder | 100.67 | 157.63 |
| Prostate | 92.38 | 89.49 |
| Testis | 17.75 | 125.93 |
| Source | Median Survival | FDR | p-value | |
|---|---|---|---|---|
| Low Expression Cohort (Months) | High Expression Cohort (Months) | |||
| All | 44.57 | 21.93 | 1% | <0.0001 |
| GSE62254 | 18.27 | 22.83 | 100% | 0.2373 |
| GSE22377 | 36.4 | 17.2 | 50% | 0.0297 |
| GSE51105 | 39.2 | 20.1 | >50% | 0.449 |
| GSE14210 | 15.9 | 7.9 | 10% | 0.0024 |
| GSE29272 | 32.6 | 18.6 | >50% | 0.0289 |
| GSE15459 | 45.1 | 22.8 | >50% | 0.0444 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-T.; Yen, C.-C.; Chang, R.; Wang, J.-T.; Chen, J.-S. CAST as a Potential Oncogene, Identified by Machine Search, in Gastric Cancer Infiltrated with Macrophages and Associated with Lgr5. Biomolecules 2022, 12, 670. https://doi.org/10.3390/biom12050670
Yang K-T, Yen C-C, Chang R, Wang J-T, Chen J-S. CAST as a Potential Oncogene, Identified by Machine Search, in Gastric Cancer Infiltrated with Macrophages and Associated with Lgr5. Biomolecules. 2022; 12(5):670. https://doi.org/10.3390/biom12050670
Chicago/Turabian StyleYang, Kuang-Tsu, Chia-Chi Yen, Renin Chang, Jui-Tzu Wang, and Jin-Shuen Chen. 2022. "CAST as a Potential Oncogene, Identified by Machine Search, in Gastric Cancer Infiltrated with Macrophages and Associated with Lgr5" Biomolecules 12, no. 5: 670. https://doi.org/10.3390/biom12050670
APA StyleYang, K.-T., Yen, C.-C., Chang, R., Wang, J.-T., & Chen, J.-S. (2022). CAST as a Potential Oncogene, Identified by Machine Search, in Gastric Cancer Infiltrated with Macrophages and Associated with Lgr5. Biomolecules, 12(5), 670. https://doi.org/10.3390/biom12050670







