Discovery of a Novel Antimicrobial Peptide, Temporin-PKE, from the Skin Secretion of Pelophylax kl. esculentus, and Evaluation of Its Structure-Activity Relationships
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Acquisition of Skin Secretion from Pelophylax kl. esculentus
2.2. ‘Shotgun’ Cloning of the Precursor-Encoding cDNA of the Novel Peptide
2.3. Isolation and Identification of the Novel Peptide
2.4. Peptide Synthesis
2.5. Purification and Identification of the Synthesised Peptides
2.6. Secondary Structure Analyses
2.7. Determination of Minimum Inhibitory (MIC) and Bactericidal (MBC) Concentrations
2.8. Haemolysis Assays
2.9. MTT Assays on HaCaT Cells
2.10. Determination of Minimum Biofilm Inhibitory (MBIC) and Eradication (MBEC) Concentrations
2.11. Time-Killing Assays
2.12. Membrane Permeability Assays
2.13. Salt and Serum Sensitivity Assays
2.14. Anti-Proliferation Assays
2.15. Resistance Induction Assays
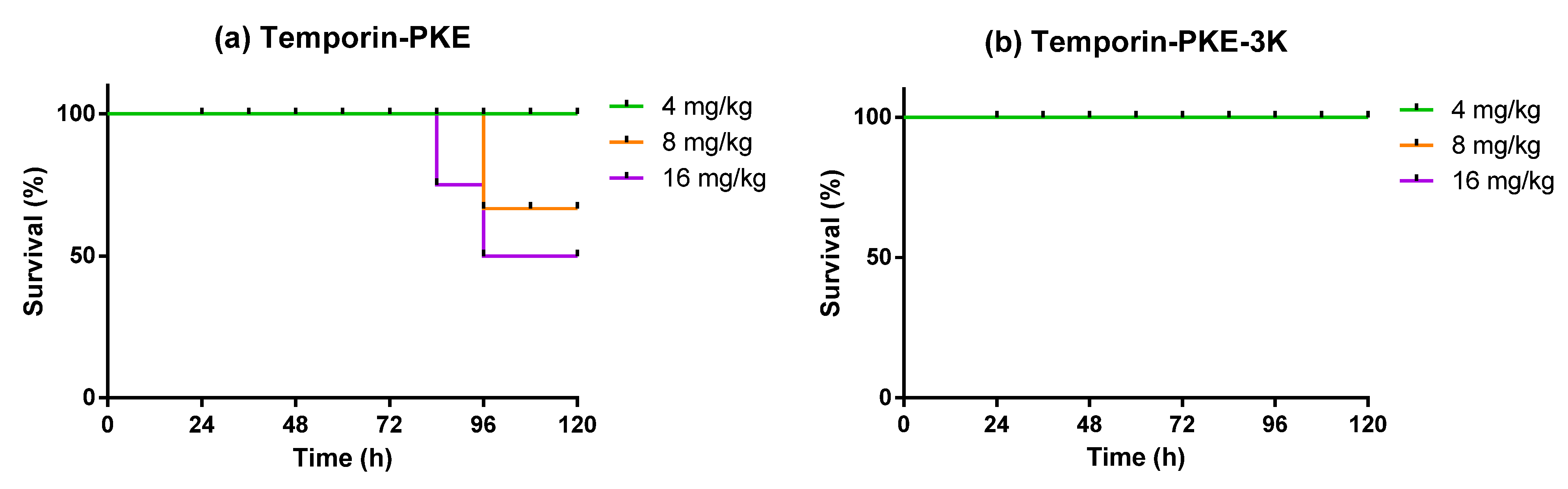
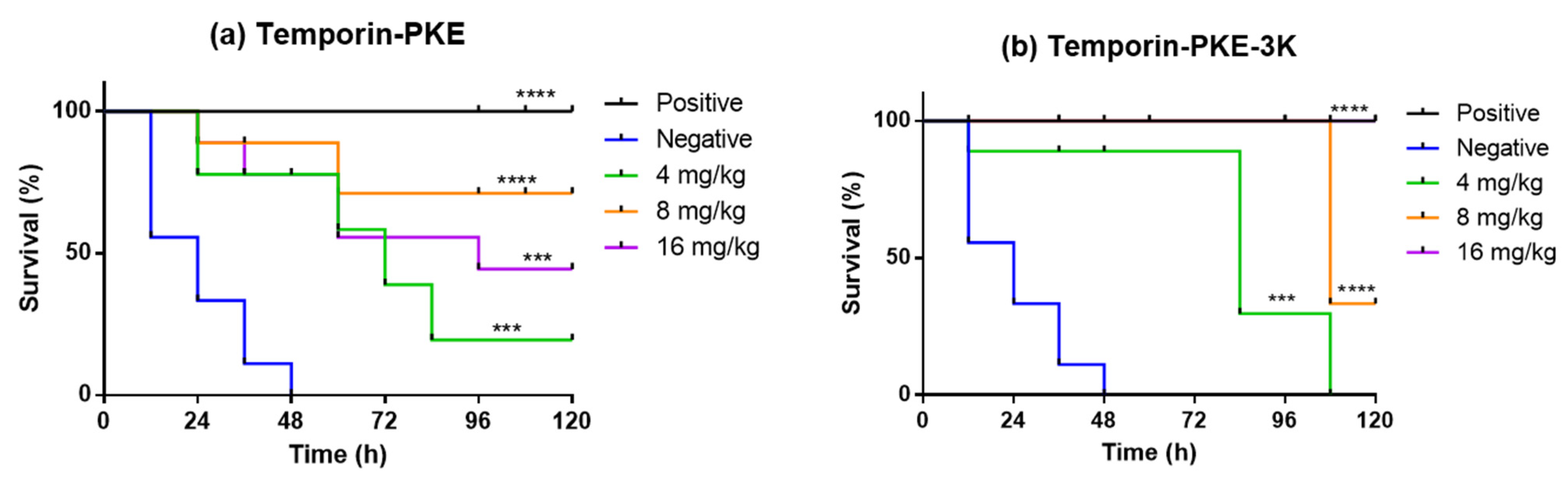
2.16. Determination of Antimicrobial Activity In Vivo
2.17. Statistical Analyses
3. Results
3.1. Molecular Cloning of the Novel Precursor-Encoding cDNA
3.2. Novel Peptide Identification and Sequence Analysis
3.3. Peptide Design
3.4. Purification and Identification of the Analogues
3.5. Secondary Structure Analyses
3.6. Antimicrobial Assays
3.7. Toxicity
3.7.1. Haemolysis Activity
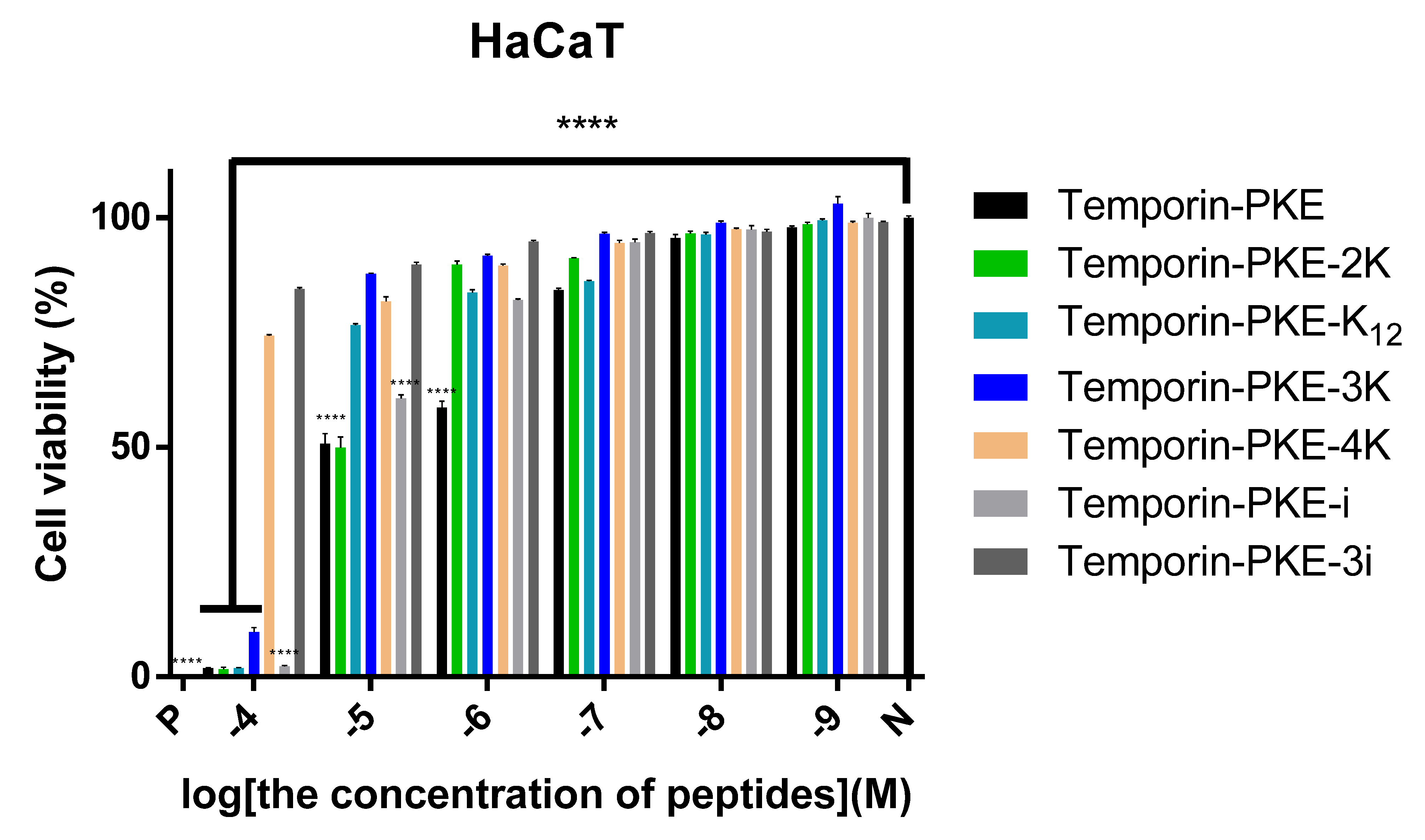
3.7.2. Cytotoxicity on HaCaT Cells
3.8. Antibiofilm Activity
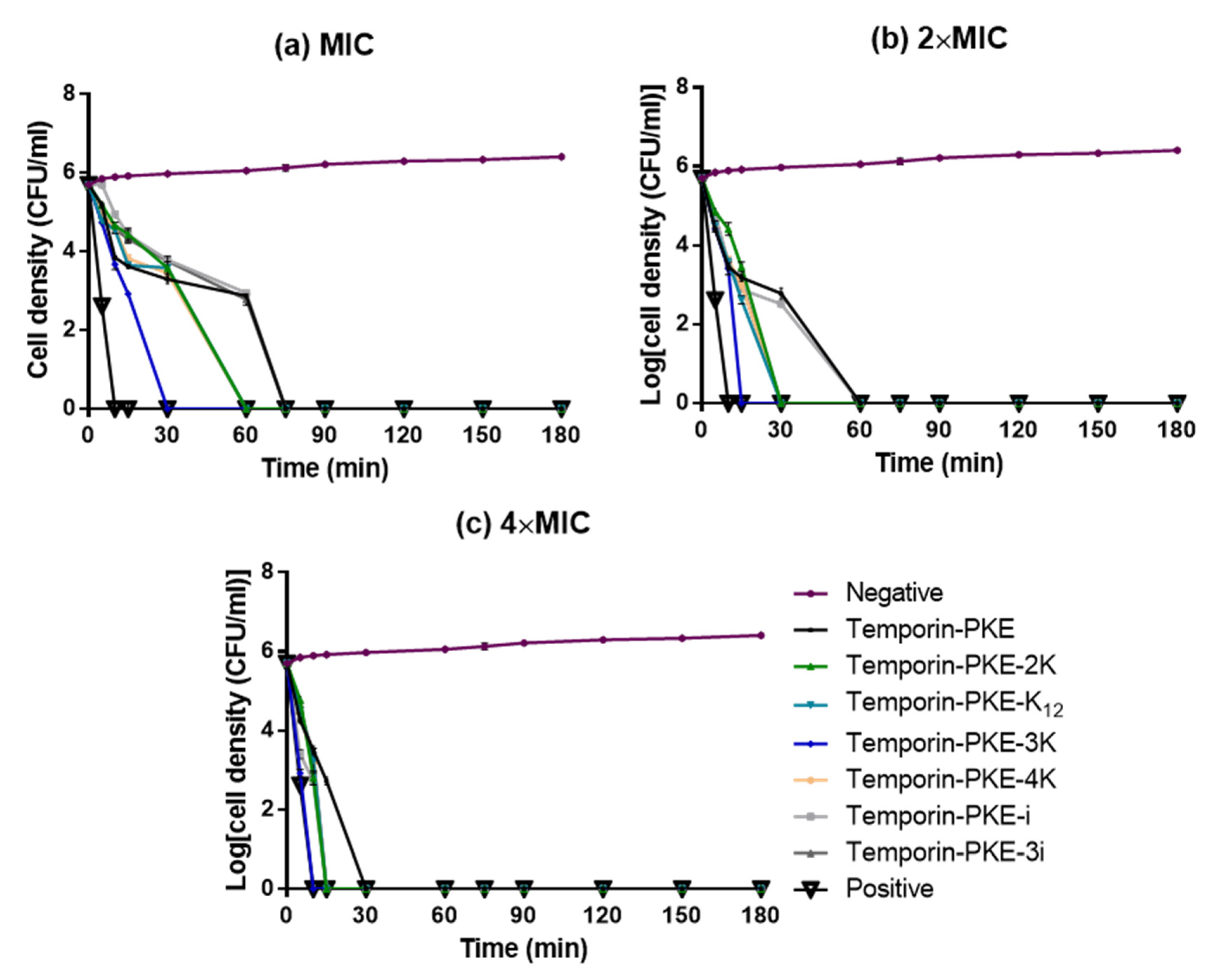
3.9. Bacteria Killing Efficiency
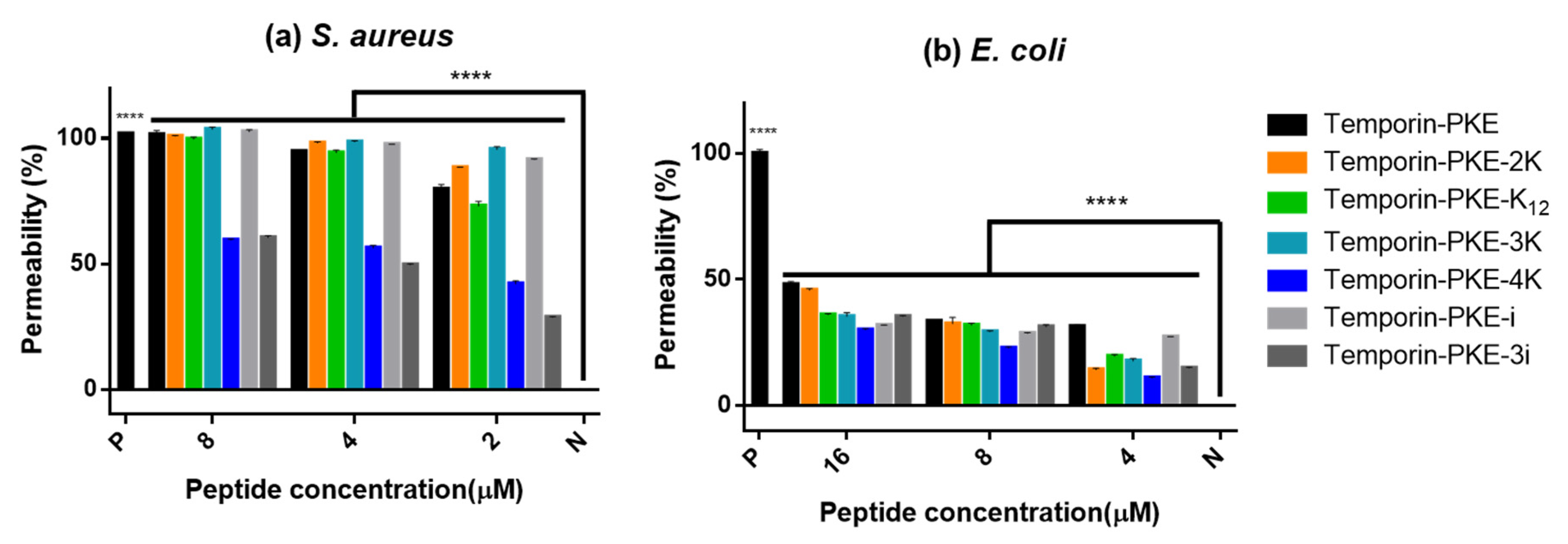
3.10. Membrane Permeability
3.11. Salt and Serum Sensitivities
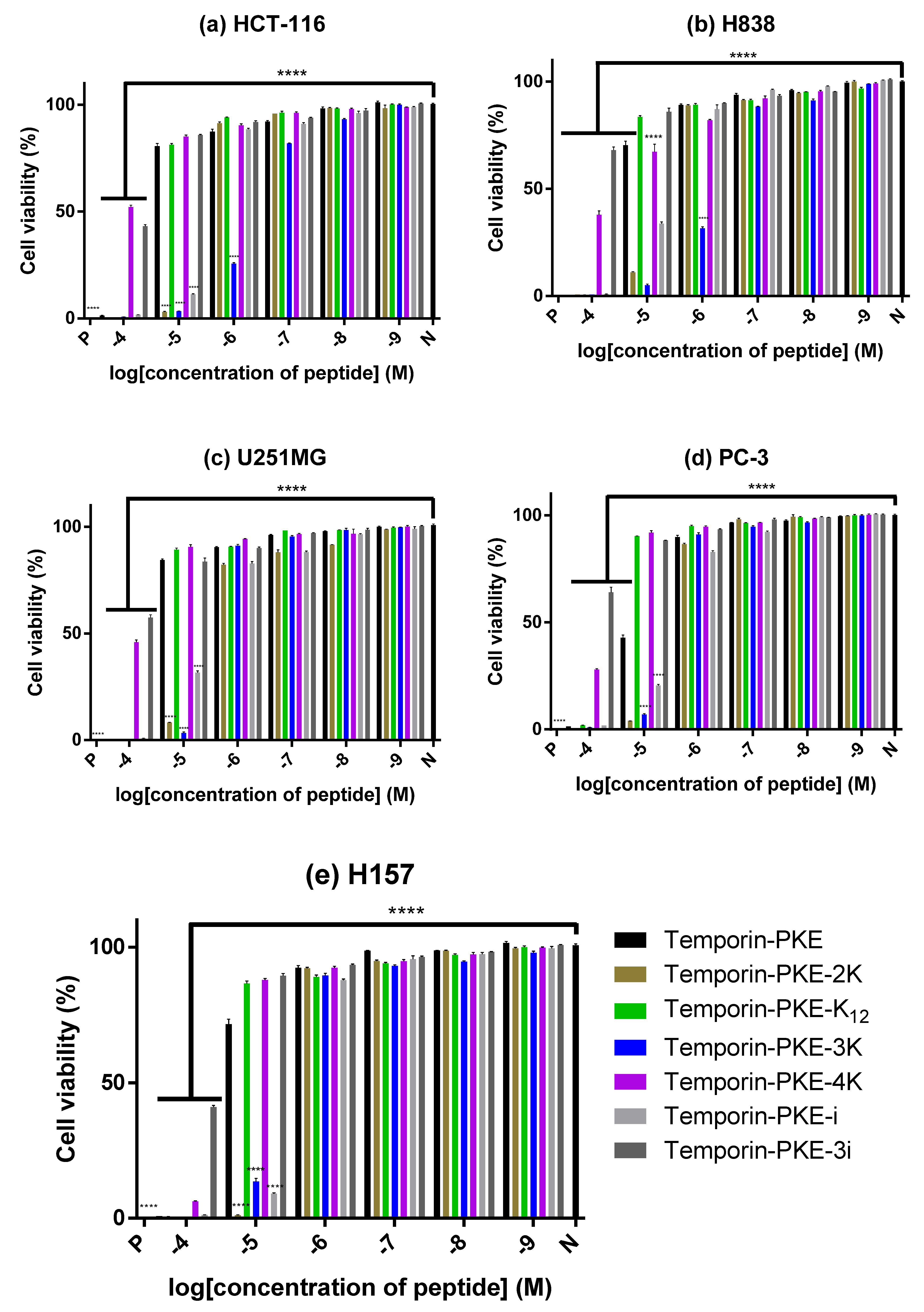
3.12. Anti-Cancer Cell Proliferattive Activity
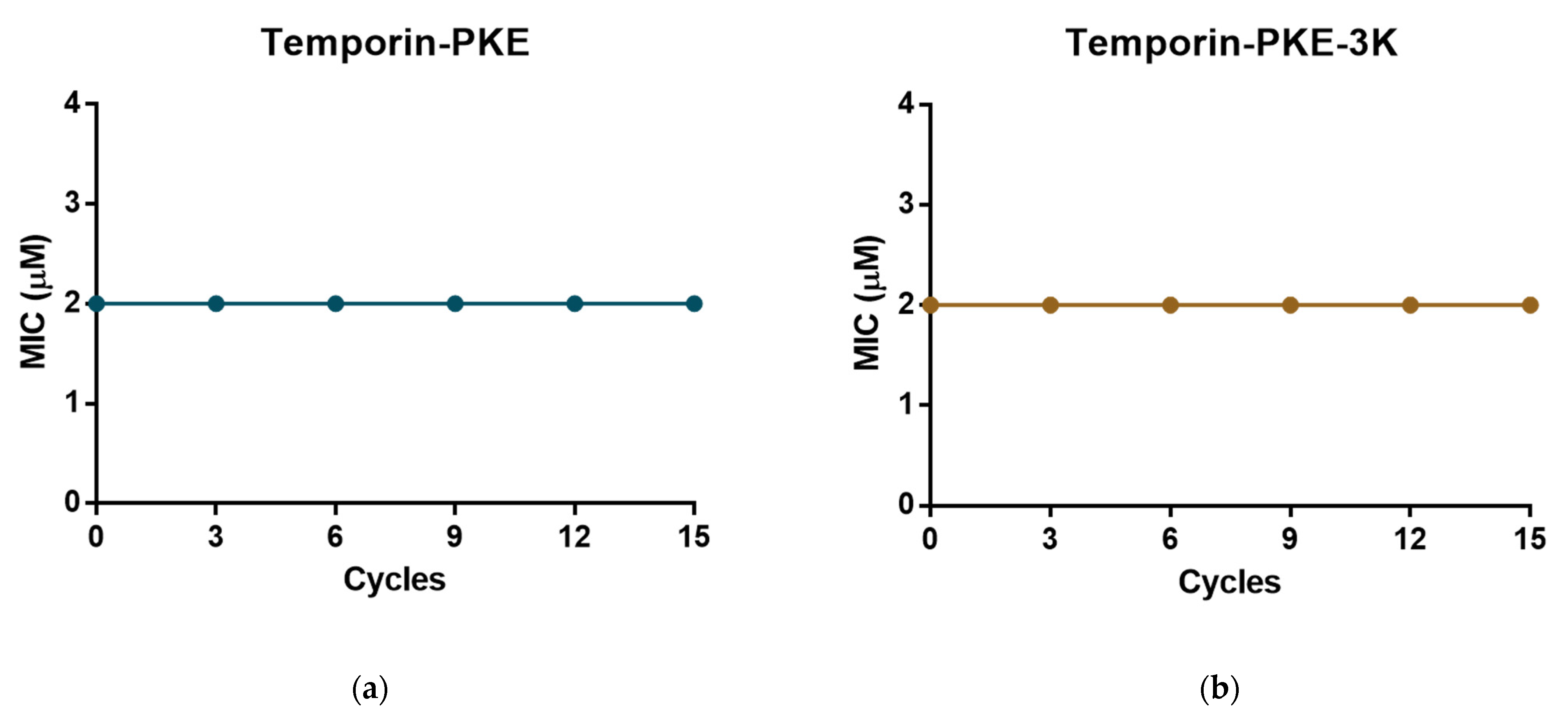
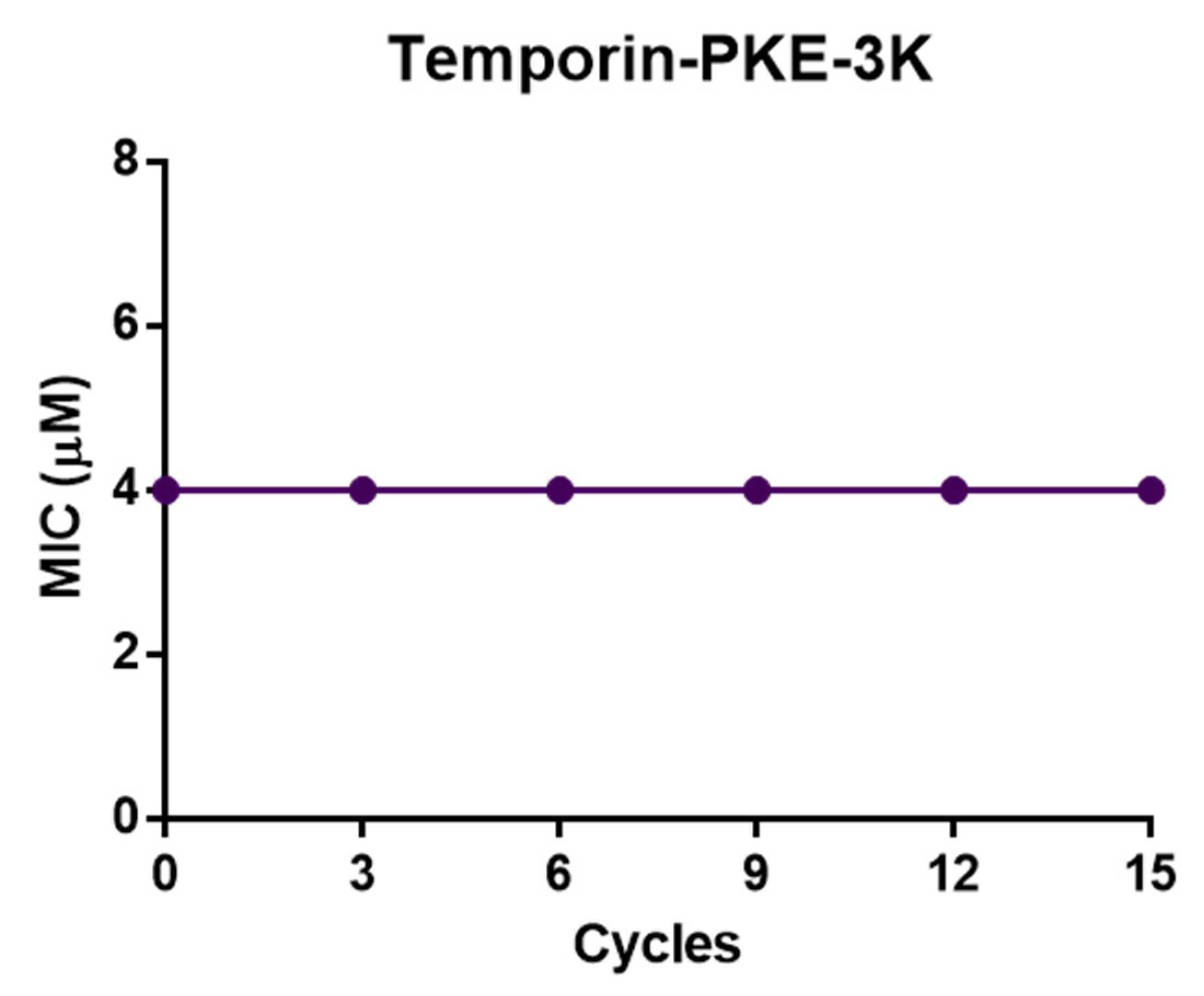
3.13. Resistance Induction of MRSA and K. pneumoniae
3.14. Determination of Antimicrobial Activity In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nii-Trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. Biomed. Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29 (Suppl. S1), 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumer, J. Treatment of pediatric Gram-positive multidrug-resistant infections. J. Infect. 2009, 59 (Suppl. S1), S51–S58. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Hartmann, K.; Lloret, A.; Ferrer, L.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Cryptococcosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 611–618. [Google Scholar] [CrossRef]
- Wencewicz, T.A. Crossroads of Antibiotic Resistance and Biosynthesis. J. Mol. Biol. 2019, 431, 3370–3399. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Antimicrobial Peptides (AMPs) with Dual Mechanisms: Membrane Disruption and Apoptosis. J. Microbiol. Biotechnol. 2015, 25, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Zhong, H.; Xie, Z.; Wei, H.; Zhang, S.; Song, Y.; Wang, M.; Zhang, Y. Antibacterial and Antibiofilm Activity of Temporin-GHc and Temporin-GHd Against Cariogenic Bacteria, Streptococcus mutans. Front. Microbiol. 2019, 10, 2854. [Google Scholar] [CrossRef] [Green Version]
- Sang, M.; Wu, Q.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Burrows, J.F.; Chen, T. Identification and target-modifications of temporin-PE: A novel antimicrobial peptide in the defensive skin secretions of the edible frog, Pelophylax kl. esculentus. Biochem. Biophys. Res. Commun. 2018, 495, 2539–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368 Pt 1, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, R.A.; Ayerra Mangado, J.; Mechkarska, M.; Kaman, W.E.; van Baarlen, P.; Conlon, J.M.; Wells, J.M. Selection of antimicrobial frog peptides and temporin-1DRa analogues for treatment of bacterial infections based on their cytotoxicity and differential activity against pathogens. Chem. Biol. Drug Des. 2020, 96, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Raja, Z.; Andre, S.; Abbassi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef] [Green Version]
- Andre, S.; Raja, Z.; Humblot, V.; Piesse, C.; Foulon, T.; Sereno, D.; Oury, B.; Ladram, A. Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus). Int. J. Mol. Sci. 2020, 21, 6713. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination With Conventional Antibiotics-A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Papo, N.; Shai, Y. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 2003, 24, 1693–1703. [Google Scholar] [CrossRef]
- Hollmann, A.; Martinez, M.; Noguera, M.E.; Augusto, M.T.; Disalvo, A.; Santos, N.C.; Semorile, L.; Maffia, P.C. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids Surf. B Biointerfaces 2016, 141, 528–536. [Google Scholar] [CrossRef]
- Duque-Salazar, G.; Mendez-Otalvaro, E.; Ceballos-Arroyo, A.M.; Orduz, S. Design of antimicrobial and cytolytic peptides by computational analysis of bacterial, algal, and invertebrate proteomes. Amino Acids 2020, 52, 1403–1412. [Google Scholar] [CrossRef]
- Ma, H.; Irudayanathan, F.J.; Jiang, W.; Nangia, S. Simulating Gram-Negative Bacterial Outer Membrane: A Coarse Grain Model. J. Phys. Chem. B 2015, 119, 14668–14682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, C.; Liang, G.; Zhang, M.; Zheng, J. Engineering antimicrobial peptides with improved antimicrobial and hemolytic activities. J. Chem. Inf. Model. 2013, 53, 3280–3296. [Google Scholar] [CrossRef]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharm. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.M.; Marcellini, L.; Luca, V.; Barra, D.; Novellino, E.; et al. The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: Identification of a potent anti-Candida peptide. Biochim. Biophys. Acta 2013, 1828, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of alpha-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, R.; Bhunia, A.; Bhattacharjya, S. Micelle-bound structures and dynamics of the hinge deleted analog of melittin and its diastereomer: Implications in cell selective lysis by D-amino acid containing antimicrobial peptides. Biochim. Biophys. Acta 2010, 1798, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.L.; Shih, Y.P.; Wang, K.T.; Yu, H.M. Enhancing the hypotensive effect and diminishing the cytolytic activity of hornet mastoparan B by D-amino acid substitution. Toxicon 2001, 39, 1561–1566. [Google Scholar] [CrossRef]
- Pei, X.; Gong, Z.; Wu, Q.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Characterisation of a novel peptide, Brevinin-1H, from the skin secretion of Amolops hainanensis and rational design of several analogues. Chem. Biol. Drug Des. 2021, 97, 273–282. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, D.; Xi, X.; Wu, Y.; Ma, C.; Zhou, M.; Wang, L.; Yang, M.; Chen, T.; Shaw, C. Identification and Characterisation of the Antimicrobial Peptide, Phylloseptin-PT, from the Skin Secretion of Phyllomedusa tarsius, and Comparison of Activity with Designed, Cationicity-Enhanced Analogues and Diastereomers. Molecules 2016, 21, 1667. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, S.; Xi, X.; Ma, C.; Wang, L.; Chen, X.; Shi, Z.; Chen, T.; Shaw, C.; Zhou, M. Study on the Structure-Activity Relationship of an Antimicrobial Peptide, Brevinin-2GUb, from the Skin Secretion of Hylarana guentheri. Antibiotics 2021, 10, 895. [Google Scholar] [CrossRef]
- Yao, A.; Ma, Y.; Chen, X.; Zhou, M.; Xi, X.; Ma, C.; Ren, S.; Chen, T.; Shaw, C.; Wang, L. Modification Strategy of D-leucine Residue Addition on a Novel Peptide from Odorrana schmackeri, with Enhanced Bioactivity and In Vivo Efficacy. Toxins 2021, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis. Molecules 2017, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, A.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Generation of truncated derivatives through in silico enzymatic digest of peptide GV30 target MRSA both in vitro and in vivo. Comput. Struct. Biotechnol. J. 2021, 19, 4984–4996. [Google Scholar] [CrossRef] [PubMed]
- Dolzani, L.; Milan, A.; Scocchi, M.; Lagatolla, C.; Bressan, R.; Benincasa, M. Sub-MIC effects of a proline-rich antibacterial peptide on clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 2019, 68, 1253–1265. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Zhang, X.; Hu, Y.; Yu, H.; Liu, J.; Sun, J. The novel antimicrobial peptides from skin of Chinese broad-folded frog, Hylarana latouchii (Anura:Ranidae). Peptides 2009, 30, 273–282. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.B.; Li, S.; Tian, L.L.; Shang, D.J. Antitumor effects and cell selectivity of temporin-1CEa, an antimicrobial peptide from the skin secretions of the Chinese brown frog (Rana chensinensis). Biochimie 2012, 94, 434–441. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Kang, Y.; Shang, D. Antimicrobial peptide temporin-1CEa isolated from frog skin secretions inhibits the proinflammatory response in lipopolysaccharide-stimulated RAW264.7 murine macrophages through the MyD88-dependent signaling pathway. Mol. Immunol. 2021, 132, 227–235. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Guo, C.; Zhang, W.; Zhang, X.; Zhang, B.; Li, S.; Ren, J.; Hu, Y.; Wang, H. Identification and functional analyses of novel antioxidant peptides and antimicrobial peptides from skin secretions of four East Asian frog species. Acta Biochim. Biophys. Sin. 2017, 49, 550–559. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Casciaro, B.; Genovese, A.; Brancaccio, D.; Marcocci, M.E.; Novellino, E.; Carotenuto, A.; Palamara, A.T.; Mangoni, M.L.; Nencioni, L. Temporin G, an amphibian antimicrobial peptide against influenza and parainfluenza respiratory viruses: Insights into biological activity and mechanism of action. FASEB J. 2021, 35, e21358. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Mocchegiani, F.; Orlando, F.; Silvestri, C.; Bozzi, A.; Di Giulio, A.; Luzi, C.; Mangoni, M.L.; et al. Interaction of antimicrobial peptide temporin L with lipopolysaccharide in vitro and in experimental rat models of septic shock caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Recupido, F.; Cirillo, A.; Romano, A.; Romanelli, A.; Caserta, S.; Guido, S.; Duilio, A. Antibiofilm Properties of Temporin-L on Pseudomonas fluorescens in Static and In-Flow Conditions. Int. J. Mol. Sci. 2020, 21, 8526. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Epand, R.F.; Rosenfeld, Y.; Peleg, A.; Barra, D.; Epand, R.M.; Shai, Y. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J. Biol. Chem. 2008, 283, 22907–22917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leptihn, S.; Har, J.Y.; Wohland, T.; Ding, J.L. Correlation of charge, hydrophobicity, and structure with antimicrobial activity of S1 and MIRIAM peptides. Biochemistry 2010, 49, 9161–9170. [Google Scholar] [CrossRef] [PubMed]
- Won, A.; Khan, M.; Gustin, S.; Akpawu, A.; Seebun, D.; Avis, T.J.; Leung, B.O.; Hitchcock, A.P.; Ianoul, A. Investigating the effects of L- to D-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim. Biophys. Acta 2011, 1808, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S. Bacterial infections: Overview. Int. Encycl. Public Health 2008, 273–282. [Google Scholar] [CrossRef]
- Mohr, K.I. History of Antibiotics Research. Curr. Top Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar]
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Annunziato, G.; Costantino, G. Antimicrobial peptides (AMPs): A patent review (2015–2020). Expert Opin. Ther. Pat. 2020, 30, 931–947. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Brancatisano, F.L.; Esin, S.; Campa, M. Use of antimicrobial peptides against microbial biofilms: Advantages and limits. Curr. Med. Chem. 2011, 18, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shao, C.; Li, G.; Shan, A.; Chou, S.; Wang, J.; Ma, Q.; Dong, N. Conversion of Broad-Spectrum Antimicrobial Peptides into Species-Specific Antimicrobials Capable of Precisely Targeting Pathogenic Bacteria. Sci. Rep. 2020, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.M.; Fuste, E.; Rabanal, F.; Vinuesa, T.; Vinas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Lakshmaiah Narayana, J.; Chen, J.Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Christiansen, D.G. Gamete types, sex determination and stable equilibria of all-hybrid populations of diploid and triploid edible frogs (Pelophylax esculentus). BMC Evol. Biol. 2009, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Daum, J.M.; Davis, L.R.; Bigler, L.; Woodhams, D.C. Hybrid advantage in skin peptide immune defenses of water frogs (Pelophylax esculentus) at risk from emerging pathogens. Infect. Genet. Evol. 2012, 12, 1854–1864. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Identification of Miscellaneous Peptides from the Skin Secretion of the European Edible Frog, Pelophylax kl. Esculentus. Protein J. 2016, 35, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Domingues, M.M.; Silva, P.M.; Franquelim, H.G.; Carvalho, F.A.; Castanho, M.A.; Santos, N.C. Antimicrobial protein rBPI21-induced surface changes on Gram-negative and Gram-positive bacteria. Nanomedicine 2014, 10, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Gopal, R.; Park, Y. Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties. Amino Acids 2016, 48, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, C.F.; Chang, T.W.; Wang, Y.J.; Liao, Y.D. Oligomerization and insertion of antimicrobial peptide TP4 on bacterial membrane and membrane-mimicking surfactant sarkosyl. PLoS ONE 2019, 14, e0216946. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhu, R.; Zhao, Y.; An, X.; Jia, F.; Peng, J.; Ma, Z.; Zhu, Y.; Wang, J.; Su, J.; et al. Antimicrobial activity and stability of protonectin with D-amino acid substitutions. J. Pept. Sci. 2017, 23, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Hakansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Aarestrup, F.M.; Hansen, E.B. Dissection of the antimicrobial and hemolytic activity of Cap18: Generation of Cap18 derivatives with enhanced specificity. PLoS ONE 2018, 13, e0197742. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a conjugate between Rose Bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef]
- Felicio, M.R.; Silva, O.N.; Goncalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Alsamghan, A.S. Molecular Mechanisms Contributing Bacterial Infections to the Incidence of Various Types of Cancer. Mediat. Inflamm. 2020, 2020, 4070419. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Lequin, O.; Piesse, C.; Goasdoue, N.; Foulon, T.; Nicolas, P.; Ladram, A. Temporin-SHf, a new type of phe-rich and hydrophobic ultrashort antimicrobial peptide. J. Biol. Chem. 2010, 285, 16880–16892. [Google Scholar] [CrossRef] [Green Version]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef] [Green Version]


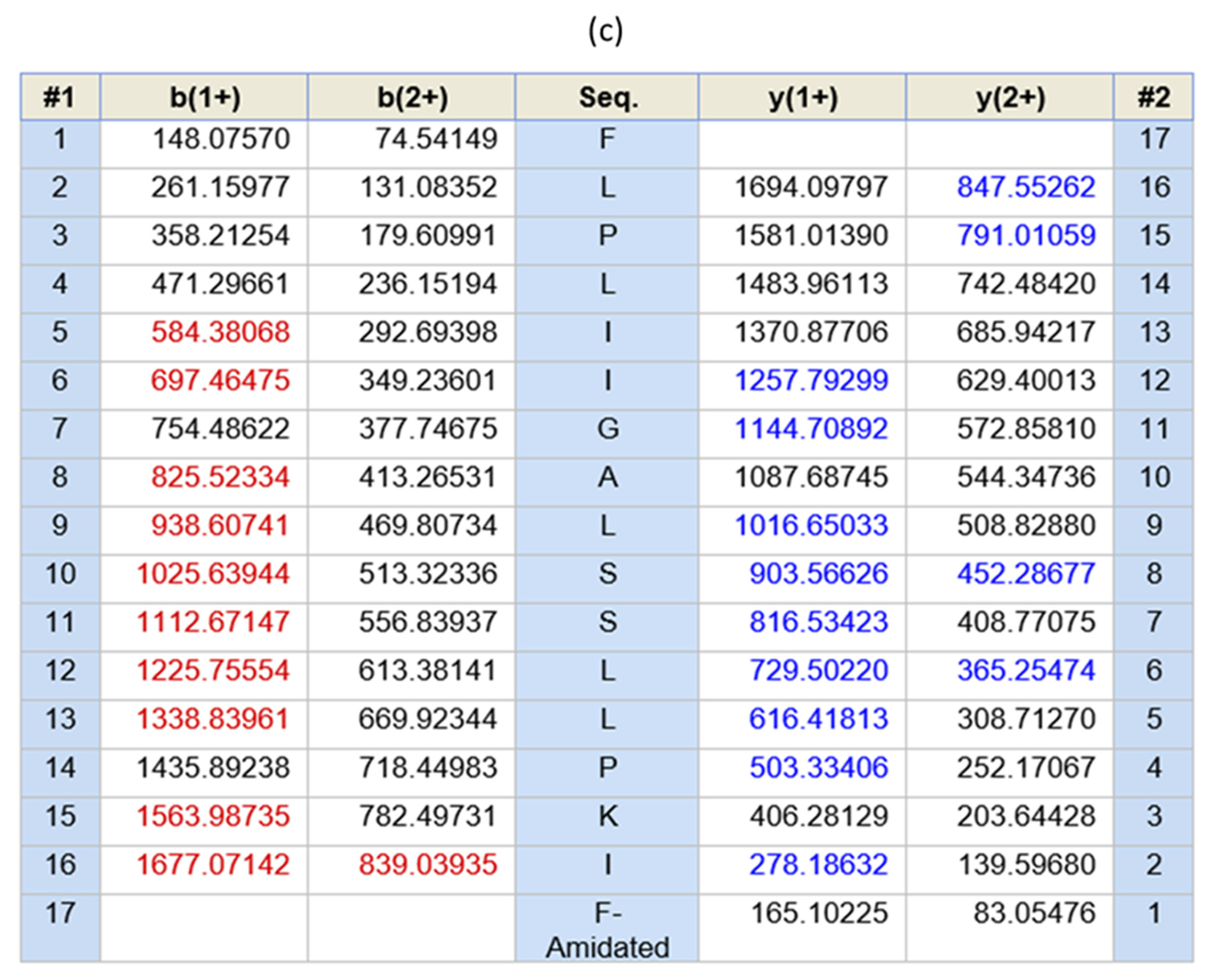
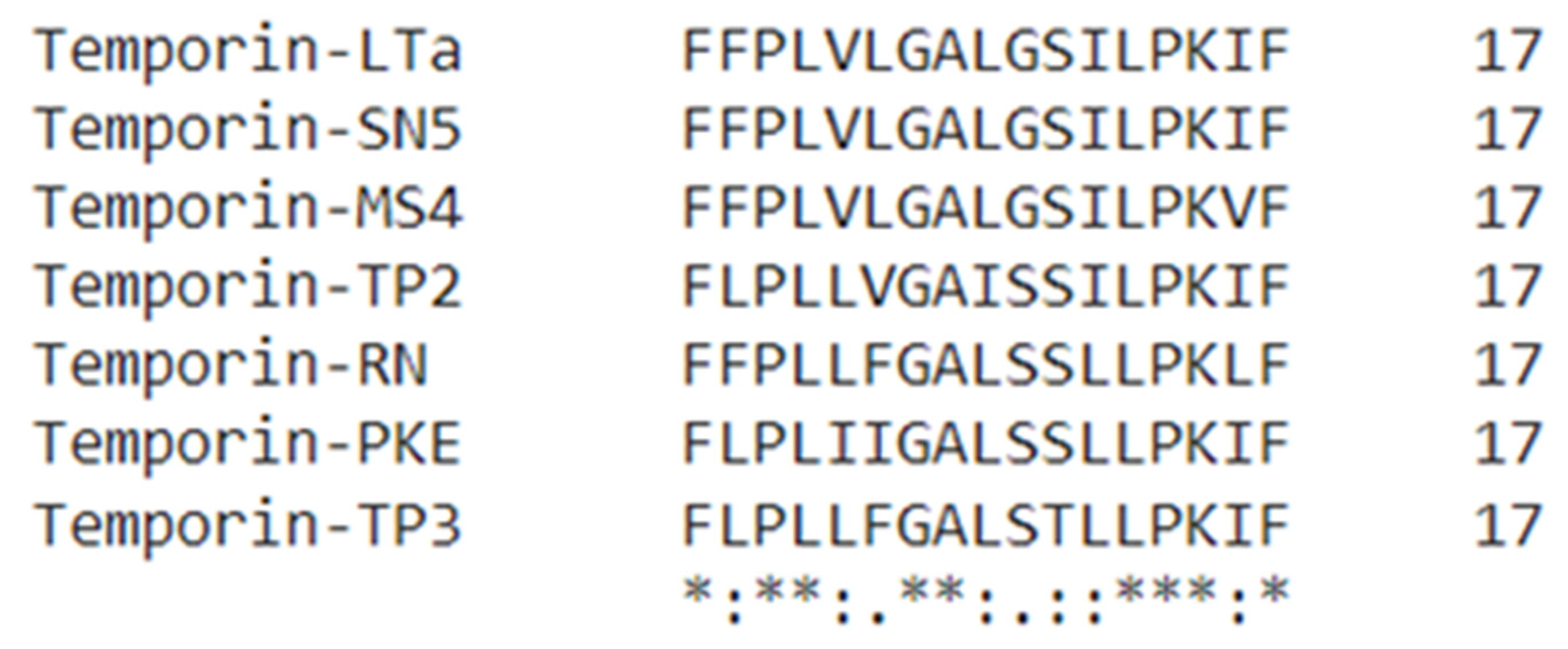
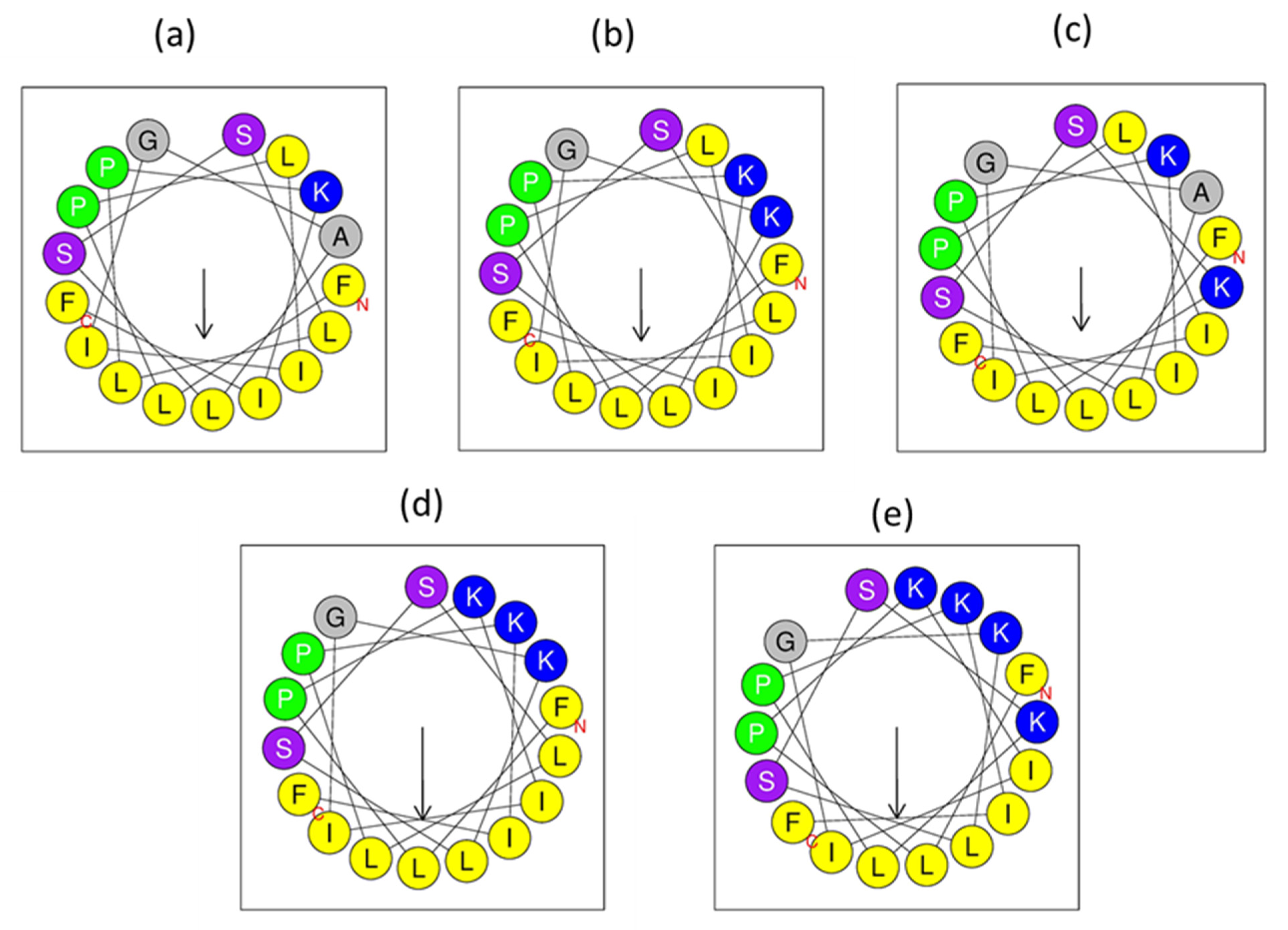


| Peptide Name | Species | Sequence | Bioactivities |
|---|---|---|---|
| temporin-LTa | Hylarana latouchii | FFPLVLGALGSILPKIF-NH2 | Antimicrobial activity against gram-positive bacteria [35] |
| temporin-1CEa | Rana chensinensis | FVDLKKIANIINSIFGK-NH2 | Broad-spectrum antimicrobial activity and antitumour activity [36]; anti-inflammatory activity [37] |
| temporin-MS1 | Hylarana maosuoensis | FLTGLIGGLMKAL-NH2 | Broad-spectrum antimicrobial activity [38] |
| temporin G | Rana temporaria | FFPVIGRILNGIL-NH2 | Antiviral activity against influenza and parainfluenza respiratory viruses [39] |
| temporin L | Rana temporaria | FVQWFSKFLGRIL-NH2 | Potent and broad-spectrum antimicrobial activity, especially against gram-negative bacteria [40]; Antibiofilm activity [41]; Antiendotoxic activity [42] |
| Peptide | Sequence | Hydrophobicity <H> | 1 HM <μH> | Net Charge (z) | 2 Theoretical MM (g/mol) | 2 Observed MM (g/mol) |
|---|---|---|---|---|---|---|
| Temporin-PKE | FLPLIIGALSSLLPKIF-NH2 | 1.068 | 0.490 | +1 | 1841 | 1841 |
| Temporin-PKE-2K | FLPLIIGKLSSLLPKIF-NH2 | 0.992 | 0.513 | +2 | 1898 | 1898 |
| Temporin-PKE-K12 | FLPLIIGALSSKLPKIF-NH2 | 0.910 | 0.443 | +2 | 1856 | 1856 |
| Temporin-PKE-3K | FLPKIIGKLSSLLPKIF-NH2 | 0.834 | 0.657 | +3 | 1913 | 1913 |
| Temporin-PKE-4K | FLPKIIGKLSSKLPKIF-NH2 | 0.675 | 0.644 | +4 | 1928 | 1928 |
| 3 Temporin-PKE-i | 4 FLPLIIGALSSLLPKiF-NH2 | - | - | +1 | 1841 | 1841 |
| 3 Temporin-PKE-3i | 4 FLPLiiGALSSLLPKiF-NH2 | - | - | +1 | 1841 | 1841 |
| Peptide | 20 mM NH4Ac | 50% TFE in NH4Ac | ||||
|---|---|---|---|---|---|---|
| Name | Helix (%) | Antiparallel (%) | Turn (%) | Helix (%) | Antiparallel (%) | Turn (%) |
| Temporin-PKE | 4.4 | 35.3 | 15.4 | 18.3 | 27.9 | 13.1 |
| Temporin-PKE-2K | 0.9 | 36.0 | 15.6 | 20.3 | 18.2 | 13.4 |
| Temporin-PKE-K12 | 3.1 | 39.2 | 14.0 | 6.8 | 8.7 | 7.1 |
| Temporin-PKE-3K | 5.0 | 24.7 | 15.3 | 72.8 | 0.0 | 2.8 |
| Temporin-PKE-4K | 2.8 | 38.7 | 14.2 | 44.6 | 3.2 | 11.7 |
| Temporin-PKE-i | 5.5 | 34.8 | 14.8 | 35.5 | 8.5 | 13.4 |
| Temporin-PKE-3i | 0.0 | 46.0 | 14.8 | 0.8 | 40.9 | 14.6 |
| Bacteria | MIC 2/MBC 3 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive 4 | Temporin-PKE | 2K | K12 | 3K | 4K | i | 3i | |
| S. aureus (ATCC 6538) | 2/2 | 2/2 | 2/2 | 4/4 | 2/2 | 32/32 | 2/2 | 32/32 |
| E. coli (ATCC CRM 8739) | 1/1 | >512 | 4/4 | 8/8 | 2/2 | 4/4 | >512 | >512 |
| C. albicans (ATCC CRM 10231) | 4/4 | >512 | >512 | >512 | 32/64 | 64/64 | >512 | 128/256 |
| MRSA (NCTC 12493) | 2/2 | 2/2 | 2/2 | 4/4 | 2/2 | 32/32 | 4/4 | 64/64 |
| K. pneumoniae (ATCC 43816) | 2/2 | >512 | 16/32 | 32/64 | 4/4 | 16/16 | >512 | >512 |
| P. aeruginosa (ATCC CRM 9027) | 2/2 | >512 | 128/256 | 256/256 | 16/16 | 64/64 | >512 | >512 |
| E. faecalis (NCTC 12697) | 4/4 | 8/8 | 2/2 | 8/8 | 2/2 | 32/32 | 4/4 | 128/128 |
| Clinical strains | ||||||||
| MRSA (B042 V2E1 A) | 2/2 | 4/8 | 2/2 | 4/4 | 2/2 | 16/16 | 4/4 | 64/64 |
| E. coli (ATCC BAA-2340) | 2/2 | >512 | 8/8 | 16/16 | 2/2 | 32/64 | >512 | >512 |
| P. aeruginosa (B004 V2S2 B) | 1/1 | >512 | 32/32 | 64/64 | 8/8 | 32/32 | >512 | >512 |
| K. pneumoniae (ATCC BAA-1705) | 2/2 | >512 | 16/16 | 64/64 | 8/8 | 32/32 | >512 | >512 |
| HC50 (μM) | 6.58 | 19.87 | 122.7 | 87.47 | 1671 | 68.37 | 64.0 | |
| G+ bacteria GM (μM) | 3.36 | 2.00 | 4.76 | 2.00 | 26.91 | 3.36 | 32.00 | |
| Overall GM 5 (μM) | - | 7.46 | 17.15 | 4.26 | 26.49 | - | - | |
| TI (Gram+ bacteria) | 1.96 | 9.94 | 25.79 | 43.74 | 62.10 | 20.33 | 6.20 | |
| TI 5,6 (Overall) | - | 2.66 | 7.16 | 20.53 | 63.08 | - | - | |
| Normal Cell Lines | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| Temporin-PKE | 2K | K12 | 3K | 4K | i | 3i | |
| HaCaT | 5.04 | 9.04 | 18.99 | 29.56 | 250.6 | 11.90 | 497.1 |
| Bacteria | MBIC 1/MBEC 2 (μM) | ||||||
|---|---|---|---|---|---|---|---|
| Temporin-PKE | 2K | K12 | 3K | 4K | i | 3i | |
| S. aureus (ATCC 6538) | 2/256 | 2/64 | 4/128 | 2/64 | 32/128 | 2/128 | 64/128 |
| E. coli (ATCC CRM 8739) | >512 | 64/>512 | 64/>512 | 16/512 | 128/>512 | >512 | >512 |
| K. pneumoniae (ATCC 43816) | >512 | 128/>512 | 512/>512 | 32/512 | 256/>512 | >512 | >512 |
| P. aeruginosa (ATCC CRM 9027) | >512 | >512 | >512 | 64/512 | 128/>512 | >512 | >512 |
| MRSA (NCTC 12493) | 4/256 | 2/64 | 4/256 | 2/128 | 32/512 | 2/128 | 64/>512 |
| E. faecalis (NCTC 12697) | 32/>512 | 16/>512 | 64/>512 | 4/>512 | 256/>512 | 4/>512 | 128/>512 |
| Peptide | MIC (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 mM NaCl | 4.5 mM KCl | 6 µM NH4Cl | 8 µM ZnCl2 | 1 mM MgCl2 | 2 mM CaCl2 | 4 µM FeCl3 | 10% FBS | Negative | |
| Temporin-PKE | 8 | 8 | 8 | 32 | 8 | 8 | 8 | 16 | 2 |
| 2K | 8 | 4 | 4 | 16 | 4 | 4 | 2 | 8 | 2 |
| K12 | 32 | 32 | 8 | 32 | 16 | 16 | 8 | 32 | 4 |
| 3K | 8 | 4 | 4 | 16 | 4 | 4 | 2 | 4 | 2 |
| 4K | 128 | 32 | 32 | 128 | 32 | 64 | 32 | 128 | 32 |
| i | 4 | 2 | 2 | 16 | 2 | 4 | 2 | 8 | 2 |
| 3i | 512 | 512 | 256 | 512 | 256 | 256 | 128 | 512 | 64 |
| Peptide | MIC (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 mM NaCl | 4.5 mM KCl | 6 µM NH4Cl | 8 µM ZnCl2 | 1 mM MgCl2 | 2 mM CaCl2 | 4 µM FeCl3 | 10% FBS | Negative | |
| Temporin-PKE | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 2K | 128 | 16 | 16 | 16 | 32 | 32 | 16 | 64 | 16 |
| K12 | 128 | 32 | 32 | 64 | 128 | 256 | 32 | 128 | 32 |
| 3K | 16 | 4 | 4 | 4 | 16 | 16 | 16 | 16 | 4 |
| 4K | 64 | 16 | 16 | 16 | 64 | 128 | 16 | 64 | 16 |
| i | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 3i | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| Cancer Cell Lines | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| Temporin-PKE | 2K | K12 | 3K | 4K | i | 3i | |
| U251MG | 23.24 | 2.49 | 25.75 | 2.83 | 85.52 | 4.46 | 120.6 |
| PC-3 | 7.29 | 2.64 | 27.32 | 3.01 | 49.50 | 3.35 | 167.0 |
| H838 | 16.38 | 3.07 | 22.67 | 0.51 | 35.28 | 5.28 | 193.4 |
| HCT-116 | 21.31 | 2.84 | 22.09 | 0.38 | 99.36 | 3.07 | 72.06 |
| H157 | 17.40 | 2.78 | 24.03 | 3.29 | 27.76 | 2.94 | 71.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Jiang, Y.; Zhao, Z.; Lu, Y.; Xi, X.; Ma, C.; Chen, X.; Zhou, M.; Chen, T.; Shaw, C.; et al. Discovery of a Novel Antimicrobial Peptide, Temporin-PKE, from the Skin Secretion of Pelophylax kl. esculentus, and Evaluation of Its Structure-Activity Relationships. Biomolecules 2022, 12, 759. https://doi.org/10.3390/biom12060759
Lin Y, Jiang Y, Zhao Z, Lu Y, Xi X, Ma C, Chen X, Zhou M, Chen T, Shaw C, et al. Discovery of a Novel Antimicrobial Peptide, Temporin-PKE, from the Skin Secretion of Pelophylax kl. esculentus, and Evaluation of Its Structure-Activity Relationships. Biomolecules. 2022; 12(6):759. https://doi.org/10.3390/biom12060759
Chicago/Turabian StyleLin, Yaxian, Yangyang Jiang, Ziwei Zhao, Yueyang Lu, Xinping Xi, Chengbang Ma, Xiaoling Chen, Mei Zhou, Tianbao Chen, Chris Shaw, and et al. 2022. "Discovery of a Novel Antimicrobial Peptide, Temporin-PKE, from the Skin Secretion of Pelophylax kl. esculentus, and Evaluation of Its Structure-Activity Relationships" Biomolecules 12, no. 6: 759. https://doi.org/10.3390/biom12060759
APA StyleLin, Y., Jiang, Y., Zhao, Z., Lu, Y., Xi, X., Ma, C., Chen, X., Zhou, M., Chen, T., Shaw, C., & Wang, L. (2022). Discovery of a Novel Antimicrobial Peptide, Temporin-PKE, from the Skin Secretion of Pelophylax kl. esculentus, and Evaluation of Its Structure-Activity Relationships. Biomolecules, 12(6), 759. https://doi.org/10.3390/biom12060759







