Effects of Electric-Toothbrush Vibrations on the Expression of Collagen and Non-Collagen Proteins through the Focal Adhesion Kinase Signaling Pathway in Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Stimulation with Electric Toothbrush
2.3. Staining of Collagen and Non-Collagen Proteins
2.4. Real-Time Reverse Transcription (RT)-PCR
2.5. ELISA
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
2.7. Transfection with Small Interfering RNA
2.8. Statistical Analysis
3. Results
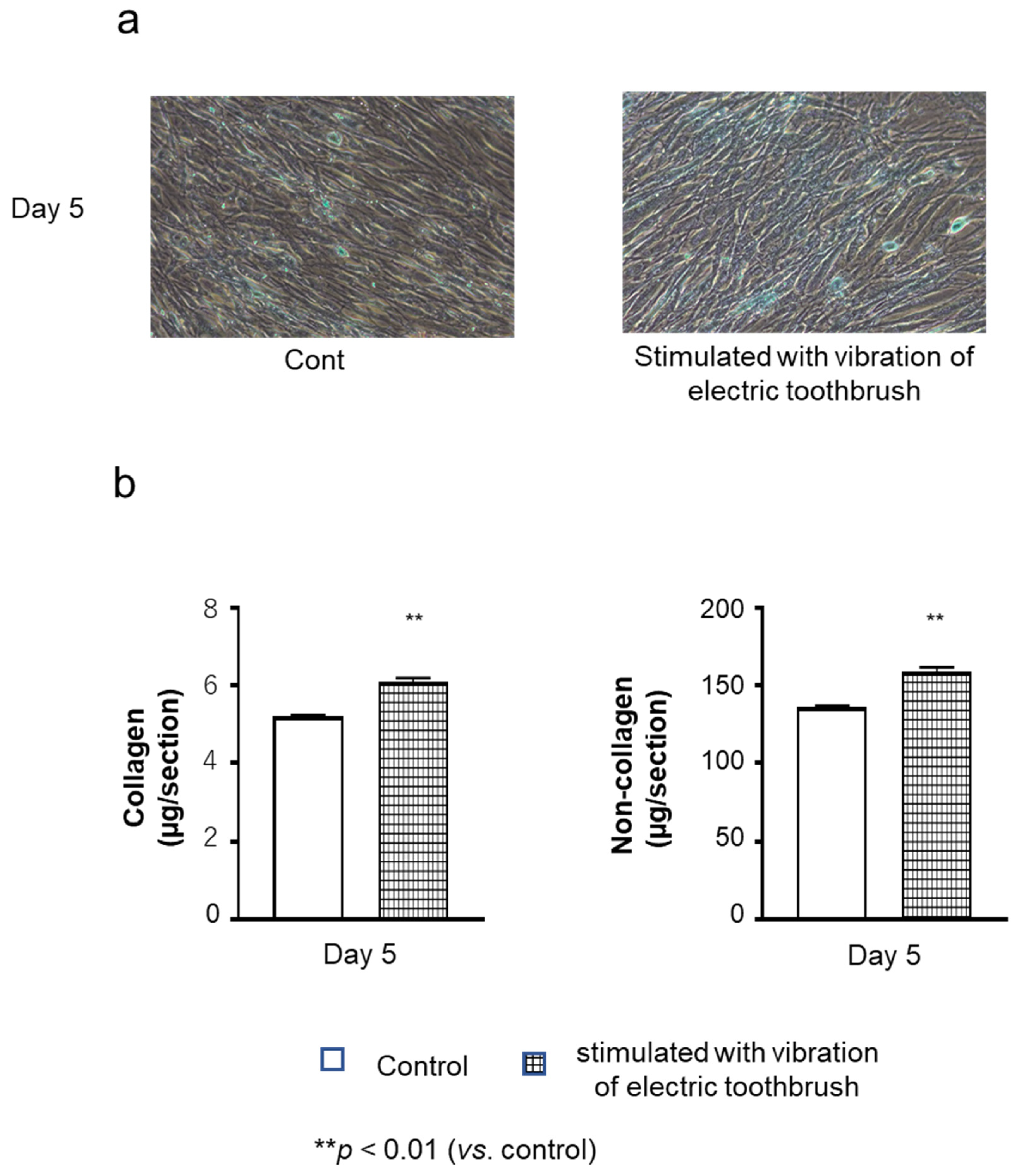
3.1. Collagenous and Non-Collagenous Proteins in the Cultured Cell Layers
3.2. Expression of ECM Molecules in HGnFs
3.3. Protein Levels of col I, col III, Elastin, and FN
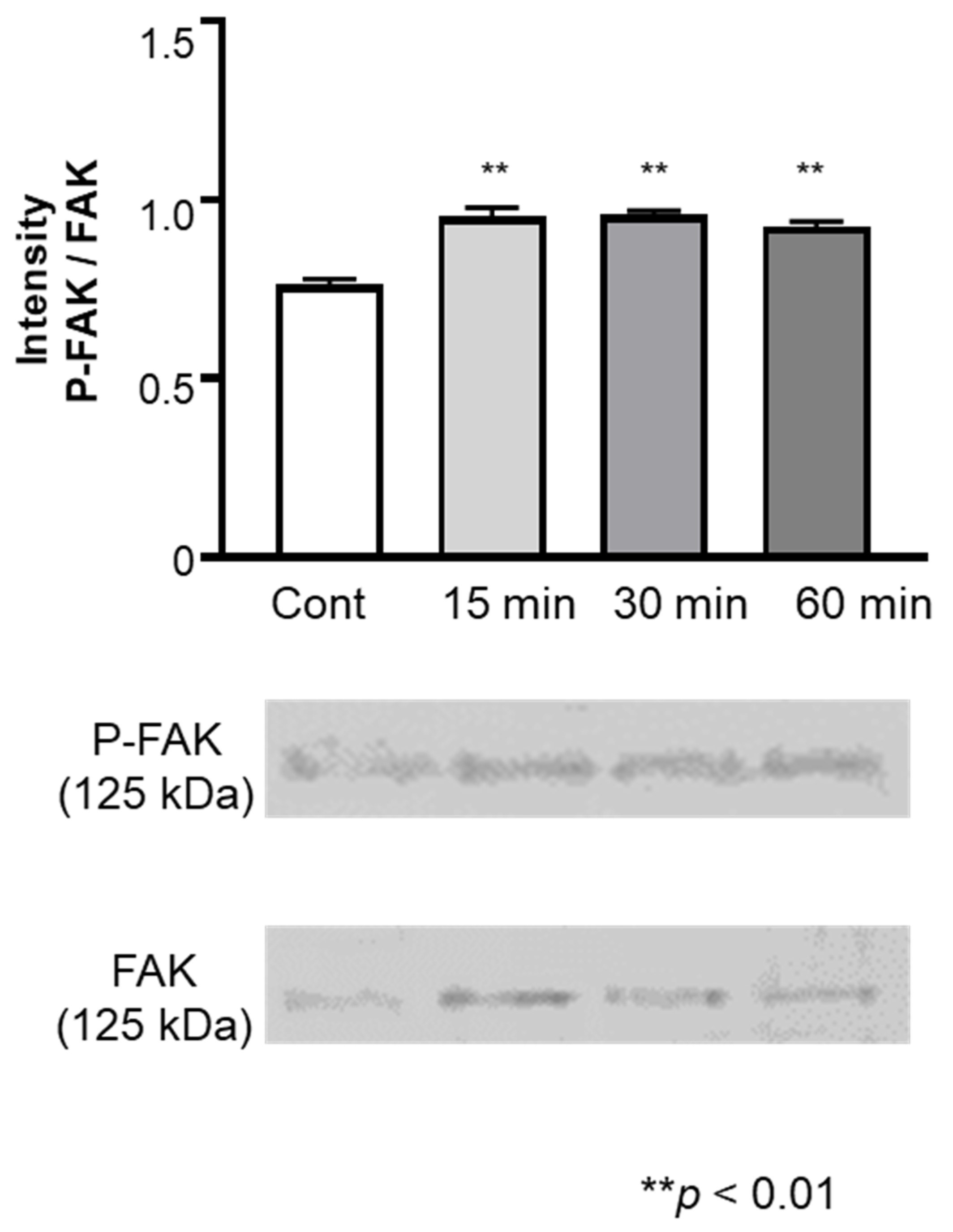
3.4. Expression of FAK and phosphorylated FAK in HGnFs
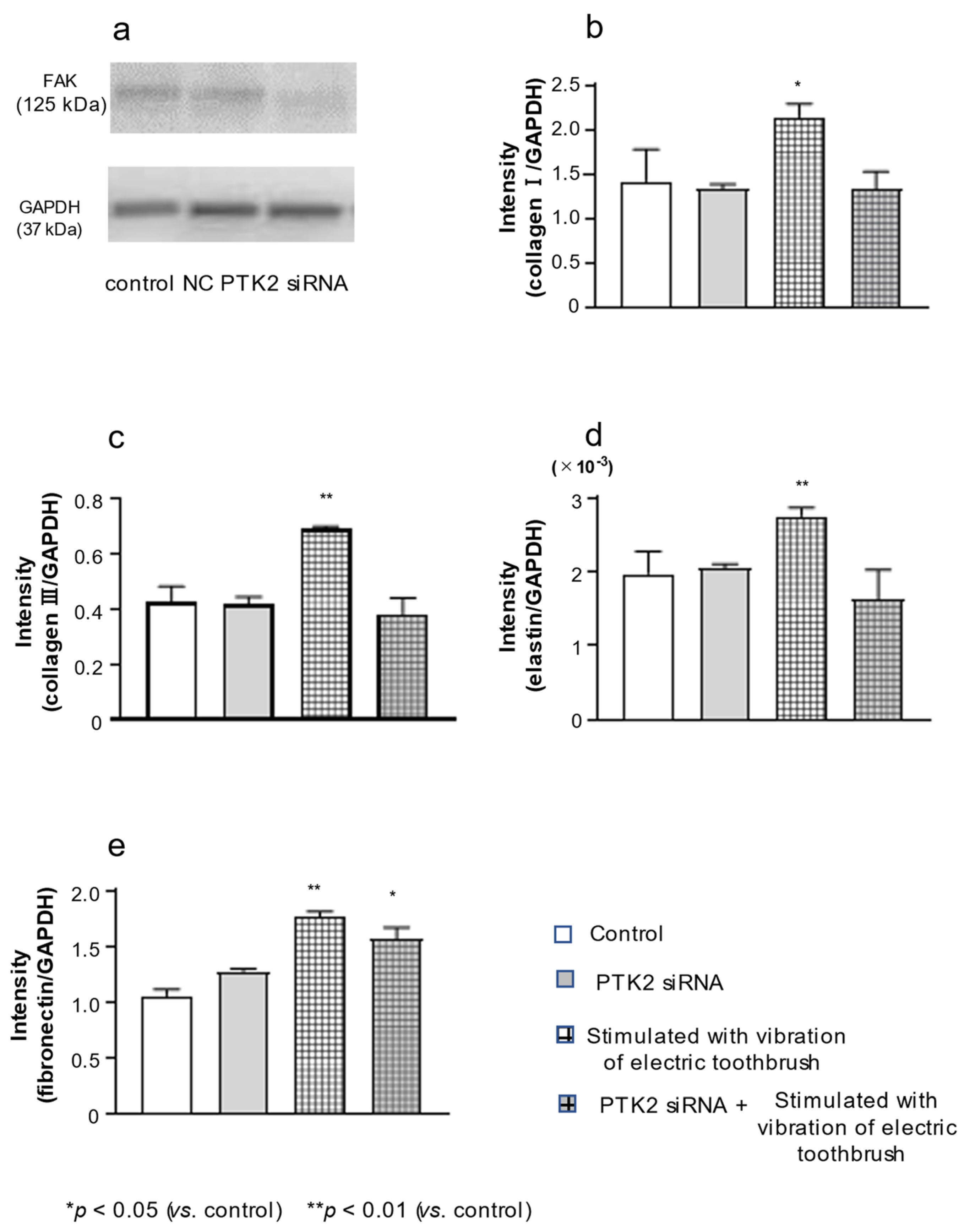
3.5. Effect of PTK2 siRNA on the Expression of FAK, col III, Elastin, and FN in HGnFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van der Weijden, F.; Slot, D.E. Oral hygiene in the prevention of periodontal diseases: The evidence. Periodontology 2000 2011, 55, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Claydon, N.C. Current concepts in toothbrushing and interdental cleaning. Periodontology 2000 2008, 48, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bhat, K.M. Acceptability of powered toothbrushes for elderly individuals. J. Public Health Dent. 2004, 64, 115–117. [Google Scholar] [CrossRef]
- Yaacob, M.; Worthington, H.V.; Deacon, S.A.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.M. Powered versus manual tooth brushing for oral health. Cochrane Database Syst. Rev. 2014, 6, CD002281. [Google Scholar] [CrossRef]
- Van der Weijden, F.A.; Slot, D.E. Efficacy of homecare regimens for mechanical plaque removal in managing gingivitis a meta review. J. Clin. Periodontol. 2015, 42, S77–S91. [Google Scholar] [CrossRef]
- Stanford, C.M.; Srikantha, R.; Wu, C.D. Efficacy of the sonicare toothbrush fluid dynamic action on removal of human supragingival plaque. J. Clin. Dent. 1997, 8, 10–14. [Google Scholar]
- Hope, C.K.; Wilson, M. Effects of dynamic fluid activity from an electric toothbrush on in vitro oral biofilms. J. Clin. Periodontol. 2003, 30, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.C.; Zaugg, C.; Weiger, R.; Walter, C. Brushing without brushing?—A review of the efficacy of powered toothbrushes in noncontact biofilm removal. Clin. Oral Investig. 2013, 17, 687–709. [Google Scholar] [CrossRef]
- Nathoo, S.; Mateo, L.R.; Chaknis, P.; Kemp, J.H.; Gatzemeyer, J.; Morrison, B.M., Jr.; Panagakos, F. Efficacy of two different toothbrush heads on a sonic power toothbrush compared to a manual toothbrush on established gingivitis and plaque. J. Clin. Dent. 2014, 25, 65–70. [Google Scholar]
- Ccahuana-Vasquez, R.A.; Adam, R.; Conde, E.; Grender, J.M.; Cunningham, P.; Goyal, C.R.; Qaqish, J. A 5-week randomized clinical evaluation of a novel electric toothbrush head with regular and tapered bristles versus a manual toothbrush for reduction of gingivitis and plaque. Int. J. Dent. Hyg. 2019, 17, 153–160. [Google Scholar] [CrossRef]
- Ikawa, T.; Mizutani, K.; Sudo, T.; Kano, C.; Ikeda, Y.; Akizuki, T.; Kobayashi, H.; Izumi, Y.; Iwata, T. Clinical comparison of an electric-powered ionic toothbrush and a manual toothbrush in plaque reduction: A randomized clinical trial. Int. J. Dent. Hyg. 2021, 19, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, S.; Mankodi, S.; Mateo, L.R.; Chaknis, P.; Panagakos, F. A clinical study comparing the supragingival plaque and gingivitis efficacy of a specially engineered sonic powered toothbrush with unique sensing and control technologies to a commercially available manual flat-trim toothbrush. J. Clin. Dent. 2012, 23, A11–A16. [Google Scholar] [PubMed]
- Berta, L.; Fazzari, A.; Ficco, A.M.; Enrica, P.M.; Catalano, M.G.; Frairia, R. Extracorporeal shock waves enhance normal fibroblast proliferation In Vitro and activate mRNA expression for TGF-beta1 and for collagen types I and III. Acta Orthop. 2009, 80, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Zaidi, M. Molecular regulation of mechanotransduction. Biochem. Biophys. Res. Commun. 2005, 18, 751–755. [Google Scholar] [CrossRef]
- Vogel, V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 459–488. [Google Scholar] [CrossRef]
- Kusano, H.; Tomofuji, T.; Azuma, T.; Sakamoto, T.; Yamamoto, T.; Watanabe, T. Proliferative response of gingival cells to ultrasonic and/or vibration toothbrushes. Am. J. Dent. 2006, 19, 7–10. [Google Scholar]
- Hortobagyi, D.; Grossmann, T.; Tschernitz, M.; Grill, M.; Kirsch, A.; Gerstenberger, C.; Gugatschka, M. In Vitro mechanical vibration down-regulates pro-inflammatory and pro-fibrotic signaling in human vocal fold fibroblasts. PLoS ONE 2020, 15, e0241901. [Google Scholar] [CrossRef]
- Yadav, S.; Dobie, T.; Assefnia, A.; Gupta, H.; Kalajzic, Z.; Nanda, R. Effect of low-frequency mechanical vibration on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 440–449. [Google Scholar] [CrossRef]
- Nishimura, M.; Chiba, M.; Ohashi, T.; Sato, M.; Shimizu, Y.; Igarashi, K.; Mitani, H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 572–583. [Google Scholar] [CrossRef]
- Akagi, H.; Nakanishi, Y.; Nakanishi, K.; Matsubara, H.; Hirose, Y.; Wang, P.-L.; Ochi, M. Influence of low-intensity pulsed ultrasound stimulation on expression of bone-related genes in rat bone marrow cells. J. Hard Tissue Biol. 2016, 25, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Shipley, T.; Farouk, K.; El-Bialy, T. Effect of high-frequency vibration on orthodontic tooth movement and bone density. J. Orthod. Sci. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Wang, D.; Chen, J. Peak loads on teeth from a generic mouthpiece of a vibration device for accelerating tooth movement. Am. J. Orthod. Dentofac. Orthop. 2021, 21, 512516. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.N.; Shivamurthy, P.G.; Sabrish, S. Effect of 125–150 Hz vibrational frequency electric toothbrush on teeth and supporting structures: A finite element method study. J. Contemp. Dent. Pract. 2021, 22, 1150–1159. [Google Scholar]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cronin, K.; Crane, J.S. Biochemistry, collagen synthesis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [CrossRef]
- Hong, H.H.; Trackman, P.C. Cytokine regulation of gingival fibroblast lysyl oxidase, collagen, and elastin. J. Periodontol. 2002, 73, 145–152. [Google Scholar] [CrossRef]
- Bailey, A.J. Collagen and elastin fibres. J. Clin. Pathol. Suppl. R. Coll. Pathol. 1978, 12, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Golinski, P.A.; Gröger, S.; Herrmann, J.M.; Bernd, A.; Meyle, J. Oral mucosa model based on a collagen-elastin matrix. J. Periodont. Res. 2011, 46, 704–711. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.A.; Kelley, D.G.; Broekelmann, T.J. Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 1982, 92, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- McDonald, J.A.; Quade, B.J.; Broekelmann, T.J.; LaChance, R.; Forsman, K.; Hasegawa, E.; Akiyama, S. Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J. Biol. Chem. 1987, 262, 2957–2967. [Google Scholar] [CrossRef]
- Clark, R.A.; Nielsen, L.D.; Welch, M.P.; McPherson, J.M. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J. Cell Sci. 1995, 108, 1251–1261. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jeon, Y.M.; Lee, H.J.; Kim, J.G.; Baek, J.A.; Lee, J.C. Activation of RhoA and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol. Cell Biochem. 2010, 335, 263–272. [Google Scholar] [CrossRef]
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N.; et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 2011, 18, 148–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7, e38069. [Google Scholar] [CrossRef]
- Nan, L.; Zheng, Y.; Liao, N.; Li, S.; Wang, Y.; Chen, Z.; Wei, L.; Zhao, S.; Mo, S. Mechanical force promotes the proliferation and extracellular matrix synthesis of human gingival fibroblasts cultured on 3D PLGA scaffolds via TGF-β expression. Mol. Med. Rep. 2019, 19, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Row, S.; Liu, Y.; Alimperti, S.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 is a novel regulator of extracellular matrix synthesis and tissue mechanics. J. Cell Sci. 2016, 129, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.; Valverde, T.; Martins-Júnior, P.; Ribeiro, H.; Kitten, G.; Carvalhaes, L. Morphological and quantitative study of collagen fibers in healthy and diseased human gingival tissues. Rom. J. Morphol. Embryol. 2015, 56, 33–40. [Google Scholar]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Wang, Y.; Mauldin, E.A.; Liechty, K.W.; Adams, S.L. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakai, K.; Tanaka, H.; Fukuzawa, K.; Nakajima, J.; Ozaki, M.; Kato, N.; Kawato, T. Effects of Electric-Toothbrush Vibrations on the Expression of Collagen and Non-Collagen Proteins through the Focal Adhesion Kinase Signaling Pathway in Gingival Fibroblasts. Biomolecules 2022, 12, 771. https://doi.org/10.3390/biom12060771
Nakai K, Tanaka H, Fukuzawa K, Nakajima J, Ozaki M, Kato N, Kawato T. Effects of Electric-Toothbrush Vibrations on the Expression of Collagen and Non-Collagen Proteins through the Focal Adhesion Kinase Signaling Pathway in Gingival Fibroblasts. Biomolecules. 2022; 12(6):771. https://doi.org/10.3390/biom12060771
Chicago/Turabian StyleNakai, Kumiko, Hideki Tanaka, Kyoko Fukuzawa, Jyunya Nakajima, Manami Ozaki, Nobue Kato, and Takayuki Kawato. 2022. "Effects of Electric-Toothbrush Vibrations on the Expression of Collagen and Non-Collagen Proteins through the Focal Adhesion Kinase Signaling Pathway in Gingival Fibroblasts" Biomolecules 12, no. 6: 771. https://doi.org/10.3390/biom12060771





