Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension
Abstract
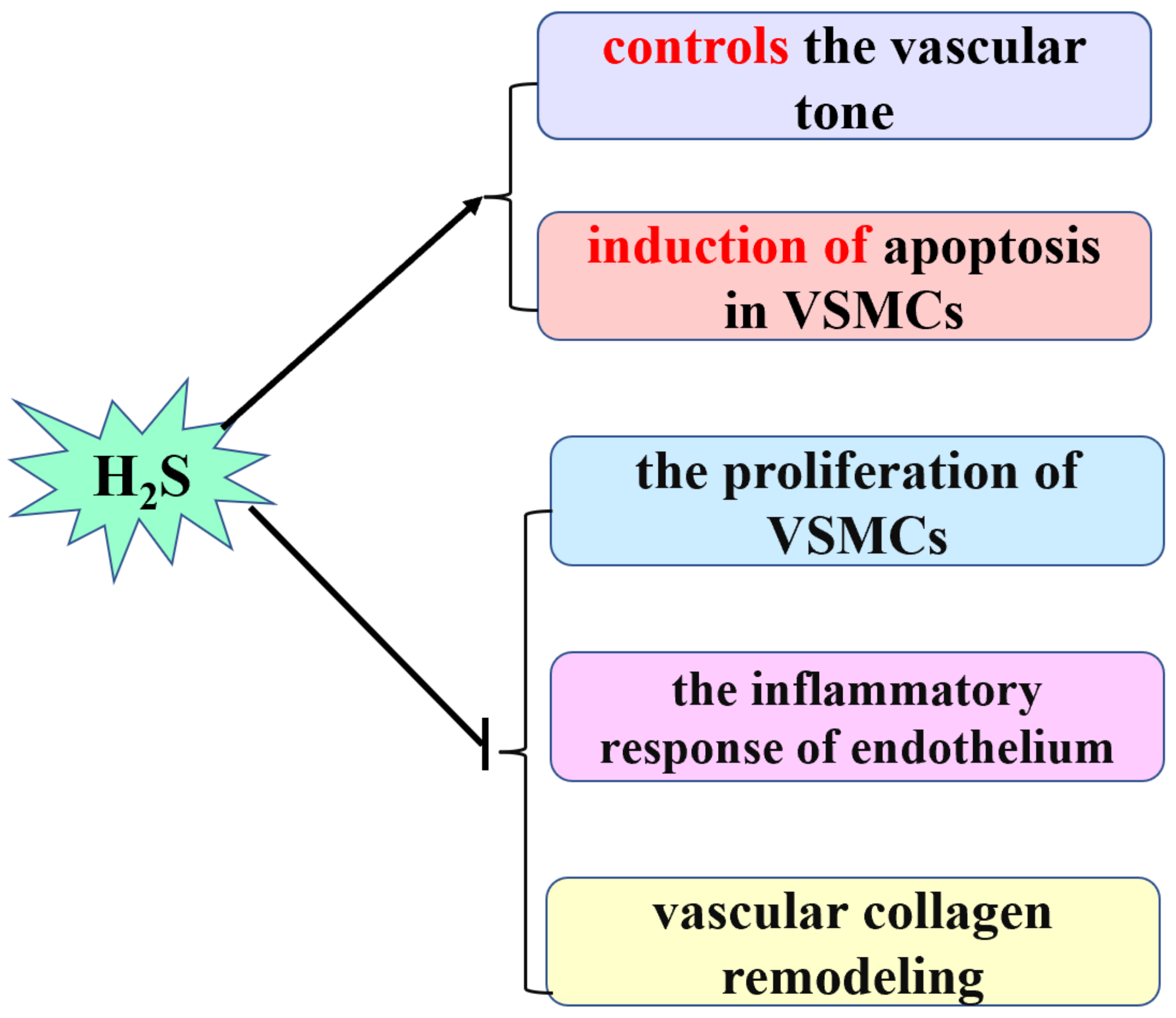
:1. Introduction
2. Biological Origin of H2S in the Cell
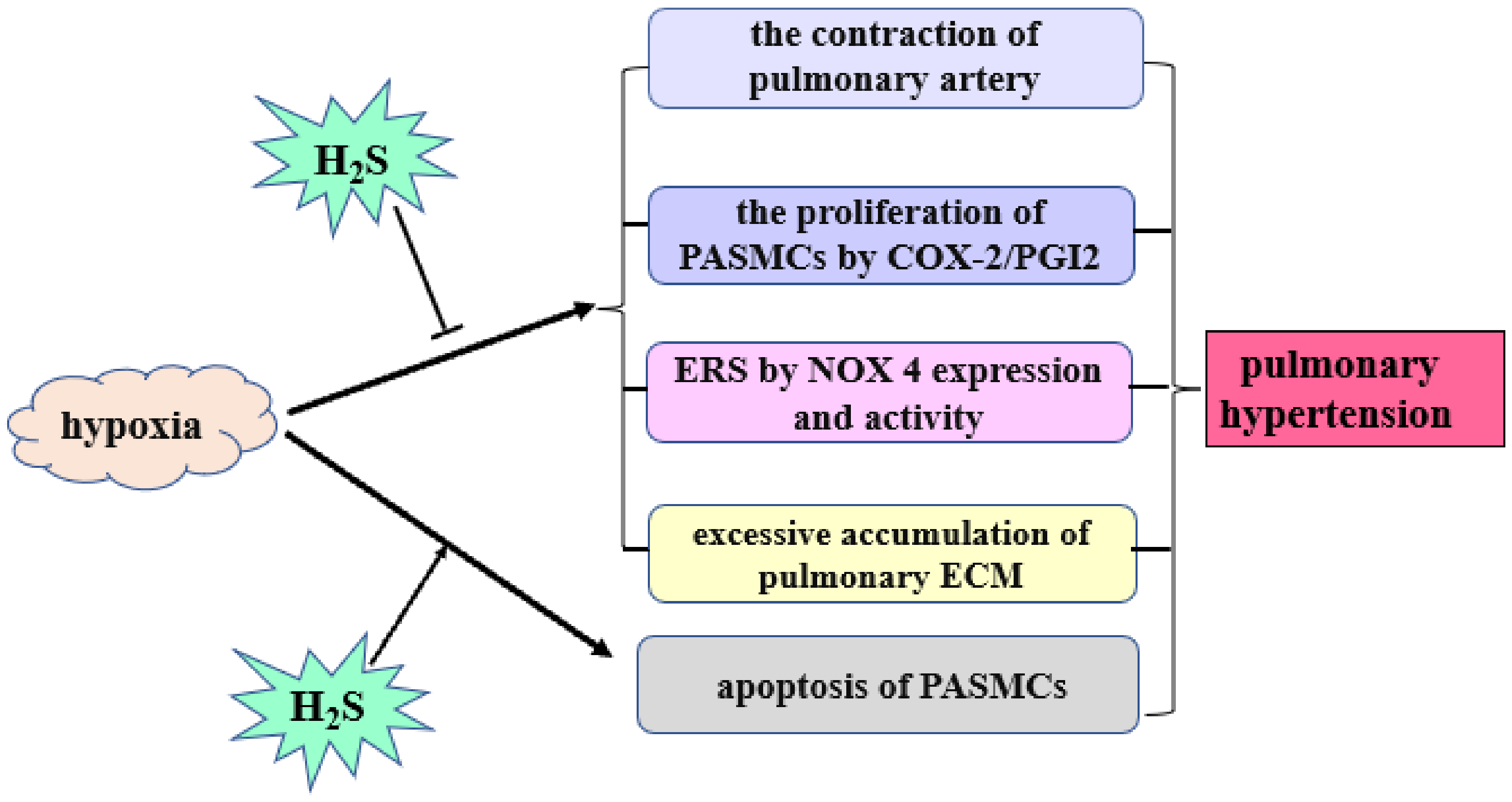
3. Role of H2S in HPH
4. Role of H2S in MCT-Induced PH
5. Role of H2S in High Pulmonary Blood Flow-Induced PH
6. Role of H2S in PH Associated with CHD
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Maron, B.A.; Abman, S.H.; Elliott, C.G.; Frantz, R.P.; Hopper, R.K.; Horn, E.M.; Nicolls, M.R.; Shlobin, O.A.; Shah, S.J.; Kovacs, G.; et al. Pulmonary Arterial Hypertension: Diagnosis, Treatment, and Novel Advances. Am. J. Respir. Crit. Care Med. 2021, 203, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Taichman, D.B.; Mandel, J. Epidemiology of Pulmonary Arterial Hypertension. Clin. Chest Med. 2013, 34, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Galie, N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: A review. JAMA Cardiol. 2016, 1, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Hansmann, G. Pulmonary Hypertension in Infants, Children, and Young Adults. J. Am. Coll. Cardiol. 2017, 69, 2551–2569. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.; Webb, G.D.; Bian, J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, H.; Fang, L.; Bian, J.; Gao, Y.; Li, C. H2S Donor and Bone Metabolism. Front. Pharmacol. 2021, 12, 661601. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective Smell of Hydrogen Sulfide and Polysulfide in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Neill, D.L.; Xian, M. Phosphonothioate-Based Hydrogen Sulfide Releasing Reagents: Chemistry and Biological Applications. Front. Pharmacol. 2017, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, C.; Organ, C.; Li, Z.; Bhushan, S.; Otsuka, H.; Pacheco, A.; Kang, J.; Aguilar, H.C.; Lefer, D.J.; et al. Design, synthesis, and cardioprotective effects of N-mercapto-based hydrogen sulfide donors. J. Med. Chem. 2015, 58, 7501–7511. [Google Scholar] [CrossRef]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation – An update. J. Adv. Res. 2020, 27, 85–97. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Tang, C. Hydrogen Sulfide as a New Endogenous Gaseous Transmitter in the Cardiovascular System. Curr. Vasc. Pharmacol. 2006, 4, 17–22. [Google Scholar]
- Roubenne, L.; Marthan, R.; Le Grand, B.; Guibert, C. Hydrogen Sulfide Metabolism and Pulmonary Hypertension. Cells 2021, 10, 1477. [Google Scholar] [CrossRef]
- Brampton, J.; Aaronson, P.I. Role of hydrogen sulfide in systemic and pulmonary hypertension: Cellular mechanisms and therapeutic implications. Cardiovasc. Hematol. Agents Med. Chem. 2016, 14, 4–22. [Google Scholar] [CrossRef]
- Jin, H.F.; Cong, B.L.; Zhao, B.; Zhang, C.Y.; Liu, X.M.; Zhou, W.J.; Shi, Y.; Tang, C.S.; Du, J.B. Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci. 2006, 78, 1299–1309. [Google Scholar]
- Bełtowski, J. Hydrogen sulfide as a biologically active mediator in the cardiovascular system. Postepy Hig. Med. Dosw. 2004, 58, 285–291. [Google Scholar]
- Łowicka, E.; Bełtowski, J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol. Rep. 2007, 59, 4–24. [Google Scholar] [PubMed]
- Skovgaard, N.; Gouliaev, A.; Aalling, M.; Simonsen, U. The role of endogenous H2S in cardiovascular physiology. Curr. Pharm. Biotechnol. 2011, 12, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Du, J.B.; Bu, D.F.; Yan, H.; Tang, X.Y.; Tang, C.S. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun. 2003, 302, 810–816. [Google Scholar]
- Zhang, Q.Y.; Du, J.B.; Zhou, W.J.; Yan, H.; Tang, C.S.; Zhang, C.Y. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem. Biophys. Res. Commun. 2004, 317, 30–37. [Google Scholar]
- Chen, J.; Zhang, H.Z.; Yu, W.C.; Chen, L.; Wang, Z.J.; Zhang, T. Expression of pulmonary arterial elastin in rats with hypoxic pulmonary hypertension using H2S. J. Recept. Signal. Transduct. Res. 2020, 40, 383–387. [Google Scholar] [CrossRef]
- Wei, H.L.; Zhang, C.Y.; Jin, H.F.; Tang, C.S.; Du, J.B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008, 29, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Pan, W.; Wang, C.; Dong, H.; Xing, L.; Hou, J.; Fang, S.; Li, H.; Yang, F.; Yu, B. H2S attenuates endoplasmic reticulum stress in hypoxia-induced pulmonary artery hypertension. Biosci. Rep. 2019, 39, BSR20190304. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, B.; Liu, D.; Nie, W.; Yuan, J.; Wang, Z.; Guo, Y. Sodium hydrosulfide prevents hypoxia-induced pulmonary arterial hypertension in broilers. Br. Poult. Sci. 2012, 53, 608–615. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, G.H.; Cai, D.Q.; Pan, B.Y.; Lin, Y.S.; Li, X.D.; Li, S.J.; Zhu, L.; Liao, X.X.; Wang, H.S. H2S inhibition of chemical hypoxia-induced proliferation of HPASMCs is mediated by the upregulation of COX-2/PGI2. Int. J. Mol. Med. 2014, 33, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.M.; Zhuan, B.; Li, P.; Zhao, X.; Wang, T.; Yang, Z. Expression of nicotinamide adenine dinucleotide phosphate-reduced oxidase-4/reactive oxygen species and cystathionine-γ-lyase/hydrogen sulfide in patients with chronic obstructive pulmonary disease-related pulmonary hypertension. Zhonghua Nei Ke Za Zhi 2019, 58, 770–776. [Google Scholar]
- Ryan, J.; Bloch, K.; Archer, S.L. Rodent models of pulmonary hypertension: Harmonisation with the world health organisation’s categorisation of human PH. Int. J. Clin. Pract. Suppl. 2011, 172, 15–34. [Google Scholar] [CrossRef]
- Wilson, D.W.; Segall, H.J.; Pan, L.C.; Lamé, M.W.; Estep, J.E.; Morin, D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit. Rev. Toxicol. 1992, 22, 307–325. [Google Scholar] [CrossRef]
- Feng, S.S.; Chen, S.Y.; Yu, W.; Zhang, D.; Zhang, C.Y.; Tang, C.S.; Du, J.B.; Jin, H.F. H2S inhibits pulmonary arterial endothelial cell inflammation in rats with monocrotaline-induced pulmonary hypertension. Lab. Investig. 2017, 97, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Sirmagul, B.; Ilgin, S.; Atli, O.; Usanmaz, S.E.; Demirel-Yilmaz, E. Assessment of the endothelial functions in monocrotaline-induced pulmonary hypertension. Clin. Exp. Hypertens. 2013, 35, 220–227. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Chen, S.; Chen, S.; Yu, W.; Liu, X.; Yang, G.; Tao, Y.; Tang, X.; Bu, D.; et al. Endogenous hydrogen sulfide sulfhydrates IKK beta at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clin. Sci. 2019, 133, 2045–2059. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, Y.; Ma, Y.; Zhang, J.; Wang, C. Protective effect of hydrogen sulfide on monocrotaline-induced pulmonary arterial hypertension via inhibition of the endothelial mesenchymal transition. Int. J. Mol. Med. 2019, 44, 2091–2102. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Ying, Z.T.; Zhao, K.Y. Effects of hydrogen sulfide on mast cell and IL-6 in rats with pulmonary hypertension induced by monocrotaline. Chin. J. Lab. Diagn. 2017, 21, 689–692. [Google Scholar]
- Wang, Y.F.; Shi, L.; Du, J.B.; Tang, C.S. Impact of L-arginine on hydrogen sulfide/cystathionine-gamma-lyase pathway in rats with high blood flow-induced pulmonary hypertension. Biochem. Biophys. Res. Commun. 2006, 345, 851–857. [Google Scholar]
- Li, X.H.; Du, J.B.; Shi, L.; Li, J.; Tang, X.Y.; Qi, J.G.; Wei, B.; Jin, H.F.; Tang, C.S. Down-regulation of endogenous hydrogen sulfide pathway in pulmonary hypertension and pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Circ. J. 2005, 69, 1418–1424. [Google Scholar]
- Li, X.H.; Du, J.B.; Bu, D.F.; Tang, X.Y.; Tang, C.S. Sodium hydrosulfide alleviated pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats1. Acta Pharmacol. Sin. 2006, 27, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Du, J.; Jin, H.; Geng, B.; Tang, C. Sodium hydrosulfide alleviates pulmonary artery collagen remodeling in rats with high pulmonary blood flow. Hear. Vessel. 2008, 23, 409–419. [Google Scholar] [CrossRef]
- Li, W.; Jin, H.-F.; Liu, D.; Sun, J.-H.; Jian, P.-J.; Li, X.-H.; Tang, C.-S.; Du, J.-B. Hydrogen sulfide induces apoptosis of pulmonary artery smooth muscle cell in rats with pulmonary hypertension induced by high pulmonary blood flow. Chin. Med. J. 2009, 122, 3032–3038. [Google Scholar]
- Luo, L.; Liu, D.; Tang, C.; Du, J.; Liu, A.D.; Holmberg, L.; Jin, H. Sulfur dioxide upregulates the inhibited endogenous hydrogen sulfide pathway in rats with pulmonary hypertension induced by high pulmonary blood flow. Biochem. Biophys. Res. Commun. 2013, 433, 519–525. [Google Scholar] [CrossRef]
- Li, X.H.; Du, J.B.; Tang, C.S. Important of hydrogen sulfide donor on pulmonary vascular structure and vasoactive peptides in rats with pulmonary hypertension induced by high pulmonary blood flow. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006, 28, 159–163. [Google Scholar]
- Sun, L.; Sun, S.; Li, Y.; Pan, W.; Xie, Y.; Wang, S.; Zhang, Z. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chin. Med. J. 2014, 127, 893–899. [Google Scholar]
- Tan, Y.; Wang, S.; Ren, X.; Zhang, C.; Xu, F. The prognostic implications of perioperative endogenous hydrogen sulfide and nitric oxide levels in children with congenital heart disease complicated by pulmonary arterial hypertension. Eur. J. Pediatr. 2021, 180, 1915–1922. [Google Scholar] [CrossRef]
- Mao, Y.G.; Chen, X.; Zhang, Y.; Chen, G. Hydrogen sulfide therapy: A narrative overview of current research and possible therapeutic implications in future. Med. Gas Res. 2020, 10, 185–188. [Google Scholar]
- Peng, W.; Zhang, M.L.; Zhang, J.; Chen, G. Potential role of hydrogen sulfide in central nervous system tumors: A narrative review. Med. Gas Res. 2022, 12, 6–9. [Google Scholar]
- Kashfi, K.; Olson, K.R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Li, Y.; Li, L.; Xu, S.; Feng, X.; Liu, S. Hydrogen sulfide (H2S)-releasing compounds: Therapeutic potential in cardiovascular diseases. Front. Pharmacol. 2018, 9, 1066. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a Novel, Water-soluble hydrogen sulfide–releasing molecule (GYY4137). Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015, 554, 143–167. [Google Scholar]
- Zhang, H.; Hao, L.Z.; Pan, J.A.; Gao, Q.; Zhang, J.F.; Kankala, R.K.; Wang, S.B.; Chen, A.Z.; Zhang, H.L. Microfluidic fabrication of inhalable large porous microspheres loaded with H2S-releasing aspirin derivative for pulmonary arterial hypertension therapy. J. Control. Release 2021, 329, 286–298. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Tang, C.; Jin, H.; Du, J. Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension. Biomolecules 2022, 12, 772. https://doi.org/10.3390/biom12060772
Sun Y, Tang C, Jin H, Du J. Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension. Biomolecules. 2022; 12(6):772. https://doi.org/10.3390/biom12060772
Chicago/Turabian StyleSun, Yan, Chaoshu Tang, Hongfang Jin, and Junbao Du. 2022. "Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension" Biomolecules 12, no. 6: 772. https://doi.org/10.3390/biom12060772
APA StyleSun, Y., Tang, C., Jin, H., & Du, J. (2022). Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension. Biomolecules, 12(6), 772. https://doi.org/10.3390/biom12060772





