The Landscape of Noncoding RNA in Pulmonary Hypertension
Abstract
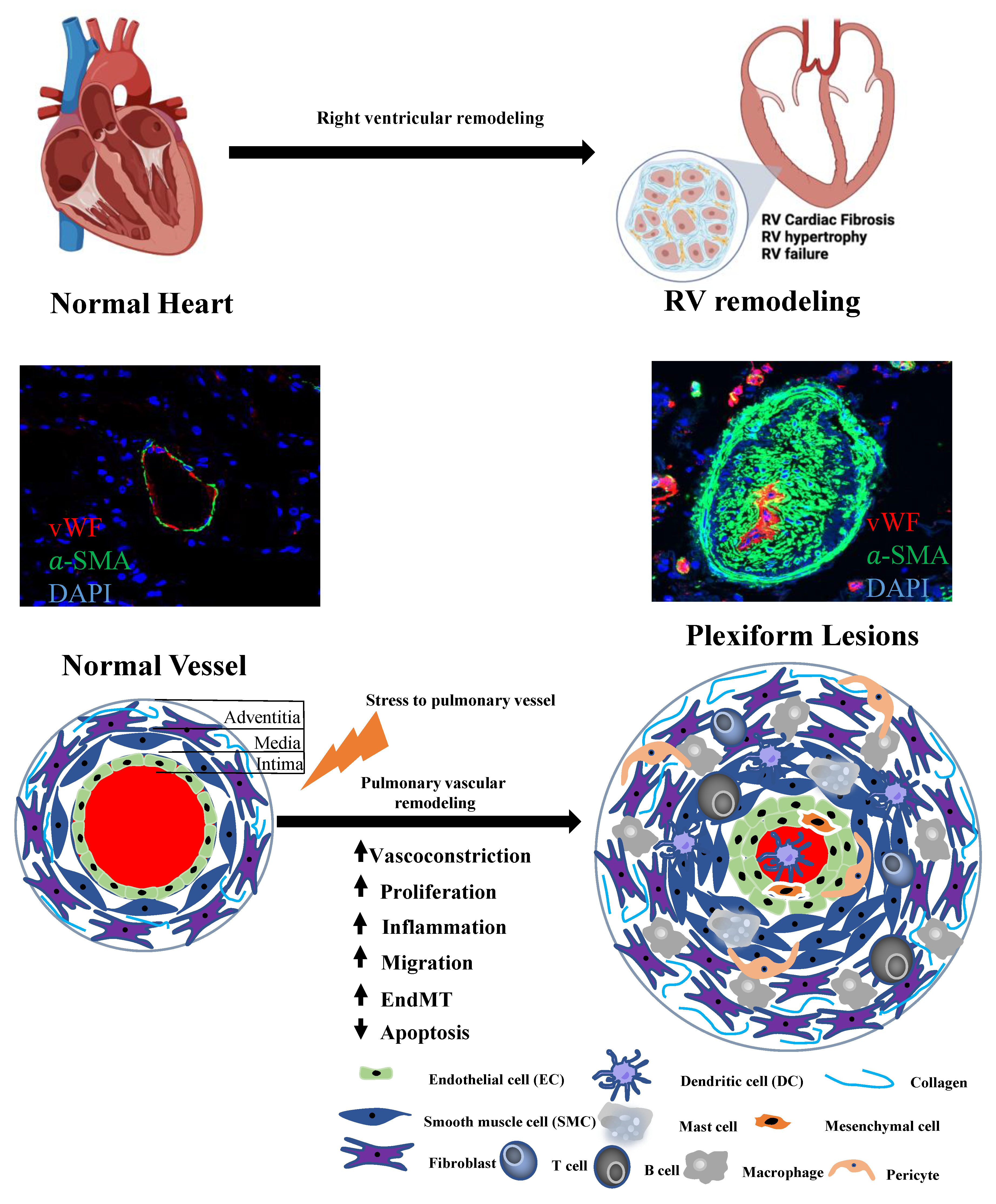
:1. The Noncoding RNA Transcriptome in Pulmonary Hypertension
2. Long Noncoding RNAs (LncRNAs) in Pulmonary Hypertension
2.1. LncRNA Biogenesis and Function
2.2. LncRNAs in Pulmonary Artery Endothelial Cells in PH
2.3. LncRNA in Pulmonary Artery Smooth Muscle Cells in PH
2.4. LncRNA Distributed in Cytoplasm and Functional as CeRNA in PASMCs
2.5. LncRNA Distributed in Both Nucleus and Cytoplasm in PASMCs
2.6. LncRNAs Localized to the Nucleus
2.7. LncRNA in Right Ventricular in Pulmonary Hypertension
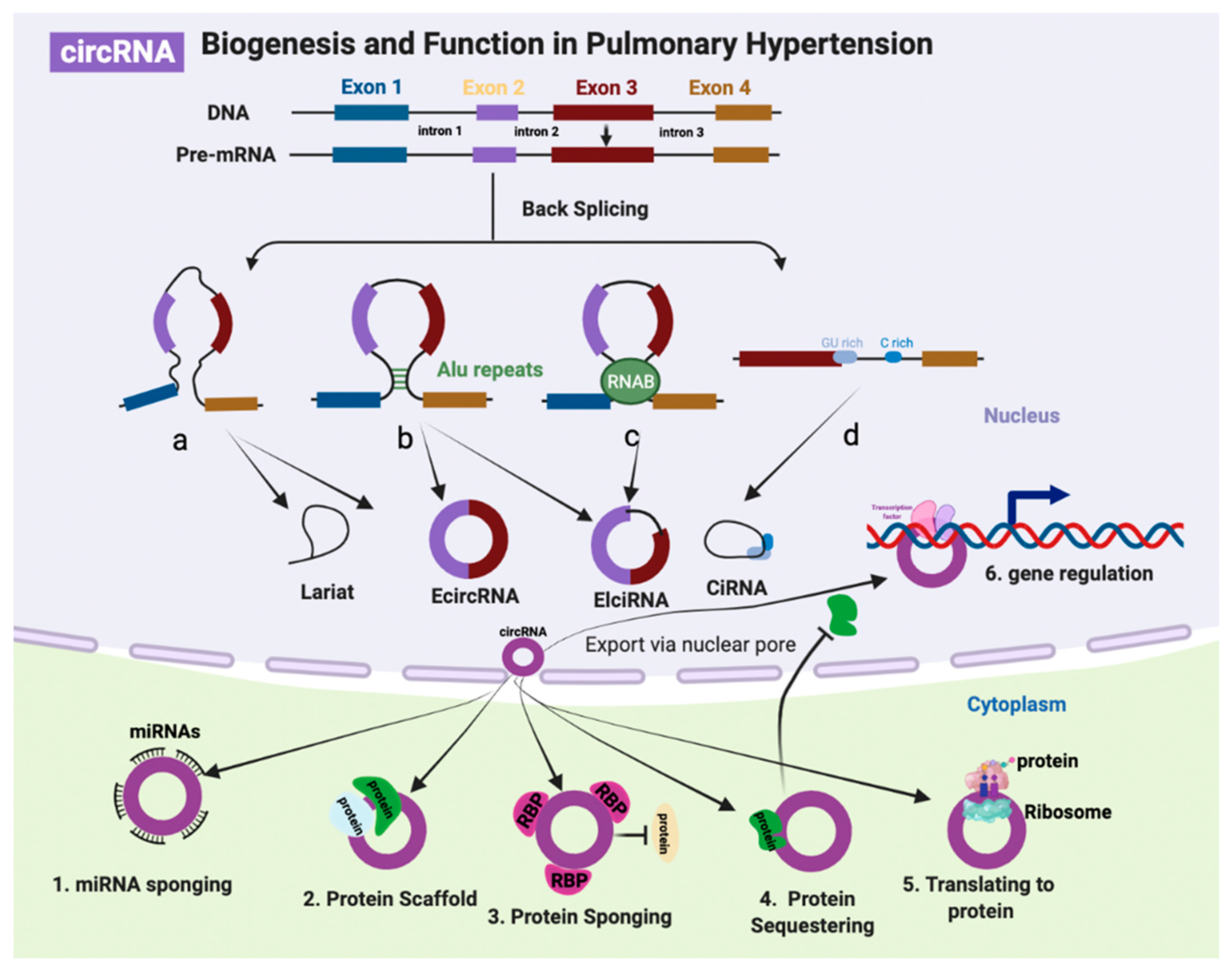
3. CircRNAs
3.1. CircRNA Biogenesis and Function
3.2. CircRNA in Pulmonary Hypertension
3.2.1. CircRNAs Identified from Patient Samples
3.2.2. CircRNAs Identified from PH Rodent Models
3.3. Forefront Areas of Non-Coding RNA Research
3.4. Single Cell and Spatial Transcriptomics
3.5. NcRNA-Protein Interactions
3.6. Biomarker of LncRNAs and CircRNAs in PH
4. NcRNA Therapeutics
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PH | pulmonary hypertension |
| PAH | pulmonary arterial hypertension |
| NcRNA | non-coding RNA |
| LncRNA | long noncoding RNA |
| SncRNA | small noncoding RNA |
| CircRNA | circular RNA |
| MiRNA | microRNA |
| PiRNA | Piwi-interacting RNA |
| RV | right ventricular |
| ceRNA | competitive endogenous RNA |
| LincRNA | long intergenic ncRNA |
| ERNA | enhancer RNA |
| PAEC | pulmonary artery endothelial cell |
| PASMC | pulmonary artery smooth muscle cell |
| PMEC | pulmonary microvascular endothelial cell |
| EPC | endothelial progenitor cell |
| PBMC | peripheral blood mononuclear cell |
| scRNA-Seq | Single-Cell RNA-Sequencing |
| spRNA-Seq | Spatial RNA-Sequencing |
References
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, T.; Raj, J.U. MicroRNAs in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2015, 52, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Pan, Y.; Fang, Y.; Zhang, J.; Xie, M.; Yang, F.; Yu, T.; Ma, P.; Li, W.; Shu, Y. The Biogenesis and Functions of piRNAs in Human Diseases. Mol. Ther. Nucleic Acids 2020, 21, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, M.; Hernandez, N. RNA polymerase III transcription as a disease factor. Genes. Dev. 2020, 34, 865–882. [Google Scholar] [CrossRef]
- Deininger, P. Alu elements: Know the SINEs. Genome Biol. 2011, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gussakovsky, D.; McKenna, S.A. Alu RNA and their roles in human disease states. RNA Biol. 2021, 18, 574–585. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Patop, I.L.; Wust, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Santer, L.; Bar, C.; Thum, T. Circular RNAs: A Novel Class of Functional RNA Molecules with a Therapeutic Perspective. Mol. Ther. 2019, 27, 1350–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.A.; Nsengimana, B.; Khan, N.H.; Song, Z.; Ngowi, E.E.; Wang, Y.; Zhang, W.; Ji, S. Chimeric Peptides/Proteins Encoded by circRNA: An Update on Mechanisms and Functions in Human Cancers. Front. Oncol. 2022, 12, 781270. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Lee, A.; McLean, D.; Choi, J.; Kang, H.; Chang, W.; Kim, J. Therapeutic implications of microRNAs in pulmonary arterial hypertension. BMB Rep. 2014, 47, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, V.; Chan, S.Y. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2017, 2, e91327. [Google Scholar] [CrossRef] [Green Version]
- Santos-Ferreira, C.A.; Abreu, M.T.; Marques, C.I.; Goncalves, L.M.; Baptista, R.; Girao, H.M. Micro-RNA Analysis in Pulmonary Arterial Hypertension: Current Knowledge and Challenges. JACC Basic Transl. Sci. 2020, 5, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Bonnet, S.; Chan, S.Y. Translational Advances in the Field of Pulmonary Hypertension. Translating MicroRNA Biology in Pulmonary Hypertension. It Will Take More Than “miR” Words. Am. J. Respir. Crit. Care Med. 2017, 195, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Errington, N.; Iremonger, J.; Pickworth, J.A.; Kariotis, S.; Rhodes, C.J.; Rothman, A.M.; Condliffe, R.; Elliot, C.A.; Kiely, D.G.; Howard, L.S.; et al. A diagnostic miRNA signature for pulmonary arterial hypertension using a consensus machine learning approach. EBioMedicine 2021, 69, 103444. [Google Scholar] [CrossRef]
- Chen, W.; Li, S. Circulating microRNA as a Novel Biomarker for Pulmonary Arterial Hypertension Due to Congenital Heart Disease. Pediatr. Cardiol. 2017, 38, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Zhao, Q.; Wu, W.; Wang, L.; Miao, Y.; Yuan, P. Circular RNAs in pulmonary hypertension: Emerging biological concepts and potential mechanism. Anim. Model Exp. Med. 2022, 5, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Zhang, Q.; Li, X. Non-Coding RNA Networks in Pulmonary Hypertension. Front. Genet. 2021, 12, 703860. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Jungreis, I.; Lagarde, J.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Armstrong, J.; Barnes, I.; et al. Gencode 2021. Nucleic Acids Res. 2021, 49, D916–D923. [Google Scholar] [CrossRef] [PubMed]
- Uszczynska-Ratajczak, B.; Lagarde, J.; Frankish, A.; Guigo, R.; Johnson, R. Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 2018, 19, 535–548. [Google Scholar] [CrossRef]
- Quinn, J.J.; Zhang, Q.C.; Georgiev, P.; Ilik, I.A.; Akhtar, A.; Chang, H.Y. Rapid evolutionary turnover underlies conserved lncRNA-genome interactions. Genes. Dev. 2016, 30, 191–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hezroni, H.; Koppstein, D.; Schwartz, M.G.; Avrutin, A.; Bartel, D.P.; Ulitsky, I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep. 2015, 11, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Bradshaw, A.C.; Baker, A.H. Role of noncoding RNA in vascular remodelling. Curr. Opin. Lipidol. 2016, 27, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: LncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes. Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Ghosal, S.; Das, S.; Balti, S.; Chakrabarti, J. Competing endogenous RNA: The key to posttranscriptional regulation. Sci. World J. 2014, 2014, 896206. [Google Scholar] [CrossRef]
- Orafidiya, F.; Deng, L.; Bevan, C.L.; Fletcher, C.E. Crosstalk between Long Non Coding RNAs, microRNAs and DNA Damage Repair in Prostate Cancer: New Therapeutic Opportunities? Cancers 2022, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ. Res. 2018, 122, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zahid, K.R.; Raza, U.; Chen, J.; Raj, U.J.; Gou, D. Pathobiology of pulmonary artery hypertension: Role of long non-coding RNAs. Cardiovasc. Res. 2020, 116, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arter. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Jurasz, P.; Courtman, D.; Babaie, S.; Stewart, D.J. Role of apoptosis in pulmonary hypertension: From experimental models to clinical trials. Pharmacol. Ther. 2010, 126, 1–8. [Google Scholar] [CrossRef]
- Michelakis, E.D. Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: Heterogeneous BMP signaling may have therapeutic implications. Circ. Res. 2006, 98, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Kuebler, W.M.; Nicolls, M.R.; Olschewski, A.; Abe, K.; Rabinovitch, M.; Stewart, D.; Chan, S.Y.; Morrell, N.W.; Archer, S.L.; Spiekerkoetter, E. A pro-con debate: Current controversies in PAH pathogenesis at the American Thoracic Society International Conference in 2017. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L502–L516. [Google Scholar] [CrossRef]
- Gu, S.; Li, G.; Zhang, X.; Yan, J.; Gao, J.; An, X.; Liu, Y.; Su, P. Aberrant expression of long noncoding RNAs in chronic thromboembolic pulmonary hypertension. Mol. Med. Rep. 2015, 11, 2631–2643. [Google Scholar] [CrossRef] [Green Version]
- Ball, M.K.; Waypa, G.B.; Mungai, P.T.; Nielsen, J.M.; Czech, L.; Dudley, V.J.; Beussink, L.; Dettman, R.W.; Berkelhamer, S.K.; Steinhorn, R.H.; et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1alpha. Am. J. Respir. Crit. Care Med. 2014, 189, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Jiang, R.; Hu, X.; Zhao, Q.; Sun, Y.; Wang, L.; Li, J.; Miao, Y.; Wu, W.; Yuan, P. Dysregulated lncRNA TUG1 in different pulmonary artery cells under hypoxia. Ann. Transl. Med. 2021, 9, 879. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Gunther, S.; et al. Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation 2017, 136, 65–79. [Google Scholar] [CrossRef]
- Stevens, T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest 2005, 128, 558S–564S. [Google Scholar] [CrossRef]
- Suzuki, T.; Carrier, E.J.; Talati, M.H.; Rathinasabapathy, A.; Chen, X.; Nishimura, R.; Tada, Y.; Tatsumi, K.; West, J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L118–L126. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Pechoux, C.; Bogaard, H.J.; Dorfmuller, P.; Remy, S.; Lecerf, F.; Plante, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [Green Version]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Antioxid. Redox Signal. 2021, 34, 891–914. [Google Scholar] [CrossRef]
- Park, J.F.; Clark, V.R.; Banerjee, S.; Hong, J.; Razee, A.; Williams, T.; Fishbein, G.; Saddic, L.; Umar, S. Transcriptomic Analysis of Right Ventricular Remodeling in Two Rat Models of Pulmonary Hypertension: Identification and Validation of Epithelial-to-Mesenchymal Transition in Human Right Ventricular Failure. Circ. Heart Fail. 2021, 14, e007058. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, Y.; Tang, Y.; Li, Q. MALAT1 Modulates TGF-beta1-Induced Endothelial-to-Mesenchymal Transition through Downregulation of miR-145. Cell Physiol. Biochem. 2017, 42, 357–372. [Google Scholar] [CrossRef]
- Tan, A.; Li, T.; Ruan, L.; Yang, J.; Luo, Y.; Li, L.; Wu, X. Knockdown of Malat1 alleviates high-glucose-induced angiogenesis through regulating miR-205-5p/VEGF-A axis. Exp. Eye Res. 2021, 207, 108585. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Chang, L.; Wei, C.; Bei, H.; Yin, Y.; Chen, M.; Wang, H.; Liang, J.; Wu, Y. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/beta-catenin signaling pathway. Lipids Health Dis. 2019, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Sun, Y.; Ye, Z.; Yang, H.; Li, L. Oxidized low density lipoprotein induces endothelial-to-mesenchymal transition by stabilizing Snail in human aortic endothelial cells. Biomed. Pharmacother. 2018, 106, 1720–1726. [Google Scholar] [CrossRef]
- Wu, H.; Liu, T.; Hou, H. Knockdown of LINC00657 inhibits ox-LDL-induced endothelial cell injury by regulating miR-30c-5p/Wnt7b/beta-catenin. Mol. Cell Biochem. 2020, 472, 145–155. [Google Scholar] [CrossRef]
- Yin, Q.; He, M.; Huang, L.; Zhang, X.; Zhan, J.; Hu, J. lncRNA ZFAS1 promotes ox-LDL induced EndMT through miR-150-5p/Notch3 signaling axis. Microvasc. Res. 2021, 134, 104118. [Google Scholar] [CrossRef]
- Umar, S.; Ruffenach, G.; Moazeni, S.; Vaillancourt, M.; Hong, J.; Cunningham, C.; Cao, N.; Navab, S.; Sarji, S.; Li, M.; et al. Involvement of Low-Density Lipoprotein Receptor in the Pathogenesis of Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e012063. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Huang, X.Q.; Jiang, Y.Y.; Li, N.; Wang, J.; Chen, S.Y. LncRNA TUG1 regulates autophagy-mediated endothelial-mesenchymal transition of liver sinusoidal endothelial cells by sponging miR-142-3p. Am. J. Transl. Res. 2020, 12, 758–772. [Google Scholar] [PubMed]
- Zhang, Y.; Fan, K.; Xu, X.; Wang, A. The TGF-beta1 Induces the Endothelial-to-Mesenchymal Transition via the UCA1/miR-455/ZEB1 Regulatory Axis in Human Umbilical Vein Endothelial Cells. DNA Cell Biol. 2020, 39, 1264–1273. [Google Scholar] [CrossRef]
- Cao, X.; Xue, L.D.; Di, Y.; Li, T.; Tian, Y.J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef]
- Shi, S.; Song, L.; Yu, H.; Feng, S.; He, J.; Liu, Y.; He, Y. Knockdown of LncRNA-H19 Ameliorates Kidney Fibrosis in Diabetic Mice by Suppressing miR-29a-Mediated EndMT. Front. Pharmacol. 2020, 11, 586895. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Jiang, Y.; Li, D.; Sun, X.; Zhang, Y.; Qin, L.; Tellides, G.; Taylor, H.S.; Huang, Y. H19/TET1 axis promotes TGF-beta signaling linked to endothelial-to-mesenchymal transition. FASEB J. 2020, 34, 8625–8640. [Google Scholar] [CrossRef]
- Chen, J.J.; Ma, W.M.; Yuan, J.L.; Cui, L.Q. PM2.5 exposure aggravates left heart failure induced pulmonary hypertension. Acta Cardiol. 2019, 74, 238–244. [Google Scholar] [CrossRef]
- Ma, K.; Li, C.; Xu, J.; Ren, F.; Xu, X.; Liu, C.; Niu, B.; Li, F. LncRNA Gm16410 regulates PM2.5-induced lung Endothelial-Mesenchymal Transition via the TGF-beta1/Smad3/p-Smad3 pathway. Ecotoxicol. Environ. Saf. 2020, 205, 111327. [Google Scholar] [CrossRef]
- He, Y.; Dan, Y.; Gao, X.; Huang, L.; Lv, H.; Chen, J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E598–E608. [Google Scholar] [CrossRef]
- Neumann, P.; Jae, N.; Knau, A.; Glaser, S.F.; Fouani, Y.; Rossbach, O.; Kruger, M.; John, D.; Bindereif, A.; Grote, P.; et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat. Commun. 2018, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Rodor, J.; Caudrillier, A.; Scanlon, J.P.; Spiroski, A.M.; Dudnakova, T.; Pfluger-Muller, B.; Shmakova, A.; von Kriegsheim, A.; Deng, L.; et al. MIR503HG Loss Promotes Endothelial-to-Mesenchymal Transition in Vascular Disease. Circ. Res. 2021, 128, 1173–1190. [Google Scholar] [CrossRef]
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef]
- Kurakula, K.; Smolders, V.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Lechartier, B.; Berrebeh, N.; Huertas, A.; Humbert, M.; Guignabert, C.; Tu, L. Phenotypic Diversity of Vascular Smooth Muscle Cells in Pulmonary Arterial Hypertension: Implications for Therapy. Chest 2022, 161, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yan, G.; Qiao, Y.; Wang, D.; Luo, E.; Hou, J.; Tang, C. Emerging role of long non-coding RNAs in pulmonary hypertension and their molecular mechanisms (Review). Exp. Ther. Med. 2020, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ali, M.K.; Dua, K.; Spiekerkoetter, E.; Mao, Y. Role of Long Non-Coding RNAs in Pulmonary Arterial Hypertension. Cells 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhao, Z.; Zhao, Q.; Yu, X.; Yan, L.; Zhang, Y.; Luo, Q.; Liu, Z. Long noncoding RNAs: Emerging roles in pulmonary hypertension. Heart Fail. Rev. 2020, 25, 795–815. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed. Pharmacother. 2019, 118, 109129. [Google Scholar] [CrossRef]
- Xing, Y.; Zheng, X.; Fu, Y.; Qi, J.; Li, M.; Ma, M.; Wang, S.; Li, S.; Zhu, D. Long Noncoding RNA-Maternally Expressed Gene 3 Contributes to Hypoxic Pulmonary Hypertension. Mol. Ther. 2019, 27, 2166–2181. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Nie, X.; Sun, S.; Dong, S.; Yuan, C.; Li, Y.; Xiao, B.; Jie, D.; Liu, Y. Long Non-Coding RNA MEG3 Downregulation Triggers Human Pulmonary Artery Smooth Muscle Cell Proliferation and Migration via the p53 Signaling Pathway. Cell Physiol. Biochem. 2017, 42, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, H.; Zhang, P.; Yu, X.; Jiang, J.; Chen, S. Down-regulation of lncRNA Gas5 promotes hypoxia-induced pulmonary arterial smooth muscle cell proliferation by regulating KCNK3 expression. Eur. J. Pharmacol. 2020, 889, 173618. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Yang, T.; Liu, Y.; Wang, X. LncRNA-GAS5/miR-382-3p axis inhibits pulmonary artery remodeling and promotes autophagy in chronic thromboembolic pulmonary hypertension. Genes. Genom. 2022, 44, 395–404. [Google Scholar] [CrossRef]
- Brock, M.; Schuoler, C.; Leuenberger, C.; Buhlmann, C.; Haider, T.J.; Vogel, J.; Ulrich, S.; Gassmann, M.; Kohler, M.; Huber, L.C. Analysis of hypoxia-induced noncoding RNAs reveals metastasis-associated lung adenocarcinoma transcript 1 as an important regulator of vascular smooth muscle cell proliferation. Exp. Biol. Med. 2017, 242, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Shen, J.; Zhang, C.; Chen, Y.; Wang, W.; Tao, K. Long-Chain Non-Coding RNA Metastasis-Related Lung Adenocarcinoma Transcript 1 (MALAT1) Promotes the Proliferation and Migration of Human Pulmonary Artery Smooth Muscle Cells (hPASMCs) by Regulating the MicroRNA-503 (miR-503)/Toll-Like Receptor 4 (TLR4) Signal Axis. Med. Sci. Monit. 2020, 26, e923123. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Wu, B.; Jiang, S.; Pan, H.; Wang, R.; Chen, J. Long noncoding RNA MALAT1 sponges miR1243p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int. J. Mol. Med. 2019, 44, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Chen, Z.; Chen, Y.; Lv, H.; Lu, H.; Yan, F.; Li, L.; Zhang, W.; Shi, J. Long non-coding RNA CASC2 suppresses pulmonary artery smooth muscle cell proliferation and phenotypic switch in hypoxia-induced pulmonary hypertension. Respir. Res. 2019, 20, 53. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Yang, C.; Gao, C.; Guo, X.; Cheng, J. LncRNA CASC2 inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation and migration by regulating the miR-222/ING5 axis. Cell Mol. Biol. Lett. 2020, 25, 21. [Google Scholar] [CrossRef]
- Yang, L.; Liang, H.; Shen, L.; Guan, Z.; Meng, X. LncRNA Tug1 involves in the pulmonary vascular remodeling in mice with hypoxic pulmonary hypertension via the microRNA-374c-mediated Foxc1. Life Sci. 2019, 237, 116769. [Google Scholar] [CrossRef]
- Wang, S.; Cao, W.; Gao, S.; Nie, X.; Zheng, X.; Xing, Y.; Chen, Y.; Bao, H.; Zhu, D. TUG1 Regulates Pulmonary Arterial Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Can. J. Cardiol. 2019, 35, 1534–1545. [Google Scholar] [CrossRef]
- Dou, X.; Ma, Y.; Qin, Y.; Dong, Q.; Zhang, S.; Tian, R.; Pan, M. NEAT1 silencing alleviates pulmonary arterial smooth muscle cell migration and proliferation under hypoxia through regulation of miR34a5p/KLF4 in vitro. Mol. Med. Rep. 2021, 24, 749. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, B.; Li, L.; Wang, D.; Qiao, Y.; Liu, B.; Luo, E.; Hou, J.; Yan, G.; Tang, C. Overexpressed lncRNA AC068039.4 Contributes to Proliferation and Cell Cycle Progression of Pulmonary Artery Smooth Muscle Cells Via Sponging miR-26a-5p/TRPC6 in Hypoxic Pulmonary Arterial Hypertension. Shock 2021, 55, 244–255. [Google Scholar] [CrossRef]
- Cheng, G.; He, L.; Zhang, Y. LincRNA-Cox2 promotes pulmonary arterial hypertension by regulating the let-7a-mediated STAT3 signaling pathway. Mol. Cell Biochem. 2020, 475, 239–247. [Google Scholar] [CrossRef]
- Wang, H.; Qin, R.; Cheng, Y. LncRNA-Ang362 Promotes Pulmonary Arterial Hypertension by Regulating miR-221 and miR-222. Shock 2020, 53, 723–729. [Google Scholar] [CrossRef]
- Lei, S.; Peng, F.; Li, M.L.; Duan, W.B.; Peng, C.Q.; Wu, S.J. LncRNA-SMILR modulates RhoA/ROCK signaling by targeting miR-141 to regulate vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H377–H391. [Google Scholar] [CrossRef]
- Su, H.; Xu, X.; Yan, C.; Shi, Y.; Hu, Y.; Dong, L.; Ying, S.; Ying, K.; Zhang, R. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2018, 19, 254. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hu, R.; Zhu, J.; Nie, X.; Jiang, Y.; Hu, P.; Liu, Y.; Sun, Z. The lncRNA PAHRF functions as a competing endogenous RNA to regulate MST1 expression by sponging miR-23a-3p in pulmonary arterial hypertension. Vasc. Pharmacol. 2021, 139, 106886. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, Y.; Yan, L.; Du, W.; Wang, S.; Zheng, X.; Zhang, M.; Zhang, J.; Qi, J.; et al. Long Noncoding RNA Rps4l Mediates the Proliferation of Hypoxic Pulmonary Artery Smooth Muscle Cells. Hypertension 2020, 76, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Sun, H.; Chen, Y.; Li, W.; Yu, X.; Zhao, X.; Zhang, L.; Yang, J.; Xin, W.; et al. lnc-Rps4l-encoded peptide RPS4XL regulates RPS6 phosphorylation and inhibits the proliferation of PASMCs caused by hypoxia. Mol. Ther. 2021, 29, 1411–1424. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yan, L.; Wang, S.; Zhang, M.; Ma, C.; Zheng, X.; Chen, H.; Zhu, D. Long noncoding RNA Hoxaas3 contributes to hypoxia-induced pulmonary artery smooth muscle cell proliferation. Cardiovasc. Res. 2019, 115, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Gao, L.F.; Zhu, X.A.; Xiang, D.K. LncRNA HOXA-AS3 Promotes the Progression of Pulmonary Arterial Hypertension through Mediation of miR-675-3p/PDE5A Axis. Biochem. Genet. 2021, 59, 1158–1172. [Google Scholar] [CrossRef]
- Jandl, K.; Thekkekara Puthenparampil, H.; Marsh, L.M.; Hoffmann, J.; Wilhelm, J.; Veith, C.; Sinn, K.; Klepetko, W.; Olschewski, H.; Olschewski, A.; et al. Long non-coding RNAs influence the transcriptome in pulmonary arterial hypertension: The role of PAXIP1-AS1. J. Pathol. 2019, 247, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, R.; Lei, S.; Yang, S.; Wu, S.J. LncRNA PAXIP1-AS1 fosters the pathogenesis of pulmonary arterial hypertension via ETS1/WIPF1/RhoA axis. J. Cell Mol. Med. 2021, 25, 7321–7334. [Google Scholar] [CrossRef]
- Zehendner, C.M.; Valasarajan, C.; Werner, A.; Boeckel, J.N.; Bischoff, F.C.; John, D.; Weirick, T.; Glaser, S.F.; Rossbach, O.; Jae, N.; et al. Long Noncoding RNA TYKRIL Plays a Role in Pulmonary Hypertension via the p53-mediated Regulation of PDGFRbeta. Am. J. Respir. Crit. Care Med. 2020, 202, 1445–1457. [Google Scholar] [CrossRef]
- Pasmant, E.; Laurendeau, I.; Heron, D.; Vidaud, M.; Vidaud, D.; Bieche, I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: Identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007, 67, 3963–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Zhao, H.Y.; Zhang, X.B.; Gao, X.L.; Peng, W.P.; Zhou, Y.; Zhao, W.H.; Yang, H.F. LncRNA ANRIL regulates cell proliferation and migration via sponging miR-339-5p and regulating FRS2 expression in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ge, S.; Gong, W.; Xu, J.; Guo, Z.; Liu, Z.; Gao, X.; Wei, X.; Ge, S. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020, 11, 435. [Google Scholar] [CrossRef]
- Hu, D.J.; Li, Z.Y.; Zhu, Y.T.; Li, C.C. Overexpression of long noncoding RNA ANRIL inhibits phenotypic switching of vascular smooth muscle cells to prevent atherosclerotic plaque development in vivo. Aging 2020, 13, 4299–4316. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Zhang, X. Downregulation of long noncoding RNA ANRIL promotes proliferation and migration in hypoxic human pulmonary artery smooth muscle cells. Mol. Med. Rep. 2020, 21, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, Y.; Yu, F.; Xu, Y.; Yanli, L.; Liu, N. Long non-coding RNA and mRNA profile analysis of metformin to reverse the pulmonary hypertension vascular remodeling induced by monocrotaline. Biomed. Pharmacother. 2019, 115, 108933. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Y.; Hu, R.; Wang, T.; Li, Y.; Liu, N. Metformin inhibits pulmonary artery smooth muscle cell proliferation by upregulating p21 via NONRATT015587.2. Int. J. Mol. Med. 2022, 49, 49. [Google Scholar] [CrossRef]
- Deng, L.; Chen, J.; Chen, B.; Wang, T.; Yang, L.; Liao, J.; Yi, J.; Chen, Y.; Wang, J.; Linneman, J.; et al. LncPTSR Triggers Vascular Remodeling in Pulmonary Hypertension by Regulating [Ca2+]i in Pulmonary Arterial Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Prisco, S.Z.; Thenappan, T.; Prins, K.W. Treatment Targets for Right Ventricular Dysfunction in Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2020, 5, 1244–1260. [Google Scholar] [CrossRef]
- Naeije, R.; Manes, A. The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2014, 23, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Yang, Y.; Wang, L.; Li, L.; Zhang, J.; Gao, X.; Dai, S.; Zhang, Y.; Guo, Q.; Peng, Y.G.; et al. Analyses of long non-coding RNA and mRNA profiles in right ventricle myocardium of acute right heart failure in pulmonary arterial hypertension rats. Biomed. Pharmacother. 2018, 106, 1108–1115. [Google Scholar] [CrossRef]
- Omura, J.; Habbout, K.; Shimauchi, T.; Wu, W.H.; Breuils-Bonnet, S.; Tremblay, E.; Martineau, S.; Nadeau, V.; Gagnon, K.; Mazoyer, F.; et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation 2020, 142, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [Green Version]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Zuo, Y.; Wang, J.; Zhang, M.Q.; Malhotra, A.; Mayeda, A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006, 34, e63. [Google Scholar] [CrossRef] [Green Version]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Chen, L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazar, P.; Papavasileiou, P.; Rajewsky, N. circBase: A database for circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef] [Green Version]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cai, Y.; Xu, J. Circular RNAs: Biogenesis, Mechanism, and Function in Human Cancers. Int. J. Mol. Sci. 2019, 20, 3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef] [Green Version]
- Miao, R.; Wang, Y.; Wan, J.; Leng, D.; Gong, J.; Li, J.; Liang, Y.; Zhai, Z.; Yang, Y. Microarray expression profile of circular RNAs in chronic thromboembolic pulmonary hypertension. Medicine 2017, 96, e7354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, X.; Wu, Y.; Cao, S.; Lou, Y.; Zhang, L.; Hu, F. Hsa_circ_0002062 Promotes the Proliferation of Pulmonary Artery Smooth Muscle Cells by Regulating the Hsa-miR-942-5p/CDK6 Signaling Pathway. Front. Genet. 2021, 12, 673229. [Google Scholar] [CrossRef]
- Miao, R.; Gong, J.; Zhang, C.; Wang, Y.; Guo, X.; Li, J.; Yang, S.; Kuang, T.; Zhong, J.; Feng, H. Hsa_circ_0046159 is involved in the development of chronic thromboembolic pulmonary hypertension. J. Thromb. Thrombolysis 2020, 49, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, H.; Li, M.; Wu, P.; Sun, L.; Liu, Y.; Zhu, K.; Zhang, B.; Sun, G.; Cao, C.; et al. Circular RNA hsa_circ_0016070 Is Associated with Pulmonary Arterial Hypertension by Promoting PASMC Proliferation. Mol. Ther. Nucleic Acids 2019, 18, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xu, Y.; Guo, M.; Sun, Y.; Ding, J.; Li, L.; Zheng, X.; Li, S.; Yuan, D.; Li, S.S. hsa_circNFXL1_009 modulates apoptosis, proliferation, migration, and potassium channel activation in pulmonary hypertension. Mol. Ther. Nucleic Acids 2021, 23, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, L.; Lian, L.; Hao, M.; Chen, S.; Hong, Y. CircATP2B4 promotes hypoxia-induced proliferation and migration of pulmonary arterial smooth muscle cells via the miR-223/ATR axis. Life Sci. 2020, 262, 118420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Yao, H.; Lie, Z.; Chen, G.; Tan, H.; Zhou, Y. Elevated serum circ_0068481 levels as a potential diagnostic and prognostic indicator in idiopathic pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894019888416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Chen, R.; Yao, X.; Zheng, Z.; Wang, C.; Chen, J.; Cheng, J. circGSAP: A New Clinical Biomarker for Idiopathic Pulmonary Hypertension? Am. J. Respir. Crit. Care Med. 2022, 205, 252–253. [Google Scholar] [CrossRef]
- Huang, Y.; Su, D.; Ye, B.; Huang, Y.; Qin, S.; Chen, C.; Zhao, Y.; Pang, Y. Expression and clinical significance of circular RNA hsa_circ_0003416 in pediatric pulmonary arterial hypertension associated with congenital heart disease. J. Clin. Lab. Anal. 2022, 36, e24273. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.C.; Kalionis, B.; Wu, J.Z.; Wang, L.L.; Ge, H.Y.; Chen, C.C.; Tang, X.D.; Song, Y.L.; He, H.; et al. Characteristics of circular RNA expression in lung tissues from mice with hypoxiainduced pulmonary hypertension. Int. J. Mol. Med. 2018, 42, 1353–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; He, B.; Sun, H.; Xiong, M.; Nie, J.; Wang, S.; Pan, Y. Novel insights into the interaction between N6-methyladenosine modification and circular RNA. Mol. Ther. Nucleic Acids 2022, 27, 824–837. [Google Scholar] [CrossRef]
- Su, H.; Wang, G.; Wu, L.; Ma, X.; Ying, K.; Zhang, R. Transcriptome-wide map of m(6)A circRNAs identified in a rat model of hypoxia mediated pulmonary hypertension. BMC Genom. 2020, 21, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, Y.; Qi, J.; Yu, X.; Ren, H.; Zhao, X.; Xin, W.W.; He, S.; Zheng, X.; Ma, C.; et al. Circ-calm4 Serves as an miR-337-3p Sponge to Regulate Myo10 (Myosin 10) and Promote Pulmonary Artery Smooth Muscle Proliferation. Hypertension 2020, 75, 668–679. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, H.; Yu, H.; Zhou, Y.; Zhang, J.; Xin, W.; Li, Y.; He, S.; Ma, C.; Zheng, X.; et al. Circular RNA Calm4 Regulates Hypoxia-Induced Pulmonary Arterial Smooth Muscle Cells Pyroptosis via the Circ-Calm4/miR-124-3p/PDCD6 Axis. Arter. Thromb. Vasc. Biol. 2021, 41, 1675–1693. [Google Scholar] [CrossRef]
- Yang, L.; Liang, H.; Meng, X.; Shen, L.; Guan, Z.; Hei, B.; Yu, H.; Qi, S.; Wen, X. mmu_circ_0000790 Is Involved in Pulmonary Vascular Remodeling in Mice with HPH via MicroRNA-374c-Mediated FOXC1. Mol. Ther. Nucleic Acids 2020, 20, 292–307. [Google Scholar] [CrossRef]
- Jing, X.; Wu, S.; Liu, Y.; Wang, H.; Huang, Q. Circular RNA Sirtuin1 represses pulmonary artery smooth muscle cell proliferation, migration and autophagy to ameliorate pulmonary hypertension via targeting microRNA-145-5p/protein kinase-B3 axis. Bioengineered 2022, 13, 8759–8771. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, M.; Ge, A.; Ge, X.; Gu, R.; Zhang, C.; Fu, Y.; Gao, J.; Wang, X.; Liu, Y.; et al. Granzyme B deficiency promotes osteoblastic differentiation and calcification of vascular smooth muscle cells in hypoxic pulmonary hypertension. Cell Death Dis. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Gu, R.; Wang, X.; He, S.; Bai, J.; Zhang, L.; Zhang, J.; Li, Q.; Qu, L.; Xin, W.; et al. circRNA CDR1as Promotes Pulmonary Artery Smooth Muscle Cell Calcification by Upregulating CAMK2D and CNN3 via Sponging miR-7-5p. Mol. Ther. Nucleic Acids 2020, 22, 530–541. [Google Scholar] [CrossRef]
- Sun, S.; Kong, Q.; Cai, Z.; Wang, M.; Zhao, H.; Zhao, C. circGrm1 promotes pulmonary artery smooth muscle cell proliferation and migration via suppression of GRM1 expression by FUS. Int. J. Mol. Med. 2021, 48, 202. [Google Scholar] [CrossRef]
- Hong, L.; Ma, X.; Liu, J.; Luo, Y.; Lin, J.; Shen, Y.; Zhang, L. Circular RNA-HIPK3 regulates human pulmonary artery endothelial cells function and vessel growth by regulating microRNA-328-3p/STAT3 axis. Pulm. Circ. 2021, 11, 20458940211000234. [Google Scholar] [CrossRef]
- Li, S.S.; Liang, S.; Long, Y.; Chen, X.; Jin, X. hsa_circWDR37_016 Regulates Hypoxia-Induced Proliferation of Pulmonary Arterial Smooth Muscle Cells. Cardiovasc. Ther. 2022, 2022, 7292034. [Google Scholar] [CrossRef]
- Guo, H.M.; Liu, Z.P. Up-regulation of circRNA_0068481 promotes right ventricular hypertrophy in PAH patients via regulating miR-646/miR-570/miR-885. J. Cell Mol. Med. 2021, 25, 3735–3743. [Google Scholar] [CrossRef]
- Kariotis, S.; Jammeh, E.; Swietlik, E.M.; Pickworth, J.A.; Rhodes, C.J.; Otero, P.; Wharton, J.; Iremonger, J.; Dunning, M.J.; Pandya, D.; et al. Biological heterogeneity in idiopathic pulmonary arterial hypertension identified through unsupervised transcriptomic profiling of whole blood. Nat. Commun. 2021, 12, 7104. [Google Scholar] [CrossRef]
- Fatemi, R.P.; Velmeshev, D.; Faghihi, M.A. De-repressing LncRNA-Targeted Genes to Upregulate Gene Expression: Focus on Small Molecule Therapeutics. Mol. Ther. Nucleic Acids 2014, 3, e196. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.Y. From bulk, single-cell to spatial RNA sequencing. Int. J. Oral. Sci. 2021, 13, 36. [Google Scholar] [CrossRef]
- Saygin, D.; Tabib, T.; Bittar, H.E.T.; Valenzi, E.; Sembrat, J.; Chan, S.Y.; Rojas, M.; Lafyatis, R. Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodor, J.; Chen, S.H.; Scanlon, J.P.; Monteiro, J.P.; Caudrillier, A.; Sweta, S.; Stewart, K.R.; Shmakova, A.; Dobie, R.; Henderson, B.E.P.; et al. Single-cell RNA-seq profiling of mouse endothelial cells in response to pulmonary arterial hypertension. Cardiovasc. Res. 2021, cvab296. [Google Scholar] [CrossRef]
- Chelladurai, P.; Savai, R.; Pullamsetti, S.S. Zooming into Cellular and Molecular Heterogeneity of Pulmonary Hypertension. What More Single-Cell Omics Can Offer. Am. J. Respir. Crit. Care Med. 2021, 203, 941–943. [Google Scholar] [CrossRef]
- Lee, J.H.; Daugharthy, E.R.; Scheiman, J.; Kalhor, R.; Ferrante, T.C.; Terry, R.; Turczyk, B.M.; Yang, J.L.; Lee, H.S.; Aach, J.; et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc. 2015, 10, 442–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Sun, Y.C.; Church, G.M.; Lee, J.H.; Zador, A.M. Efficient in situ barcode sequencing using padlock probe-based BaristaSeq. Nucleic Acids Res. 2018, 46, e22. [Google Scholar] [CrossRef] [Green Version]
- Stahl, P.L.; Salmen, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangelmaier, E.; Lal, A. Adaptor proteins in long noncoding RNA biology. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194370. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Han, S.; Peng, C.; Li, Y. Long Noncoding RNA and Protein Interactions: From Experimental Results to Computational Models Based on Network Methods. Int. J. Mol. Sci. 2019, 20, 1284. [Google Scholar] [CrossRef] [Green Version]
- Bai, Q.; Bai, Z.; Sun, L. Detection of RNA-binding Proteins by In Vitro RNA Pull-down in Adipocyte Culture. J. Vis. Exp. 2016, 113, e54207. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Bierhoff, H. Analysis of lncRNA-Protein Interactions by RNA-Protein Pull-Down Assays and RNA Immunoprecipitation (RIP). Methods Mol. Biol. 2018, 1686, 241–250. [Google Scholar] [CrossRef]
- Ramanathan, M.; Porter, D.F.; Khavari, P.A. Methods to study RNA-protein interactions. Nat. Methods 2019, 16, 225–234. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, A.; Verma, I.M. Gene therapy: Promises and problems. Annu. Rev. Genom. Hum. Genet. 2001, 2, 177–211. [Google Scholar] [CrossRef]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes. Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- Sprangers, A.J.; Hao, L.; Banga, R.J.; Mirkin, C.A. Liposomal Spherical Nucleic Acids for Regulating Long Noncoding RNAs in the Nucleus. Small 2017, 13, 1602753. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Wang, X.; Tang, J.; Liu, Y.; Mei, L.; Li, M.; Yang, M.; Tang, L.; Gao, H.; et al. Efficient siRNA transfer to knockdown a placenta specific lncRNA using RGD-modified nano-liposome: A new preeclampsia-like mouse model. Int. J. Pharm. 2018, 546, 115–124. [Google Scholar] [CrossRef]
- Muraki, Y.; Yamasaki, M.; Takeuchi, H.; Tohyama, K.; Sano, N.; Matsuo, T. Fluorescent Imaging Analysis for Distribution of Fluorescent Dye Labeled- or Encapsulated-Liposome in Monocrotaline-Induced Pulmonary Hypertension Model Rat. Chem. Pharm. Bull. 2018, 66, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Coelho, T.; Adams, D.; Conceicao, I.; Waddington-Cruz, M.; Schmidt, H.H.; Buades, J.; Campistol, J.; Berk, J.L.; Polydefkis, M.; Wang, J.J.; et al. A phase II, open-label, extension study of long-term patisiran treatment in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. Orphanet. J. Rare Dis. 2020, 15, 179. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Connerty, P.; Moles, E.; de Bock, C.E.; Jayatilleke, N.; Smith, J.L.; Meshinchi, S.; Mayoh, C.; Kavallaris, M.; Lock, R.B. Development of siRNA-Loaded Lipid Nanoparticles Targeting Long Non-Coding RNA LINC01257 as a Novel and Safe Therapeutic Approach for t(8;21) Pediatric Acute Myeloid Leukemia. Pharmaceutics 2021, 13, 1681. [Google Scholar] [CrossRef]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjug. Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef]
- Corboz, M.R.; Li, Z.; Malinin, V.; Plaunt, A.J.; Konicek, D.M.; Leifer, F.G.; Chen, K.J.; Laurent, C.E.; Yin, H.; Biernat, M.C.; et al. Preclinical Pharmacology and Pharmacokinetics of Inhaled Hexadecyl-Treprostinil (C16TR), a Pulmonary Vasodilator Prodrug. J. Pharmacol. Exp. Ther. 2017, 363, 348–357. [Google Scholar] [CrossRef] [Green Version]
- McLendon, J.M.; Joshi, S.R.; Sparks, J.; Matar, M.; Fewell, J.G.; Abe, K.; Oka, M.; McMurtry, I.F.; Gerthoffer, W.T. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control. Release 2015, 210, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, R.; Yokota, T. Knocking Down Long Noncoding RNAs Using Antisense Oligonucleotide Gapmers. Methods Mol. Biol. 2020, 2176, 49–56. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR-Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef]
| CircRNA | Interacting miRNA | Target mRNA | Expression in PH | Functions in PH |
|---|---|---|---|---|
| Has-circ_0002062 | miR-942-5p [129] | CDK6 | Increase | Promote cell proliferation and migration |
| Circ_0068481 | miR-646/570/885 [149] | EYA3 | Increase | Biomarker of disease severity of PH |
| Circ-calm4 | miR-337-5p [140] miR-124-3p [141] | Myo10 PDCD6 | Increase | Promote cell proliferation, migration, cell cycle, and pyroptosis |
| Circ-CDR1 | miR-7-5p [145] | CNN3-CAMK2D | Increase | Promote PASMC from contractile to osteogenic phenotype |
| CircSIRT1 | miR-145-5p [143] | AKT3 | Increase | Promote cell proliferation, migration and autophagy |
| Has_circ_0016070 | miR-942 [131] | CCND1 | Increase | Promote cell proliferation and cell cycle arrest |
| Has_circNFXL1_009 | miR-29b-5p [132] | KCNB1 | Decrease | Inhibition cell proliferation and migration; induce apoptosis |
| CircATP2B4 | miR-223 [133] | ATR | Increase | Promote cell proliferation and migration |
| CircWDR37 | miR-138-5p [148] | Increase | Promote cell proliferation, migration, cell cycle; apoptosis resistance | |
| Mmu_circ_0000790 | miR-373c [142] | FOXC1 | Increase | Promote cell proliferation, apoptosis resistance |
| CircHIPK3 | miR-328-3p [147] | STAT3 | Increase | Inhibit proliferation, migration, angiogenesis |
| Has_circ_0026480 | miR-27a-3p [130] | ATXN1 | Decrease | |
| Has_circ_0046159 | miR-1226-3p [130] | ATA2A2 | Increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Han, X.; Wang, Z.; Nie, X.; Bian, J. The Landscape of Noncoding RNA in Pulmonary Hypertension. Biomolecules 2022, 12, 796. https://doi.org/10.3390/biom12060796
Deng L, Han X, Wang Z, Nie X, Bian J. The Landscape of Noncoding RNA in Pulmonary Hypertension. Biomolecules. 2022; 12(6):796. https://doi.org/10.3390/biom12060796
Chicago/Turabian StyleDeng, Lin, Xiaofeng Han, Ziping Wang, Xiaowei Nie, and Jinsong Bian. 2022. "The Landscape of Noncoding RNA in Pulmonary Hypertension" Biomolecules 12, no. 6: 796. https://doi.org/10.3390/biom12060796
APA StyleDeng, L., Han, X., Wang, Z., Nie, X., & Bian, J. (2022). The Landscape of Noncoding RNA in Pulmonary Hypertension. Biomolecules, 12(6), 796. https://doi.org/10.3390/biom12060796








