Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis
Abstract
1. Introduction
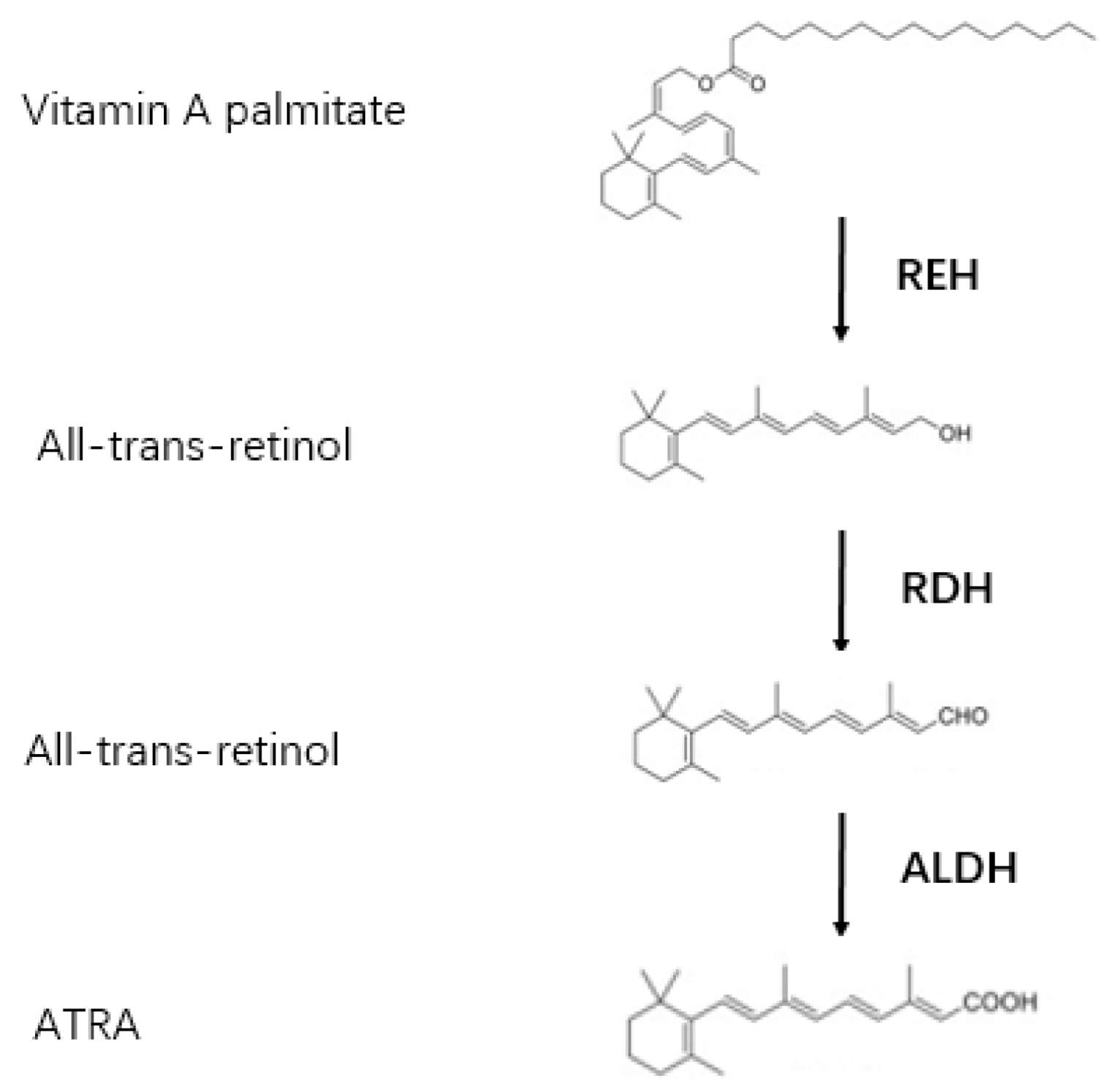
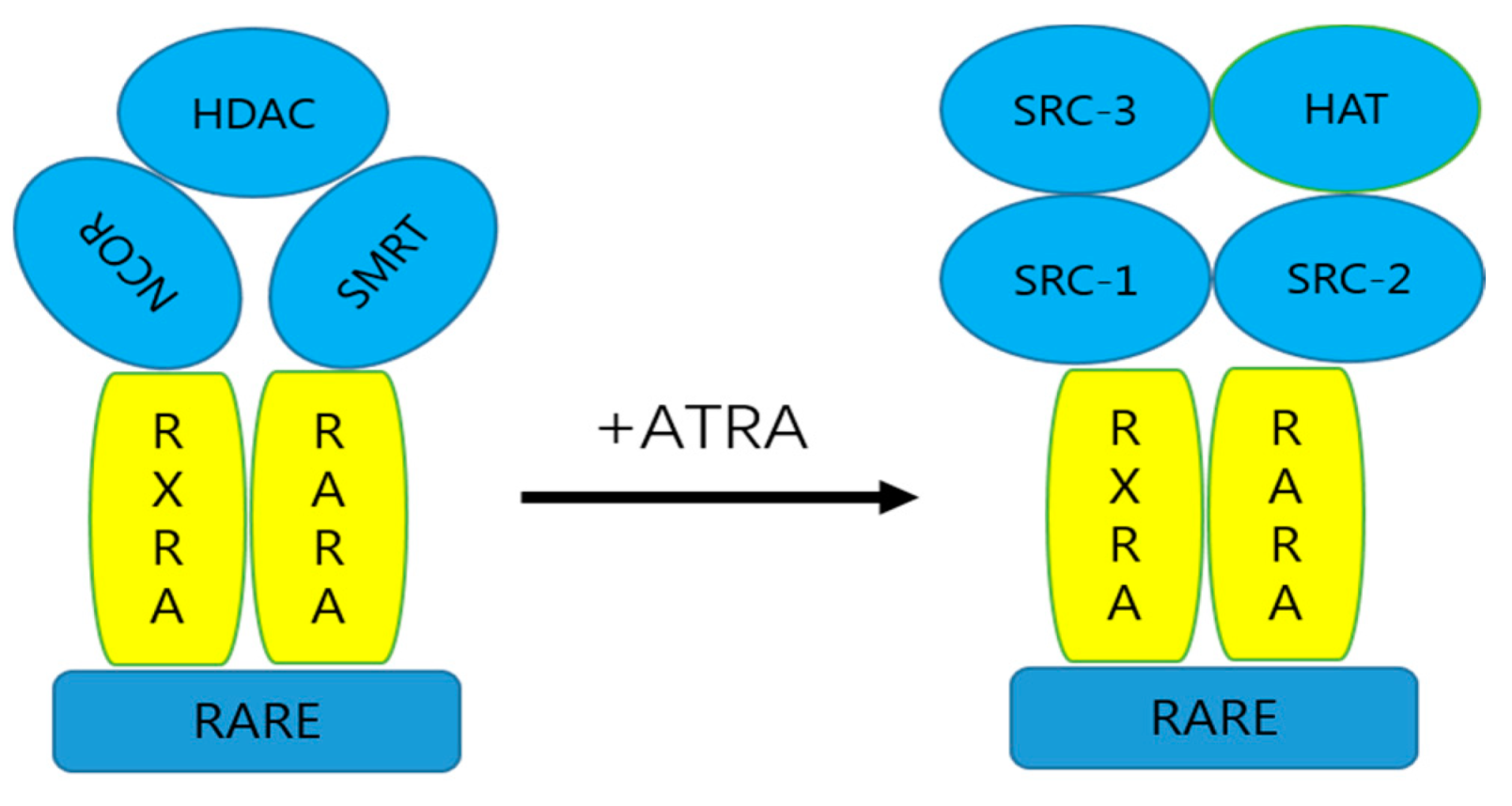
2. Metabolism of ATRA
3. ATRA and Adipocytes
4. Effect of All-Trans Retinoic Acid on Atherosclerosis
4.1. ATRA and Macrophages
4.2. ATRA and SMC
4.2.1. ATRA and SMC Proliferation and Migration
4.2.2. ATRA and SMC Differentiation
4.3. ATRA and Neutrophils
4.4. ATRA and T Cell
4.5. ATRA and Endotheliocyte
4.6. ATRA and Other Pathologic Mechanisms Associated with Atherosclerosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Cannon, B. Cardiovascular disease: Biochemistry to behaviour. Nature 2013, 493, S2–S3. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Bécherel, P.-A.; Mossalayi, M.; Goff, L.; Francès, C.; Chosidow, O.; Debré, P.; Arock, M. Mechanism of anti-inflammatory action of retinoids on keratinocytes. Lancet 1994, 344, 1570–1571. [Google Scholar] [CrossRef]
- Soprano, D.R.; Qin, P.; Soprano, K.J. Retinoic Acid Receptors and Cancers. Annu. Rev. Nutr. 2004, 24, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Avvisati, G.; Tallman, M.S. All-trans retinoic acid in acute promyelocytic leukaemia. Best Pract. Res. Clin. Haematol. 2003, 16, 419–432. [Google Scholar] [CrossRef]
- Zhou, B.; Pan, Y.; Hu, Z.; Wang, X.; Han, J.; Zhou, Q.; Zhai, Z.; Wang, Y. All-trans-Retinoic Acid Ameliorated High Fat Diet-Induced Atherosclerosis in Rabbits by Inhibiting Platelet Activation and Inflammation. J. Biomed. Biotechnol. 2012, 2012, 259693. [Google Scholar] [CrossRef]
- Zarei, L.; Bahrami, M.; Farhad, N.; Froushani, S.M.A.; Abbasi, A. All-trans retinoic acid effectively reduces atheroma plaque size in a rabbit model of high-fat-induced atherosclerosis. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, P.J.; Barry, W.L.; McPherson, J.A.; McNamara, C.A.; Gimple, L.W.; Sanders, J.M.; Sarembock, I.J. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: A favorable effect on vessel remodeling. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, E.; Sato, S.; Honda, S.; Nakazawa, A. All trans retinoic acid alleviates coronary stenosis by regulating smooth muscle cell function in a mouse model of Kawasaki disease. Sci. Rep. 2021, 11, 13856. [Google Scholar] [CrossRef]
- Ross, S.A.; McCaffery, P.; Dräger, U.C.; De Luca, L.M. Retinoids in Embryonal Development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef]
- Petkovich, P.M. Retinoic acid metabolism. J. Am. Acad. Dermatol. 2001, 45, S136–S142. [Google Scholar] [CrossRef]
- Cunningham, T.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef]
- Fujii, H.; Sato, T.; Kaneko, S.; Gotoh, O.; Fujii-Kuriyama, Y.; Osawa, K.; Kato, S.; Hamada, H. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. EMBO J. 1997, 16, 4163–4173. [Google Scholar] [CrossRef]
- Gudas, L.J. Retinoids and vertebrate development. J. Biol. Chem. 1994, 269, 15399–15402. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Heyman, R.A.; Mangelsdorf, D.; Dyck, J.A.; Stein, R.B.; Eichele, G.; Evans, R.; Thaller, C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68, 397–406. [Google Scholar] [CrossRef]
- Connolly, R.M.; Nguyen, N.K.; Sukumar, S. Molecular Pathways: Current Role and Future Directions of the Retinoic Acid Pathway in Cancer Prevention and Treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Ziouzenkova, O.; Plutzky, J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2007, 582, 32–38. [Google Scholar] [CrossRef]
- Napoli, J.L. [13] Quantification of physiological levels of retinoic acid. Methods Enzymol. 1986, 123, 112–124. [Google Scholar] [CrossRef]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Rochel, N.; Moras, D. Architecture of DNA Bound RAR Heterodimers. Biochem. Retin. Acid Recept. I Struct. Act. Funct. Mol. Level 2014, 70, 21–36. [Google Scholar] [CrossRef]
- Bastien, J.; Rochette-Egly, C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 2004, 328, 1–16. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef]
- Landrier, J.-F.; Marcotorchino, J.; Tourniaire, F. Lipophilic Micronutrients and Adipose Tissue Biology. Nutrients 2012, 4, 1622–1649. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Okuno, M.; Tannous, L.; Piantedosi, R.; Allan, M.; Goodman, D.S.; Blaner, W.S. Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 1992, 267, 1805–1810. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. CMLS 2010, 67, 1423–1445. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Hanada, H.; Hirota, K.; Nonaka, M.; Ikeda, C. All-trans retinoic acid displays multiple effects on the growth, lipogenesis and adipokine gene expression of AML-I preadipocyte cell line. Cell Biol. Int. 2012, 37, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zovich, D.; Orologa, A.; Okuno, M.; Kong, L.; Talmage, D.; Piantedosi, R.; Goodman, D.; Blaner, W. Differentiation-dependent expression of retinoid-binding proteins in BFC-1 beta adipocytes. J. Biol. Chem. 1992, 267, 13884–13889. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Felipe, F.; Palou, A. Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. CMLS 2003, 60, 1311–1321. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Palou, A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 177–189. [Google Scholar] [CrossRef]
- Brandebourg, T.D.; Hu, C.Y. Regulation of differentiating pig preadipocytes by retinoic acid. J. Anim. Sci. 2005, 83, 98–107. [Google Scholar] [CrossRef]
- Mercader, J.; Madsen, L.; Felipe, F.; Palou, A.; Kristiansen, K.; Bonet, M.L. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2007, 20, 1061–1072. [Google Scholar] [CrossRef]
- Tourniaire, F.; Musinovic, H.; Gouranton, E.; Astier, J.; Marcotorchino, J.; Arreguin, A.; Bernot, D.; Palou, A.; Bonet, M.L.; Ribot, J.; et al. All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid Res. 2015, 56, 1100–1109. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Sun, X.; Jia, H.; Zhao, C.; Xu, C. All-trans retinoic acid controls differentiation, proliferation, and lipolysis in isolated subcutaneous adipocytes from peripartal Holstein cows. J. Dairy Sci. 2021, 104, 4999–5008. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Between brown and white: Novel aspects of adipocyte differentiation. Ann. Med. 2011, 43, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, M.; Chmielowska, M.; Martyńska, L.; Domańska, A.; Bik, W.; Litwiniuk, A. All-trans-retinoic acid ameliorates atherosclerosis, promotes perivascular adipose tissue browning, and increases adiponectin production in Apo-E mice. Sci. Rep. 2021, 11, 4451. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- Villarroya, F.; Iglesias, R.; Giralt, M. Retinoids and Retinoid Receptors in the Control of Energy Balance: Novel Pharmacological Strategies in Obesity and Diabetes. Curr. Med. Chem. 2004, 11, 795–805. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.; Thiele, C.; Tall, A.R. ATP-binding Cassette Transporter A1 (ABCA1) Functions as a Cholesterol Efflux Regulatory Protein. J. Biol. Chem. 2001, 276, 23742–23747. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Investig. 2007, 117, 3900–3908. [Google Scholar] [CrossRef]
- Ayaori, M.; Yakushiji, E.; Ogura, M.; Nakaya, K.; Hisada, T.; Uto-Kondo, H.; Takiguchi, S.; Terao, Y.; Sasaki, M.; Komatsu, T.; et al. Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 561–572. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Yu, M.; Amengual, J.; Menon, A.; Kamaly, N.; Zhou, F.; Xu, X.; Farokhzad, O.C. Targeted Nanotherapeutics Encapsulating Liver X Receptor Agonist GW3965 Enhance Antiatherogenic Effects without Adverse Effects on Hepatic Lipid Metabolism in Ldlr(−/−) Mice. Adv. Healthc. Mater. 2017, 6, 1700313. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Barrett, T. Monocytes and macrophages in atherogenesis. Curr. Opin. Lipidol. 2019, 30, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.-J.; Xiang, S.-F.; Chen, Y.-Q.; Zhang, N.; Cao, J.; Zhu, H.; Yang, B.; Zhou, Q.; Ying, M.-D.; He, Q.-J. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol. Sin. 2019, 40, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Vellozo, N.S.; Pereira-Marques, S.T.; Cabral-Piccin, M.P.; Filardy, A.A.; Ribeiro-Gomes, F.L.; Rigoni, T.S.; DosReis, G.A.; Lopes, M.F. All-Trans Retinoic Acid Promotes an M1- to M2-Phenotype Shift and Inhibits Macrophage-Mediated Immunity to Leishmania major. Front. Immunol. 2017, 8, 1560. [Google Scholar] [CrossRef]
- Alatshan, A.; Kovács, G.E.; Aladdin, A.; Czimmerer, Z.; Tar, K.; Benkő, S. All-Trans Retinoic Acid Enhances Both the Signaling for Priming and the Glycolysis for Activation of NLRP3 Inflammasome in Human Macrophage. Cells 2020, 9, 1591. [Google Scholar] [CrossRef]
- Dubland, J.A.; Francis, G.A. So Much Cholesterol: The unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr. Opin. Lipidol. 2016, 27, 155–161. [Google Scholar] [CrossRef]
- Peclo, M.M.; Printseva, O.Y. Retinoic acid enhances the proliferation of smooth muscle cells. Experientia 1987, 43, 196–198. [Google Scholar] [CrossRef]
- Hagiwara, H.; Inoue, A.; Nakajo, S.; Nakaya, K.; Kojima, S.; Hirose, S. Inhibition of Proliferation of Chondrocytes by Specific Receptors in Response to Retinoids. Biochem. Biophys. Res. Commun. 1996, 222, 220–224. [Google Scholar] [CrossRef]
- Miano, J.M.; Topouzis, S.; Majesky, M.W.; Olson, E.N. Retinoid Receptor Expression and all-trans Retinoic Acid–Mediated Growth Inhibition in Vascular Smooth Muscle Cells. Circulation 1996, 93, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Suzuki, T.; Tajima, S. Modulations of Elastin Expression and Cell Proliferation by Retinoids in Cultured Vascular Smooth Muscle Cells. J. Biochem. 1995, 117, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pakala, R.; Benedict, C.R. RAR gamma agonists inhibit proliferation of vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2000, 35, 302–308. [Google Scholar] [CrossRef]
- Takeda, K.; Ichiki, T.; Funakoshi, Y.; Ito, K.; Takeshita, A. Downregulation of Angiotensin II Type 1 Receptor by All- trans Retinoic Acid in Vascular Smooth Muscle Cells. Hypertension 2000, 35, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gardner, D.G. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J. Clin. Investig. 1998, 102, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, C.; Sasaguri, T.; Komiyama, Y.; Takahashi, H. All-trans Retinoic Acid Inhibits Vascular Smooth Muscle Cell Proliferation Targeting Multiple Genes for Cyclins and Cyclin-Dependent Kinases. Hypertens. Res. 2001, 24, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Manabe, I.; Fukushima, Y.; Tobe, K.; Aizawa, K.; Miyamoto, S.; Kawai-Kowase, K.; Moriyama, N.; Imai, Y.; Kawakami, H.; et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002, 8, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Sakamaki, T.; Kanda, T.; Hoshino, Y.I.; Sawada, Y.; Sato, M.; Kurabayashi, M. Smooth muscle cell outgrowth from coronary atherectomy specimens in vitro is associated with less time to restenosis and expression of a key Transcription factor KLF5/BTEB2. Cardiology 2003, 100, 80–85. [Google Scholar] [CrossRef]
- Tran-Lundmark, K.; Tannenberg, P.; Rauch, B.H.; Ekstrand, J.; Tran, P.-K.; Hedin, U.; Kinsella, M.G. Perlecan Heparan Sulfate Is Required for the Inhibition of Smooth Muscle Cell Proliferation by All-trans-Retinoic Acid. J. Cell. Physiol. 2014, 230, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Streb, J.W.; Long, X.; Lee, T.-H.; Sun, Q.; Kitchen, C.M.; Georger, M.A.; Slivano, O.J.; Blaner, W.S.; Carr, D.W.; Gelman, I.H.; et al. Retinoid-Induced Expression and Activity of an Immediate Early Tumor Suppressor Gene in Vascular Smooth Muscle Cells. PLoS ONE 2011, 6, e18538. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yin, X.; Gao, Z.; Zhang, H.; Liu, X.; Pan, X.; Li, N.; Yu, Z. Retinoic acid remodels extracellular matrix (ECM) of cultured human fetal palate mesenchymal cells (hFPMCs) through down-regulation of TGF-β/Smad signaling. Toxicol. Lett. 2014, 225, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Neuville, P.; Yan, Z.; Gidlöf, A.; Pepper, M.S.; Hansson, G.K.; Gabbiani, G.; Sirsjo, A. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARalpha-dependent signaling pathway. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1430–1436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gidlöf, A.C.; Ocaya, P.; Krivospitskaya, O.; Sirsjö, A. Vitamin A: A drug for prevention of restenosis/reocclusion after percutaneous coronary intervention? Clin. Sci. 2008, 114, 19–25. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, B.; Jiang, X.; Cai, M.; Liu, N.; Zhang, S.; Tan, Y.; Huang, G.; Jin, W.; Liu, B.; et al. All-Trans-Retinoic Acid Suppresses Neointimal Hyperplasia and Inhibits Vascular Smooth Muscle Cell Proliferation and Migration via Activation of AMPK Signaling Pathway. Front. Pharmacol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Fei, X.; Song, Z.; Xie, F.; Yang, F.; Liu, X.; Xu, Z.; Wang, G. All-Trans Retinoic Acid Prevented Vein Grafts Stenosis by Inhibiting Rb-E2F Mediated Cell Cycle Progression and KLF5-RARα Interaction in Human Vein Smooth Muscle Cells. Cardiovasc. Drugs Ther. 2020, 35, 103–111. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; deBlois, D.; O’Brien, E.R. The intima. Soil for atherosclerosis and restenosis. Circ. Res. 1995, 77, 445–465. [Google Scholar] [CrossRef]
- Axel, D.I.; Frigge, A.; Dittmann, J.; Runge, H.; Spyridopoulos, I.; Riessen, R.; Viebahn, R.; Karsch, K.R. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Herdeg, C.; Oberhoff, M.; Baumbach, A.; Schroeder, S.; Leitritz, M.; Blattner, A.; Siegel-Axel, D.I.; Meisner, C.; Karsch, K.R. Effects of local all-trans-retinoic acid delivery on experimental atherosclerosis in the rabbit carotid artery. Cardiovasc. Res. 2003, 57, 544–553. [Google Scholar] [CrossRef][Green Version]
- Johst, U.; Betsch, A.; Wiskirchen, J.; Schöber, W.; Vonthein, R.; Rinkert, N.; Kehlbach, R.; Claussen, C.D.; Duda, S.H. All-Trans and 9-cis Retinoid Acids Inhibit Proliferation, Migration, and Synthesis of Extracellular Matrix of Human Vascular Smooth Muscle Cells by Inducing Differentiation In Vitro. J. Cardiovasc. Pharmacol. 2003, 41, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Lindschau, C.; Quass, P.; Distler, A.; Luft, F.C. Differentiation of Vascular Smooth Muscle Cells and the Regulation of Protein Kinase C-α. Circ. Res. 1995, 76, 21–29. [Google Scholar] [CrossRef]
- Gollasch, M.; Haase, H.; Ried, C.; Lindschau, C.; Morano, I.; Luft, F.C.; Haller, H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 593–601. [Google Scholar] [CrossRef]
- Colbert, M.C.; Kirby, M.L.; Robbins, J. Endogenous Retinoic Acid Signaling Colocalizes With Advanced Expression of the Adult Smooth Muscle Myosin Heavy Chain Isoform during Development of the Ductus Arteriosus. Circ. Res. 1996, 78, 790–798. [Google Scholar] [CrossRef]
- Neuville, P.; Bochaton-Piallat, M.-L.; Gabbiani, G. Retinoids and Arterial Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2000, 20, 1882–1888. [Google Scholar] [CrossRef]
- Blank, R.S.; Swartz, E.A.; Thompson, M.M.; Olson, E.N.; Owens, G.K. A Retinoic Acid–Induced Clonal Cell Line Derived From Multipotential P19 Embryonal Carcinoma Cells Expresses Smooth Muscle Characteristics. Circ. Res. 1995, 76, 742–749. [Google Scholar] [CrossRef]
- Wright, G.L.; Wang, S.; Bailey, G.; Reichenbecher, V. Effect of retinoic acid on contractile competence of vascular smooth muscle. Am. J. Physiol. Circ. Physiol. 1996, 270, H1363–H1370. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Chen, J.; Nallamshetty, S.; Pham, T.; Goto, S.; Muehlschlegel, J.D.; Libby, P.; Aikawa, M.; Aikawa, E.; Plutzky, J. Retinoids Repress Human Cardiovascular Cell Calcification with Evidence for Distinct Selective Retinoid Modulator Effects. Arter. Thromb. Vasc. Biol. 2020, 40, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Nombela, F.; Vivancos, J.; Hamilton, J.A.; Corbí, Á.L.; Lizasoain, I.; Moro, M.A. N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the PPARγ agonist rosiglitazone. Stroke A J. Cereb. Circ. 2013, 44, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Giugliano, G.; Brevetti, G.; Lanero, S.; Schiano, V.; Laurenzano, E.; Chiariello, M. Leukocyte count in peripheral arterial disease: A simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis 2010, 210, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D.; Klatsky, A.L.; Siegelaub, A.B. The Leukocyte Count as a Predictor of Myocardial Infarction. N. Engl. J. Med. 1974, 290, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Megens, R.T.A.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.M.J.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, J.; Hu, M.; Chen, X.; Lu, Z.; Bellanti, J.A.; Zheng, S.G. All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J. Neuroinflammation 2019, 16, 175. [Google Scholar] [CrossRef]
- Mallat, Z.; Taleb, S.; Ait-Oufella, H.; Tedgui, A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 2009, 50, S364–S369. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Immune-inflammatory responses in atherosclerosis: Role of an adaptive immunity mainly driven by T and B cells. Immunobiology 2016, 221, 1014–1033. [Google Scholar] [CrossRef]
- Cardilo-Reis, L.; Gruber, S.; Schreier, S.M.; Drechsler, M.; Papac-Milicevic, N.; Weber, C.; Wagner, O.; Stangl, H.; Soehnlein, O.; Binder, C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012, 4, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- King, V.L.; Szilvassy, S.J.; Daugherty, A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor-/-mice. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 456–461. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Iwata, M.; Eshima, Y.; Kagechika, H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003, 15, 1017–1025. [Google Scholar] [CrossRef]
- Brown, C.C.; Esterhazy, D.; Sarde, A.; London, M.; Pullabhatla, V.; Osma-Garcia, I.; Al-Bader, R.; Ortiz, C.; Elgueta, R.; Arno, M.; et al. Retinoic Acid Is Essential for Th1 Cell Lineage Stability and Prevents Transition to a Th17 Cell Program. Immunity 2015, 42, 499–511. [Google Scholar] [CrossRef]
- Taleb, S.; Tedgui, A.; Mallat, Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 258–264. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.F.L.; Ranjbaran, H.; Gallo, A.; Tellides, G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e6. [Google Scholar] [CrossRef]
- Pastrana, J.L.; Sha, X.; Virtue, A.; Mai, J.; Cueto, R.; Lee, I.A.; Yang, X.F. Regulatory T cells and Atherosclerosis. J. Clin. Exp. Cardiol. 2012, 2012, 2. [Google Scholar] [CrossRef]
- Sun, C.-M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- von Boehmer, H. Oral tolerance: Is it all retinoic acid? J. Exp. Med. 2007, 204, 1737–1739. [Google Scholar] [CrossRef]
- Coombes, J.L.; Maloy, K.J. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin. Immunol. 2007, 19, 116–126. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Pino-Lagos, K.; Guo, Y.; Brown, C.; Alexander, M.P.; Elgueta, R.; Bennett, K.A.; De Vries, V.; Nowak, E.; Blomhoff, R.; Sockanathan, S.; et al. A retinoic acid–dependent checkpoint in the development of CD4+ T cell–mediated immunity. J. Exp. Med. 2011, 208, 1767–1775. [Google Scholar] [CrossRef]
- Shevach, E.M.; Rosenthal, A.S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J. Exp. Med. 1973, 138, 1213–1229. [Google Scholar] [CrossRef]
- Mills, C.D.; Ley, K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef]
- Lin, J.-D.; Nishi, H.; Poles, J.; Niu, X.; McCauley, C.; Rahman, K.; Brown, E.J.; Yeung, S.T.; Vozhilla, N.; Weinstock, A.; et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 2019, 4, e124574. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Sugino, I.; Kuboki, K.; Matsumoto, T.; Murakami, E.; Nishimura, C.; Yoshino, G. Influence of fatty liver on plasma small, dense LDL- cholesterol in subjects with and without metabolic syndrome. J. Atheroscler. Thromb. 2011, 18, 1–7. [Google Scholar] [CrossRef]
- Bilbija, D.; Elmabsout, A.A.; Sagave, J.; Haugen, F.; Bastani, N.; Dahl, C.P.; Gullestad, L.; Sirsjö, A.; Blomhoff, R.; Valen, G. Expression of retinoic acid target genes in coronary artery disease. Int. J. Mol. Med. 2014, 33, 677–686. [Google Scholar] [CrossRef]
- Grace, V.B.; Siddikuzzaman; Rimashree, B. Liposome encapsulated all trans retinoic acid (ATRA) has enhanced immunomodulatory and inflammation reducing activities in mice model. Anti-Cancer Agents Med. Chem. 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Reriani, M.; Raichlin, E.; Prasad, A.; Mathew, V.; Pumper, G.M.; Nelson, R.E.; Lennon, R.; Rihal, C.; Lerman, L.O.; Lerman, A. Long-Term Administration of Endothelin Receptor Antagonist Improves Coronary Endothelial Function in Patients With Early Atherosclerosis. Circulation 2010, 122, 958–966. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Achan, V.; Tran, C.T.; Arrigoni, F.; Whitley, G.S.; Leiper, J.M.; Vallance, P. all-trans-Retinoic acid increases nitric oxide synthesis by endothelial cells: A role for the induction of dimethylarginine dimethylaminohydrolase. Circ. Res. 2002, 90, 764–769. [Google Scholar] [CrossRef]
- Rhee, E.-J.; Nallamshetty, S.; Plutzky, J. Retinoid metabolism and its effects on the vasculature. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 230–240. [Google Scholar] [CrossRef]
- Yokota, J.; Kawana, M.; Hidai, C.; Aoka, Y.; Ichikawa, K.-I.; Iguchi, N.; Okada, M.; Kasanuki, H. Retinoic acid suppresses endothelin-1 gene expression at the transcription level in endothelial cells. Atherosclerosis 2001, 159, 491–496. [Google Scholar] [CrossRef]
- Tao, L.; Nie, Y.; Wang, G.; Ding, Y.; Ding, J.; Xiong, F.; Tang, S.; Wang, Y.; Zhou, B.; Zhu, H. All-trans retinoic acid reduces endothelin-1 expression and increases endothelial nitric oxide synthase phosphorylation in rabbits with atherosclerosis. Mol. Med. Rep. 2017, 17, 2619–2625. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhou, Q.; Wang, Y.; Zhou, J.; Jiang, Q.; Wang, Y.; Zhu, H. ATRA improves endothelial dysfunction in atherosclerotic rabbits by decreasing CAV-1 expression and enhancing eNOS activity. Mol. Med. Rep. 2018, 17, 6796–6802. [Google Scholar] [CrossRef]
- Amisten, S.; Al-Amily, I.M.; Soni, A.; Hawkes, R.; Atanes, P.; Persaud, S.J.; Rorsman, P.; Salehi, A. Anti-diabetic action of all-trans retinoic acid and the orphan G protein coupled receptor GPRC5C in pancreatic β-cells. Endocr. J. 2017, 64, 325–338. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Cabrera-Valladares, G.; German, M.S.; Matschinsky, F.M.; Wang, J.; Fernandez-Mejia, C. Effect of Retinoic Acid on Glucokinase Activity and Gene Expression and on Insulin Secretion in Primary Cultures of Pancreatic Islets. Endocrinology 1999, 140, 3091–3096. [Google Scholar] [CrossRef]
- Said, E.; Mousa, S.; Fawzi, M.; Sabry, N.; Farid, S. Combined effect of high-dose vitamin A, vitamin E supplementation, and zinc on adult patients with diabetes: A randomized trial. J. Adv. Res. 2020, 28, 27–33. [Google Scholar] [CrossRef]
- Bilbija, D.; Haugen, F.; Sagave, J.; Baysa, A.; Bastani, N.; Levy, F.O.; Sirsjö, A.; Blomhoff, R.; Valen, G. Retinoic Acid Signalling Is Activated in the Postischemic Heart and May Influence Remodelling. PLoS ONE 2012, 7, e44740. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Azevedo, P.S.; Minicucci, M.F.; Rafacho, B.P.M.; Duarte, D.R.; Matsubara, L.S.; Matsubara, B.B.; Paiva, S.; Zornoff, L.A.M. Retinoic acid prevents ventricular remodelling induced by tobacco smoke exposure in rats. Acta Cardiol. 2011, 66, 3–7. [Google Scholar] [CrossRef]
- Subramanian, U.; Nagarajan, D. All-Trans Retinoic Acid supplementation prevents cardiac fibrosis and cytokines induced by Methylglyoxal. Glycoconj. J. 2017, 34, 255–265. [Google Scholar] [CrossRef]
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early inhibition of endothelial retinoid uptake upon myocardial infarction restores cardiac function and prevents cell, tissue, and animal death. J. Mol. Cell. Cardiol. 2018, 126, 105–117. [Google Scholar] [CrossRef]
- Koshiuka, K.; Elstner, E.; Williamson, E.; Said, J.W.; Tada, Y.; Koeffler, H.P. Novel therapeutic approach: Organic arsenical (melarsoprol) alone or with all-trans-retinoic acid markedly inhibit growth of human breast and prostate cancer cells in vitro and in vivo. Br. J. Cancer 2000, 82, 452–458. [Google Scholar] [CrossRef]
- Lin, L.-M.; Li, B.-X.; Xiao, J.-B.; Lin, D.-H.; Yang, B.-F. Synergistic effect of all-trans-retinoic acid and arsenic trioxide on growth inhibition and apoptosis in human hepatoma, breast cancer, and lung cancer cells in vitro. World J. Gastroenterol. 2005, 11, 5633–5637. [Google Scholar] [CrossRef]
- Verhagen, H.J.M.P.; Smit, M.A.; Rutten, A.; Denkers, F.; Poddighe, P.J.; Merle, P.A.; Ossenkoppele, G.J.; Smit, L. Primary acute myeloid leukemia cells with overexpression of EVI-1 are sensitive to all-trans retinoic acid. Blood 2016, 127, 458–463. [Google Scholar] [CrossRef]
- Ni, X.; Hu, G.; Cai, X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit. Rev. Food Sci. Nutr. 2018, 59, S71–S80. [Google Scholar] [CrossRef]
- Yi, X.; Wang, Y.; Jia, Z.; Hiller, S.; Nakamura, J.; Luft, J.C.; Tian, S.; DeSimone, J.M. Retinoic Acid-Loaded Poly(lactic-co-glycolic acid) Nanoparticle Formulation of ApoB-100-Derived Peptide 210 Attenuates Atherosclerosis. J. Biomed. Nanotechnol. 2020, 16, 467–480. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Chen, J. Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis. Biomolecules 2022, 12, 869. https://doi.org/10.3390/biom12070869
Deng Q, Chen J. Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis. Biomolecules. 2022; 12(7):869. https://doi.org/10.3390/biom12070869
Chicago/Turabian StyleDeng, Qile, and Jixiang Chen. 2022. "Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis" Biomolecules 12, no. 7: 869. https://doi.org/10.3390/biom12070869
APA StyleDeng, Q., & Chen, J. (2022). Potential Therapeutic Effect of All-Trans Retinoic Acid on Atherosclerosis. Biomolecules, 12(7), 869. https://doi.org/10.3390/biom12070869





