Modulation of Vasomotor Function by Perivascular Adipose Tissue of Renal Artery Depends on Severity of Arterial Dysfunction to Nitric Oxide and Severity of Metabolic Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Metabolic Parameters
2.3. Vascular Functions
2.4. Quantitative Real-Time PCR Assay
2.5. Data Analyses
2.6. Drugs
3. Results
3.1. Metabolic Parameters
3.2. Changes in PVAT Response on Vasomotor Functions
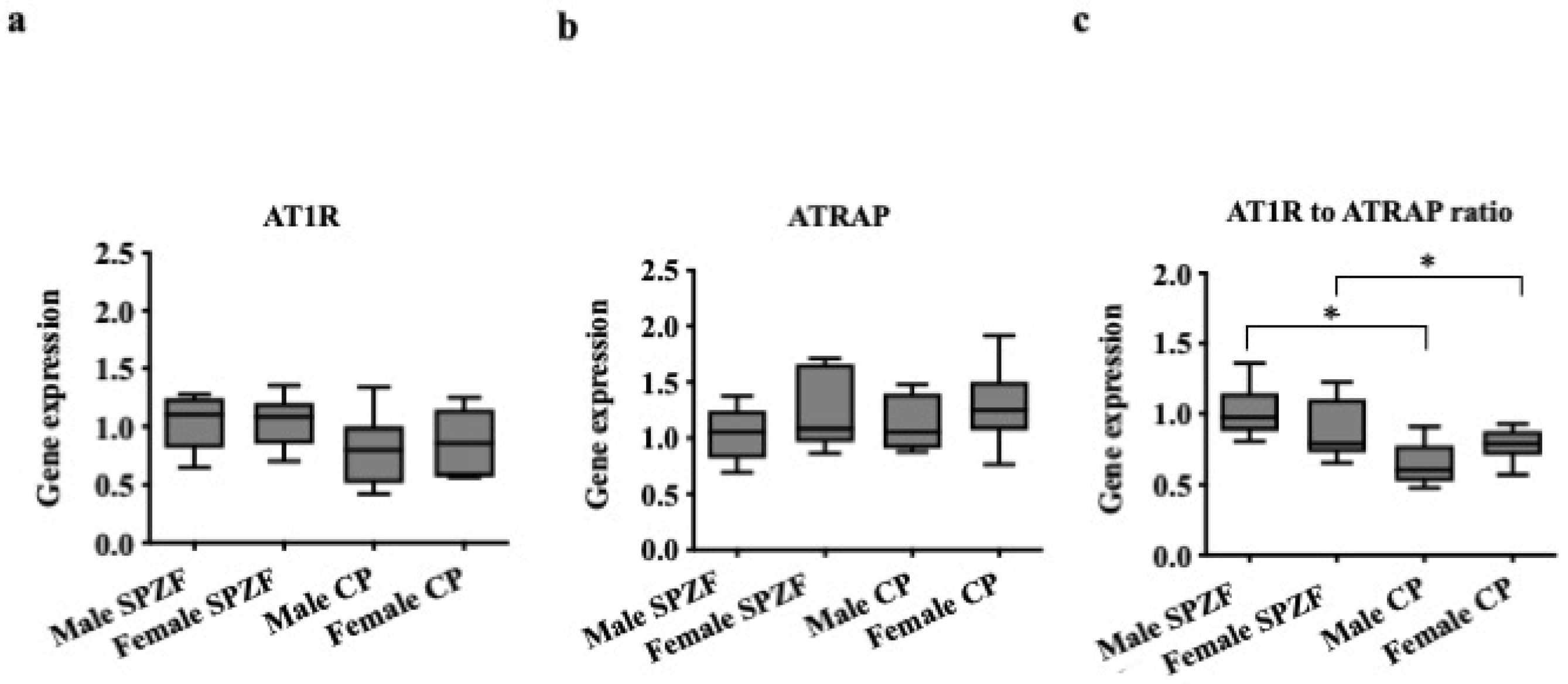
3.3. mRNA Levels of AT1R and ATRAP in PVAT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.G.; O’Malley, E.J.; Ho, W.S.V. Pro-contractile effects of perivascular fat in health and disease. Br. J. Pharmacol. 2017, 174, 3482–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicka, M.; Janowska, J.; Chudek, J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int. J. Cardiol. 2016, 222, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Alqahtani, A.; Khan, N.A.; Alqahtani, T.; Thangathirupathi, A.; Jagadeesh, G. The physiologic and physiopathologic roles of perivascular adipose tissue and its interactions with blood vessels and the renin-angiotensin system. Pharmacol. Res. 2021, 173, 105890. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Nerlekar, N.; Yuvaraj, J.; Fernandes, K.; Jiang, C.; Nicholls, S.J.; Dey, D.; Wong, D.T.L. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: A cross-sectional study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 298–306. [Google Scholar] [CrossRef]
- Fontes, M.T.; Paula, S.M.; Lino, C.A.; Senger, N.; Couto, G.K.; Barreto-Chaves, M.L.M.; Mill, J.G.; Rossoni, L.V. Renin-angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure. Clin. Sci. (Lond.) 2020, 134, 3195–3211. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Takagi, H.; Fukuda, N.; Takahashi, A.; Yao, E.H.; Mitsumata, M.; Hiraoka-Yamamoto, J.; Ikeda, K.; Matsumoto, K.; Yamori, Y. Cardiovascular remodeling and metabolic abnormalities in SHRSP.Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome. Hypertens. Res. 2008, 31, 1021–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka-Yamamoto, J.; Nara, Y.; Yasui, N.; Onobayashi, Y.; Tsuchikura, S.; Ikeda, K. Establishment of a new animal model of metabolic syndrome: SHRSP fatty (fa/fa) rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Iwata, S.; Maruyama, K.; McGuire, J.J.; Shinozuka, K. Time-Dependent Differences in the Influence of Perivascular Adipose Tissue on Vasomotor Functions in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 233–239. [Google Scholar] [CrossRef]
- Kagota, S.; Maruyama-Fumoto, K.; Iwata, S.; Shimari, M.; Koyanagi, S.; Shiokawa, Y.; McGuire, J.J.; Shinozuka, K. Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl(-)l-Cysteine-Sensitive Factor(s). Int. J. Mol. Sci. 2018, 20, 106. [Google Scholar] [CrossRef] [Green Version]
- Kagota, S.; Futokoro, R.; Maruyama-Fumoto, K.; McGuire, J.J.; Shinozuka, K. Perivascular Adipose Tissue Compensation for Endothelial Dysfunction in the Superior Mesenteric Artery of Female SHRSP.Z-Leprfa/IzmDmcr Rats. J. Vasc. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Cassis, L.A.; Police, S.B.; Yiannikouris, F.; Thatcher, S.E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008, 10, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Azushima, K.; Kinguchi, S.; Wakui, H.; Yamaji, T. ATRAP, a receptor-interacting modulator of kidney physiology, as a novel player in blood pressure and beyond. Hypertens. Res. 2022, 45, 32–39. [Google Scholar] [CrossRef]
- Wakui, H.; Tamura, K.; Tanaka, Y.; Matsuda, M.; Bai, Y.; Dejima, T.; Masuda, S.; Shigenaga, A.; Maeda, A.; Mogi, M.; et al. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension 2010, 55, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Wakui, H.; Dejima, T.; Tamura, K.; Uneda, K.; Azuma, K.; Maeda, A.; Ohsawa, M.; Kanaoka, T.; Azushima, K.; Kobayashi, R.; et al. Activation of angiotensin II type 1 receptor-associated protein exerts an inhibitory effect on vascular hypertrophy and oxidative stress in angiotensin II-mediated hypertension. Cardiovasc. Res. 2013, 100, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Tamura, K.; Wakui, H.; Dejima, T.; Ohsawa, M.; Azushima, K.; Kanaoka, T.; Uneda, K.; Matsuda, M.; Yamashita, A.; et al. Angiotensin receptor-binding protein ATRAP/Agtrap inhibits metabolic dysfunction with visceral obesity. J. Am. Heart Assoc. 2013, 2, e000312. [Google Scholar] [CrossRef] [Green Version]
- Azushima, K.; Ohki, K.; Wakui, H.; Uneda, K.; Haku, S.; Kobayashi, R.; Haruhara, K.; Kinguchi, S.; Matsuda, M.; Maeda, A.; et al. Adipocyte-Specific Enhancement of Angiotensin II Type 1 Receptor-Associated Protein Ameliorates Diet-Induced Visceral Obesity and Insulin Resistance. J. Am. Heart Assoc. 2017, 6, e004488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Lian, G.; Cai, X.; Lin, Z.; Xie, L. Effect of prehypertensive losartan therapy on AT1R and ATRAP methylation of adipose tissue in the later life of highfatfed spontaneously hypertensive rats. Mol. Med. Rep. 2018, 17, 1753–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restini, C.B.A.; Ismail, A.; Kumar, R.K.; Burnett, R.; Garver, H.; Fink, G.D.; Watts, S.W. Renal perivascular adipose tissue: Form and function. Vasc. Pharmacol. 2018, 106, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Enyedi, M.; Nedelea, A.S.; Nica, A.E.; et al. Impact of adipose tissue in chronic kidney disease development (Review). Exp. Ther. Med. 2021, 21, 539. [Google Scholar] [CrossRef]
- Koletsky, S. Animal model: Obese hypertensive rat. Am. J. Pathol. 1975, 81, 463–466. [Google Scholar]
- Kagota, S.; Yamaguchi, Y.; Tanaka, N.; Kubota, Y.; Kobayashi, K.; Nejime, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Disturbances in nitric oxide/cyclic guanosine monophosphate system in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006, 78, 1187–1196. [Google Scholar] [CrossRef]
- McNamee, C.J.; Kappagoda, C.T.; Kunjara, R.; Russell, J.C. Defective endothelium-dependent relaxation in the JCR:LA-corpulent rat. Circ. Res. 1994, 74, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Nakagami, H.; Pang, Z.; Shimosato, T.; Moritani, T.; Kurinami, H.; Koriyama, H.; Tenma, A.; Shimamura, M.; Morishita, R. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertens. Res. 2014, 37, 629–635. [Google Scholar] [CrossRef]
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Ren. Physiol. 2019, 317, F456–F462. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, Y.; Umegaki, K.; Kagota, S.; Tanaka, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29, 1756–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagota, S.; Maruyama, K.; Wakuda, H.; McGuire, J.J.; Yoshikawa, N.; Nakamura, K.; Shinozuka, K. Disturbance of vasodilation via protease-activated receptor 2 in SHRSP.Z-Lepr fa/IzmDmcr rats with metabolic syndrome. Vasc. Pharmacol. 2014, 63, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, C.G.; Rand, M.J. Mechanisms of nitric oxide-independent relaxations induced by carbachol and acetylcholine in rat isolated renal arteries. Br. J. Pharmacol. 2000, 130, 1191–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.Y.; Gao, Y.; Di, Y.W.; Pan, L.L.; Zhou, Y.; Ye, J.M. High-fat feeding reduces endothelium-dependent vasodilation in rats: Differential mechanisms for saturated and unsaturated fatty acids? Clin. Exp. Pharmacol. Physiol. 2006, 33, 708–713. [Google Scholar] [CrossRef]
- Kagota, S.; Yamaguchi, Y.; Nakamura, K.; Kunitomo, M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen. Pharmacol. 2000, 34, 201–209. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, B.; Mustafa, S.J. Sex Difference in Coronary Endothelial Dysfunction in Apolipoprotein E Knockout Mouse: Role of NO and A2A Adenosine Receptor. Microcirculation 2015, 22, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Shaw, L.J.; Bugiardini, R.; Merz, C.N. Women and ischemic heart disease: Evolving knowledge. J. Am. Coll. Cardiol. 2009, 54, 1561–1575. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, R.; Wakui, H.; Azushima, K.; Uneda, K.; Haku, S.; Ohki, K.; Haruhara, K.; Kinguchi, S.; Matsuda, M.; Ohsawa, M.; et al. An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model. Kidney Int. 2017, 91, 1115–1125. [Google Scholar] [CrossRef]
- Medina, D.; Mehay, D.; Arnold, A.C. Sex differences in cardiovascular actions of the renin-angiotensin system. Clin. Auton. Res. 2020, 30, 393–408. [Google Scholar] [CrossRef]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Amer, D.A.; Li, V.K.; Goudie, E.B.; Johnston, N.R.; Sharma, S.; Neilson, I.; Webb, D.J.; Megson, I.L.; et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008, 52, 908–913. [Google Scholar] [CrossRef] [Green Version]




| Group (n) | SPZF | CP | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Body weight (g) | 493 ± 7 | 447 ± 5 b,* | 582 ± 13 a | 469 ± 12 a,b,* |
| Waist circumference–body length ratio (cm/cm) | 1.04 ± 0.01 | 1.09 ± 0.01 | 1.03 ± 0.01 | 1.05 ± 0.02 |
| sBP (mmHg) | 246 ± 7 | 200 ± 5 b,* | 176 ± 11 a | 169 ± 6 a,b,* |
| Serum levels | ||||
| Glucose (mg/100 mL) | 356 ± 25 | 261 ± 28 | 321 ± 38 | 357 ± 35 |
| Insulin (ng/mL) | 37.1 ± 4.9 | 54.8 ± 7.0 * | 30.4 ± 6.3 a | 20.1 ± 4.3 a |
| Urine levels | ||||
| Glucose (score) | 0.18 ± 0.18 | 0.63 ± 0.24 | 0.08 ± 0.07 | 0.14 ± 0.13 |
| Protein (score) | 3.27 ± 0.14 | 3.78 ± 0.15 b | 3.30 ± 0.21 | 2.23 ± 0.20 b,* |
| Agonist and Activity | CRC Parameter | PVAT State | Sex | |
|---|---|---|---|---|
| Male | Female | |||
| Acetylcholine-induced relaxation | −Log EC50 | PVAT (−) | 7.53 ± 0.11 | 7.29 ± 0.15 |
| PVAT (+) | 7.70 ± 0.17 | 7.61 ± 0.09 | ||
| Emax (%) | PVAT (−) | 65.8 ± 7.2 b | 39.4 ± 6.9 b | |
| PVAT (+) | 75.1 ± 7.4 | 50.3 ± 8.7 | ||
| Sodium nitroprusside-induced relaxation | −Log EC50 | PVAT (−) | 7.16 ± 0.17 b | 6.92 ± 0.08 b |
| PVAT (+) | 6.98 ± 0.14 | 6.86 ± 0.06 | ||
| Emax (%) | PVAT (−) | 93.2 ± 3.1 b | 66.1 ± 5.4 b | |
| PVAT (+) | 99.0 ± 6.5 | 66.9 ± 7.2 | ||
| Phenylephrine-induced contraction | −Log EC50 | PVAT (−) | 6.33 ± 0.09 | 6.44 ± 0.09 |
| PVAT (+) | 6.27 ± 0.12 | 6.50 ± 0.08 | ||
| Emax (g) | PVAT (−) | 0.487 ± 0.047 | 0.497 ± 0.078 | |
| PVAT (+) | 0.485 ± 0.020 | 0.425 ± 0.078 | ||
| Agonist and Activity | CRC Parameter | PVAT State | Sex | |
|---|---|---|---|---|
| Male | Female | |||
| Acetylcholine-induced relaxation | −Log EC50 | PVAT (−) | 7.38 ± 0.11 | 7.27 ± 0.20 |
| PVAT (+) | 7.48 ± 0.09 | 7.41 ± 0.12 | ||
| Emax (%) | PVAT (−) | 58.7 ± 5.9 a,b | 29.2 ± 8.6 a,b | |
| PVAT (+) | 81.4 ± 5.4 * | 57.4 ± 6.3 * | ||
| Sodium nitroprusside-induced relaxation | −Log EC50 | PVAT (−) | 7.43 ± 0.13 | 7.18 ± 0.04 |
| PVAT (+) | 7.15 ± 0.09 | 6.88 ± 0.16 | ||
| Emax (%) | PVAT (−) | 80.9 ± 5.4 | 79.7 ± 4.7 | |
| PVAT (+) | 72.6 ± 3.8 | 83.4 ± 11.1 | ||
| Phenylephrine-induced contraction | −Log EC50 | PVAT (−) | 6.11 ± 0.10 | 5.95 ± 0.32 |
| PVAT (+) | 5.38 ± 0.55 | 5.93 ± 0.07 | ||
| Emax (g) | PVAT (−) | 0.508 ± 0.062 | 0.363 ± 0.070 | |
| PVAT (+) | 0.506 ± 0.118 | 0.404 ± 0.041 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagota, S.; Futokoro, R.; McGuire, J.J.; Maruyama-Fumoto, K.; Shinozuka, K. Modulation of Vasomotor Function by Perivascular Adipose Tissue of Renal Artery Depends on Severity of Arterial Dysfunction to Nitric Oxide and Severity of Metabolic Parameters. Biomolecules 2022, 12, 870. https://doi.org/10.3390/biom12070870
Kagota S, Futokoro R, McGuire JJ, Maruyama-Fumoto K, Shinozuka K. Modulation of Vasomotor Function by Perivascular Adipose Tissue of Renal Artery Depends on Severity of Arterial Dysfunction to Nitric Oxide and Severity of Metabolic Parameters. Biomolecules. 2022; 12(7):870. https://doi.org/10.3390/biom12070870
Chicago/Turabian StyleKagota, Satomi, Risa Futokoro, John J. McGuire, Kana Maruyama-Fumoto, and Kazumasa Shinozuka. 2022. "Modulation of Vasomotor Function by Perivascular Adipose Tissue of Renal Artery Depends on Severity of Arterial Dysfunction to Nitric Oxide and Severity of Metabolic Parameters" Biomolecules 12, no. 7: 870. https://doi.org/10.3390/biom12070870






