Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach
Abstract
:1. Introduction
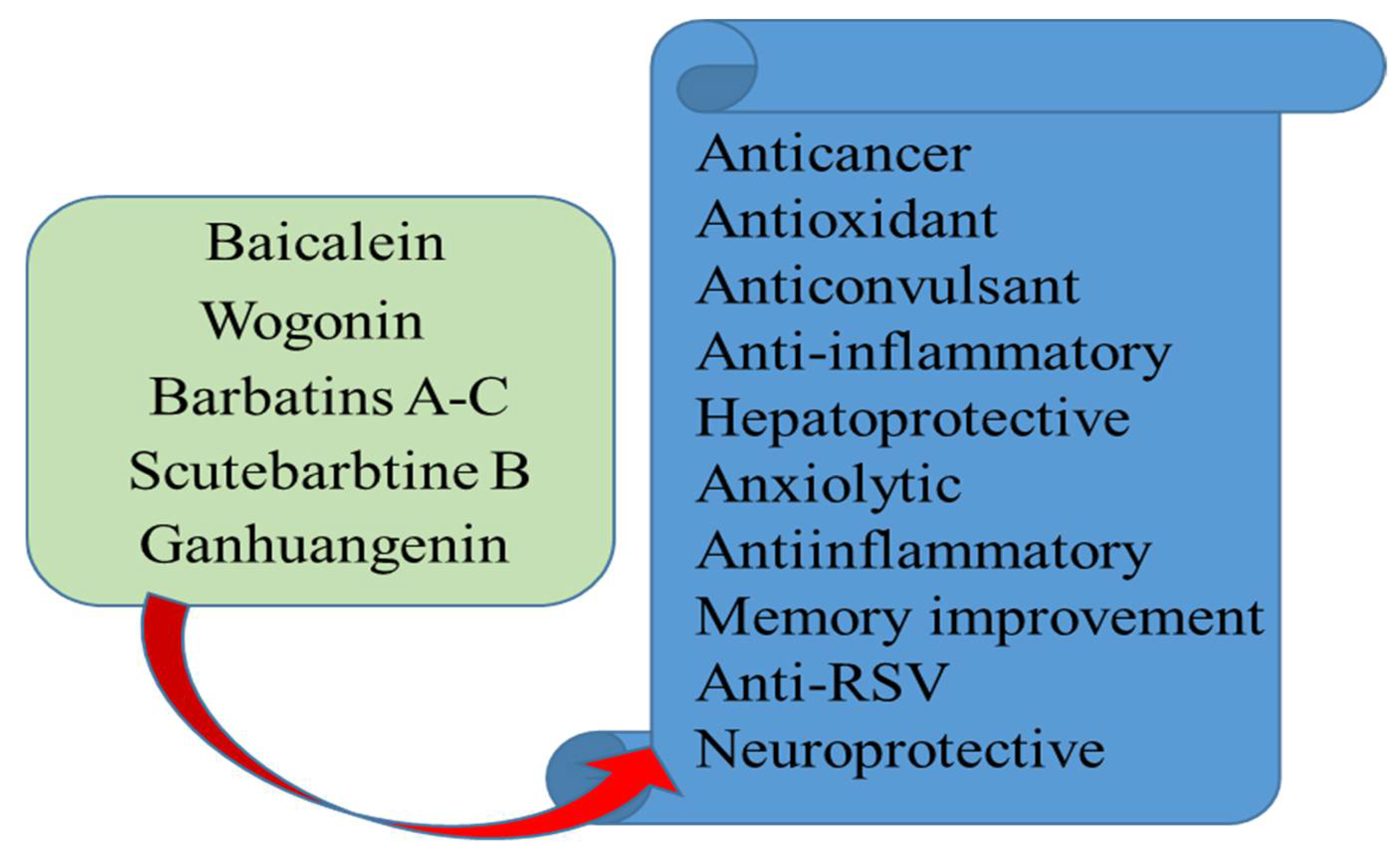
2. Compounds with Biological Activities from Genus Scutellaria Species
3. Most Prominent Compounds of Genus Scutellaria
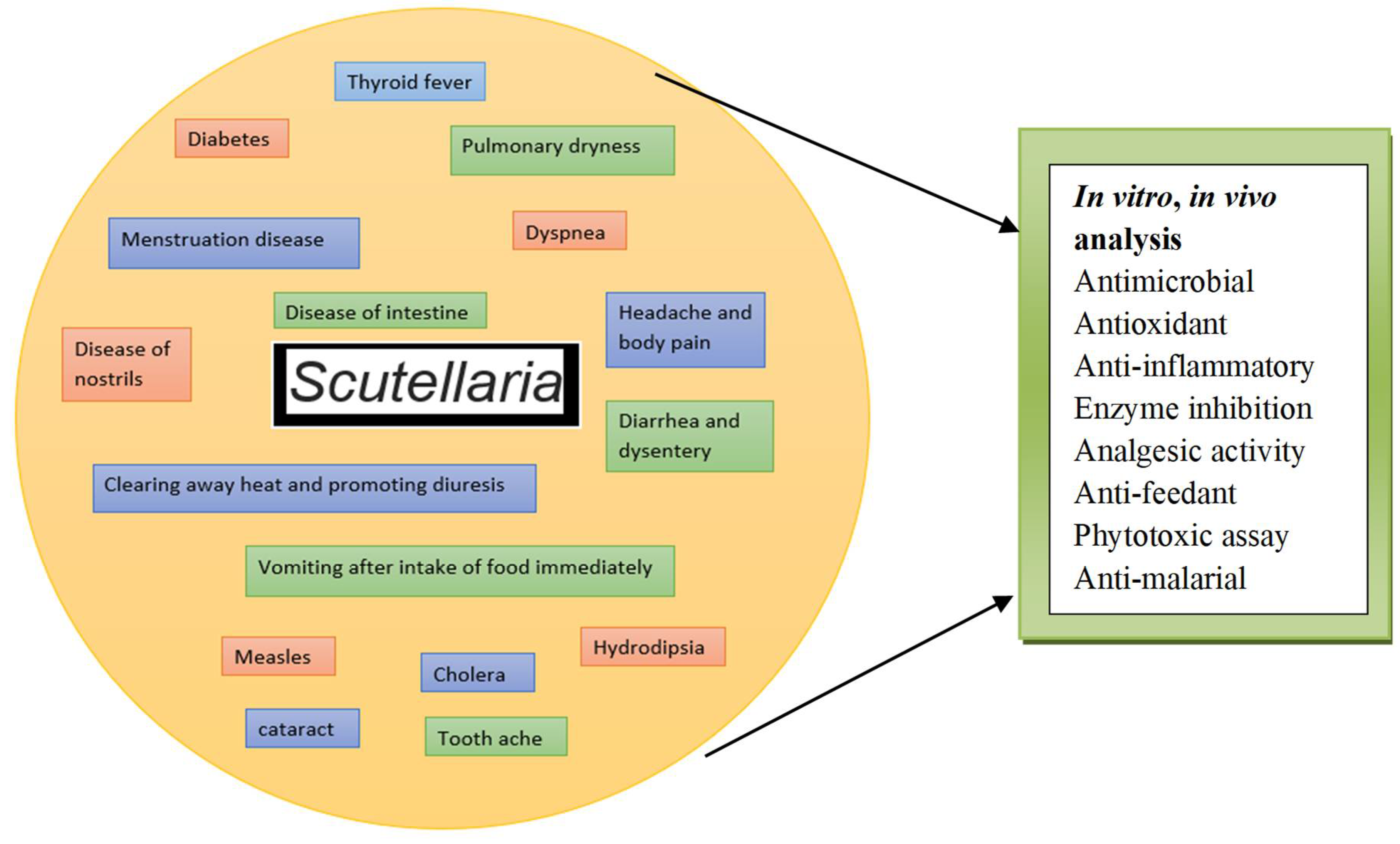
4. Phytochemical and Biological Assessment of Scutellaria
5. General Extraction Process
6. Biological Activities of Genus Scutellaria
6.1. Antimicrobial Capabilities
6.2. Enzyme Inhibitory Potential
6.3. Anti-Fungal Significance
6.4. Anticancer Implication of Genus Scutellaria
6.5. Anti-Inflammatory Potential
6.6. Analgesic Significance
7. Clinical Significance
8. Current and Future Perspective
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.; Rahman, H.; Khan, A.; Bibi, S.; Ullah, O.; Ullah, S.; Ur Rehman, N.; Murad, W.; Al-Harrasi, A. Identification of α-Glucosidase Inhibitors from Scutellaria edelbergii: ESI-LC-MS and Computational Approach. Molecules 2022, 27, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-Kott, A.F. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef] [PubMed]
- Napagoda, M.; Wijesundara, D. 1 Medicinal plants as sources of novel therapeutics: The history, present, and future. Chem. Nat. Prod. Phytochem. Pharm. Med. Plants 2022, 3, 4–18. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Murad, W.; Ur Rehman, N.; Mubin, S.; Al-Sabahi, J.N.; Ahmad, M.; Zahoor, M.; Ullah, O.; Waqas, M.; Ullah, S. GC-MS analysis and biomedical therapy of oil from n-hexane fraction of Scutellaria edelbergii Rech. f.: In vitro, in vivo, and in silico approach. Molecules 2021, 26, 7676. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhu, S.; Wu, G.; Hassan, S.S.U.; Xie, Y.; Ishaq, M.; Sun, Y.; Yan, S.-K.; Qian, X.-P.; Jin, H.-Z. Chemical constituents of vernonia parishii. Chem. Nat. Compd. 2020, 56, 134–136. [Google Scholar] [CrossRef]
- Minareci, E.; Pekönür, S. An important Euroasian genus: Scutellaria L. Int. J. Sec. Met. 2017, 4, 35–46. [Google Scholar]
- Sripathi, R.; Ravi, S. Ethnopharmacology, phytoconstituents, essential oil composition and biological activities of the genus Scutellaria. J. Pharm. Sci. Res. 2017, 9, 275. [Google Scholar]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Murad, W.; Ur Rehman, N.; Halim, S.A.; Ahmed, M.; Rehman, H.; Zahoor, M.; Mubin, S.; Khan, A.; Nassan, M.A. Biomedical applications of Scutellaria edelbergii Rech. f.: In vitro and in vivo approach. Molecules 2021, 26, 3740. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi.(Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and cancer treatment: Recent progress and perspectives in biomedical and clinical studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, J.; Cui, M.-Y.; Liu, J.; Fang, Y.; Yan, M.; Qiu, W.; Shang, H.; Xu, Z.; Yidiresi, R. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant 2019, 12, 935–950. [Google Scholar] [CrossRef] [Green Version]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Majid, A.; Ahmad, H.; Saqib, Z.; Ali, H. Potential distribution of endemic Scutellaria chamaedrifolia; geographic information system and statistical model approach. Pak. J. Bot 2015, 47, 51–56. [Google Scholar]
- Chen, L.; Batjikh, I.; Hurh, J.; Han, Y.; Huo, Y.; Ali, H.; Li, J.F.; Rupa, E.J.; Ahn, J.C.; Mathiyalagan, R. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik 2019, 184, 324–329. [Google Scholar] [CrossRef]
- Senol, F.; Orhan, İ.; Yilmaz, G.; Cicek, M.; Sener, B. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.S.; Li, X.; Kim, H.H.; Arasu, M.V.; Al-Dhabi, N.A.; Lee, S.Y.; Park, S.U. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. 2016, 11, 345–425. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Yong, Z.; Chunying, L.; Yushi, Z.; Yang, B.; Yalan, Y.; Chen, P.; Lianmei, W.; Aihua, L. Potential chronic liver toxicity in rats orally administered an ethanol extract of Huangqin (Radix Scutellariae baicalensis). J. Trad. Chin. Med. 2018, 38, 242–256. [Google Scholar] [CrossRef]
- Lin, Y.-L. Aurantiamide from the aerial parts of Scutellaria rivularis. Planta Med. 1987, 53, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Kim, Y.T. Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Molecules 2013, 18, 5804–5813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gousiadou, C.; Karioti, A.; Heilmann, J.; Skaltsa, H. Iridoids from Scutellaria albida ssp. albida. Phytochemistry 2007, 68, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-D.; Kim, J.H.; Pang, Q.Q.; Jung, P.-M.; Cho, E.J.; Lee, S. Antioxidant activity and acteoside analysis of Abeliophyllum Distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Bruno, M.; Cruciata, M.; Bondi, M.L.; Piozzi, F.; María, C.; Rodríguez, B.; Servettaz, O. Neo-clerodane diterpenoids from Scutellaria Lateriflora. Phytochemistry 1998, 48, 687–691. [Google Scholar] [CrossRef]
- Torrenegra, R.D.; Rodríguez, J.; Rodríguez, O.; Palau, V.E.; Méndez, G.M. Antiproliferative activity of 3, 5, 7-trihydroxy-6-methoxy flavone obtained from Chromolaena leivensis (Hieron) on cancer cell lines of breast, prostate, lung, colon and cervix. Pharmacology 2016, 1, 7–11. [Google Scholar]
- Dai, S.-J.; Tao, J.-Y.; Liu, K.; Jiang, Y.-T.; Shen, L. Neo-clerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Phytochemistry 2006, 67, 1326–1330. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Hu, P.; Ji, J.; Li, X.; Chen, J. Neoclerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2020, 34, 1345–1351. [Google Scholar] [CrossRef]
- Bruno, M.; Piozzi, F.; Rodríguez, B.; María, C.; Vassallo, N.; Servettaz, O. Neo-clerodane diterpenoids from S. altissima and S. albida. Phytochemistry 1996, 42, 1059–1064. [Google Scholar]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stability after in vitro simulated digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef]
- Dehkordi, F.J.; Kharazian, N.; Lorigooini, Z. Characterization of flavonoid components in Scutellaria L. species (Lamiaceae) using finger-printing analysis. Acta Biol. Crac. S. Botan. 2020, 62, 79–96. [Google Scholar]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramza, A.; Korczak, J.; Amarowicz, R. Tea polyphenols-their antioxidant properties and biological activity-a review. Pol. J. Food. Nutr. Sci. 2005, 14, 219. [Google Scholar]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Trad. Complement. Med. 2017, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging cytotoxic alkaloids in the battle against cancer: Overview of molecular mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, V.U.; Anwar, S.; Miana, G.A.; Krohn, K. Chemical constituents of Scutellaria linearis. Biochem. Syst. Eecol. 2008, 5, 490–492. [Google Scholar] [CrossRef]
- Stevens, J.F.; Miranda, C.L.; Buhler, D.R.; Deinzer, M.L. Chemistry and biology of hop flavonoids. J. Am. Soc. Brew. Chem. 1998, 56, 136–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirotani, M.; Yoshikawa, T.; Furuya, T. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry 1997, 44, 83–87. [Google Scholar] [CrossRef]
- Calis, İ.; Kirmizibekmez, H.; Beutler, J.A.; Donmez, A.A.; Yalçin, F.N.; Kilic, E.; Ozalp, M.; Ruedi, P.; Tasdemir, D. Secondary metabolites of Phlomis viscosa and their biological activities. Turk. J. Chem. 2005, 29, 71–82. [Google Scholar]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Comp. Rev. Food Sci. Food Safe. 2016, 15, 542–567. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-H.; Zhang, Y.-J.; Yang, C.-R. New flavonoid glycosides from Scutellaria amoena. Stud. Plant Sci. 1999, 6, 305–310. [Google Scholar]
- Esselen, M.; Barth, S.W. Food-borne topoisomerase inhibitors: Risk or benefit. Advan. Mol. Toxicol. 2014, 8, 123–171. [Google Scholar]
- Zhang, Q.; Liu, A.; Wang, Y. Scrophularia ningpoensis Hemsl: A review of its phytochemistry, pharmacology, quality control and pharmacokinetics. J. Pharm. Pharmacol. 2021, 73, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants 2022, 11, 22–38. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M. Anti-tumor and anti-metastatic actions of wogonin isolated from Scutellaria baicalensis roots through anti-lymphangiogenesis. Phytomedicine 2013, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Tomimori, T.; Miyaichi, Y.; Imoto, Y.; Kizu, H.; Namba, T. Studies on the nepese crude drugs. VI.: On the flavonoid constituents of the root of Scutellaria discolor colebr.(2). Chem. Pharm. Bull. 1986, 34, 406–408. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in biosynthesis, pharmacology, and pharmacokinetics of pinocembrin, a promising natural small-molecule drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, Y.; Miyaichi, Y.; Tomimori, T. Studies on the Nepalese crude drugs. XIII. on the flavonoid and iridoid constituents of the root of Scutellaria grossa wall. Chem. Pharm. Bull. 1991, 39, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Bhat, G.; Ganai, B.A.; Shawl, A.S. New phenolics from the root of Scutellaria prostrata Jacq. ex Benth. Nat. Prod. Res. 2014, 28, 1685–1690. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Kuo, Y.-H.; Cheng, M.-C.; Wang, Y. Structures of scutellones D and E determined from X-ray diffraction, spectral and chemical evidence. Neoclerodane-type diterpenoids from Scutellaria rivularis Wall. Chem. Pharm. Bull. 1988, 36, 2642–2646. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Rahman, K.; Wang, S.-J.; Zhou, S.; Zhang, H. Scutellaria barbata: A review on chemical constituents, pharmacological activities and clinical applications. Curr. Pharm. Des. 2020, 26, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.M.; Maria, C.; Rodríguez, B.; Simmonds, M.S.; Blaney, W.M. Neo-clerodane insect antifeedants from Scutellaria alpina subsp. javalambrensis. Phytochemistry 1997, 44, 593–597. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Liu, Y. Studies on the structures of new flavonoids from the root of Scutellaria amoena. Acta Pharm. Sini. 1989, 24, 200–206. [Google Scholar]
- Salini, S.; Chubicka, T.; Sasidharan, N.; Sindhu, E.; Babu, T. Cytotoxic and antioxidant properties of selected Scutellaria species from the Western Ghats of Peninsular India. Pharm. Biol. 2013, 51, 152–159. [Google Scholar] [CrossRef]
- Takagi, S.; Yamaki, M.; Inoue, K. Studies on the water-soluble constituents of the roots of Scutellaria baicalensis Georgi (Wogonin)(author’s transl). J. Pharm. Soc. Jpn. 1980, 100, 1220–1224. [Google Scholar] [CrossRef]
- Li, T.; Yang, W.Z.; Song, T.X.; Liu, C.J.; Jiang, M.M. Integrating chemical profiling and network pharmacology analysis based on anti-inflammatory effects for quality control of Scutellaria Barb. Phytochem. Anal. 2021, 32, 1141–1151. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, A.; Goswami, R.; Basu, S. Hypoglycemic activity of the antioxidant saponarin, characterized as α-glucosidase inhibitor present in Tinospora cordifolia. J. Enz Inh. Med. Chem. 2009, 24, 684–690. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Miyaichi, Y.; Tomimori, T. Studies on nepalese crude drugs. XIV. new flavonoids from the root of Scutellaria prostrata Jacq. ex benth. Chem. Pharm. Bull. 1991, 39, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Long, H.-L.; Xu, G.-Y.; Deng, A.-J.; Li, Z.-H.; Ma, L.; Lu, Y.; Zhang, Z.-H.; Wu, F.; Qin, H.-L. Two new flavonoids from the roots of Scutellaria Baicalensis. J. Asian Nat. Prod. Res. 2015, 17, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-J.; Peng, W.-B.; Shen, L.; Zhang, D.-W.; Ren, Y. New norditerpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2011, 25, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Aboutabl, E.; Hashem, F.A.; Sleem, A.; Maamoon, A. Flavonoids, anti-inflammatory activity and cytotoxicity of Macfadyena unguis-cati L. Afr. J. Trad. Complement. Altern. Med. 2008, 5, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.; Piozzi, F.; Maggio, A.M.; Simmonds, M.S. Antifeedant activity of neoclerodane diterpenoids from two Sicilian species of Scutellaria. Biochem. Syst. Ecol. 2002, 8, 793–799. [Google Scholar] [CrossRef]
- Wang, C.-M.; Yeh, K.-L.; Tsai, S.-J.; Jhan, Y.-L.; Chou, C.-H. Anti-proliferative activity of triterpenoids and sterols isolated from Alstonia scholaris against non-small-cell lung carcinoma cells. Molecules 2017, 22, 2119. [Google Scholar] [CrossRef] [Green Version]
- Maria, C.; Rodríguez, B.; Bruno, M.; Piozzi, F.; Savona, G.; Vassallo, N.; Servettaz, O. Neo-clerodane diterpenoids from Scutellaria Alp. Phytochem. 1995, 38, 181–187. [Google Scholar]
- Erdemoglu, N.; Ozkan, S.; Duran, A.; Tosun, F. GC-MS analysis and antimicrobial activity of alkaloid extract from Genista vuralii. Pharm. Biol. 2009, 47, 81–85. [Google Scholar] [CrossRef]
- Tomimori, T.; Miyaichi, Y.; Imoto, Y.; Kizu, H.; Namba, T. Studies on Nepalese crude drugs. V. On the flavonoid constituents of the root of Scutellaria discolor Colebr.(1). Chem. Pharm. Bull. 1985, 33, 4457–4463. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cacherat, B.; Hu, Q.; Ma, D. Recent advances in the synthesis of ent-kaurane diterpenoids. Nat. Prod. Rep. 2021, 39, 119–138. [Google Scholar] [CrossRef]
- Gong, G.; Wang, H.; Kong, X.; Duan, R.; Dong, T.T.; Tsim, K.W. Flavonoids are identified from the extract of Scutellariae Radix to suppress inflammatory-induced angiogenic responses in cultured RAW 264.7 macrophages. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Bozov, P.; Girova, T.; Prisadova, N.; Hristova, Y.; Gochev, V. Antimicrobial activity of neo-clerodane diterpenoids isolated from Lamiaceae species against pathogenic and food spoilage microorganisms. Nat. Prod. Commun. 2015, 10, 578–601. [Google Scholar] [CrossRef] [Green Version]
- De Smet, P. Scutellaria species. In Adverse Effects of Herbal Drugs 2; Springer: Berlin/Heidelberg, Germany, 1993; pp. 289–296. [Google Scholar]
- Rashid, M.; Fareed, M.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and their biological secrets. Plant Hum. Health 2019, 2, 579–605. [Google Scholar]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef]
- Malikov, V.; Yuldashev, M. Phenolic compounds of plants of the Scutellaria L. genus. Distribution, structure, and properties. Chem. Nat. Compd. 2002, 38, 358–406. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in the pancreatic cancer cell (PANC-1). Art. Cells Nanomed. Biotech. 2019, 47, 1617–1627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar]
- Yuan, Y.; Wu, C.; Liu, Y.; Yang, J.; Huang, L. The Scutellaria baicalensis R2R3-MYB transcription factors modulates flavonoid biosynthesis by regulating GA metabolism in transgenic tobacco plants. PLoS ONE 2013, 8, 77275. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Huang, T.-S.; Wong, C.-H.; Hong, C.-L.; Tsai, Y.-H.; Liang, C.-C.; Lu, F.-J.; Chang, W.-H. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem. Toxicol. 2009, 47, 638–644. [Google Scholar] [CrossRef]
- Hui, K.M.; Huen, M.S.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.T.-F.; Xue, H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef]
- Cole, I.B.; Saxena, P.K.; Murch, S.J. Medicinal biotechnology in the genus Scutellaria. Vitr. Cell. Develop. Biol. Plant 2007, 43, 318–327. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria Barb. Phytochem. 2004, 65, 881–884. [Google Scholar] [CrossRef]
- Pant, C.C.; Melkani, A.B.; Mohan, L.; Dev, V. Composition and antibacterial activity of essential oil from Scutellaria grossa Wall ex Benth. Nat. Prod. Res. 2012, 26, 190–192. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Kyriazopoulos, P.; Golegou, S.; Triantaphyllidis, S.; Sokovic, M.; Kypriotakis, Z. Composition and antimicrobial activity of the essential oils of Scutellaria sieberia Benth. and Scutellaria rupestris Boiss. et Heldr. ssp. adenotricha (Boiss. et Heldr.) Greuter et Burdet from Greece. J. Essent. Oil Res. 2005, 17, 232–235. [Google Scholar] [CrossRef]
- Dereboylu, A.; Sarikahya, N.; Sengonca, N.; Kirmizigul, S.; Yasa, I.; Gucel, S.; Guvensen, A. Glandular trichomes morphology, chemical composition and antimicrobial activity of the essential oil of three endemic Scutellaria taxa (Lamiaceae). Asian J. Chem. 2012, 24, 4911. [Google Scholar]
- Kim, H.I.; Hong, S.H.; Ku, J.M.; Lim, Y.S.; Lee, S.J.; Song, J.; Kim, T.Y.; Cheon, C.; Ko, S.-G. Scutellaria radix promotes apoptosis in non-small cell lung cancer cells via induction of AMPK-dependent autophagy. Am. J. Chin. Med. 2019, 47, 691–705. [Google Scholar] [CrossRef]
- Nan, Y.; Yuan, L.; Zhou, L.; Niu, Y. Study on the optimization of the technology for the extraction and purification of total flavone in Scutellaria baicalensis and its antibacterial activity. Afr. J. Microbiol. Res. 2011, 5, 5689–5696. [Google Scholar]
- Yu, T.-T.; Guo, K.; Chen, H.-C.; Lan, C.-Z.; Wang, J.; Huang, L.-L.; Wang, X.-H.; Zhang, Z.; Gao, S. Effects of traditional Chinese medicine Xin-Ji-Er-Kang formula on 2K1C hypertensive rats: Role of oxidative stress and endothelial dysfunction. BMC Complement. Altern. Med. 2013, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.C.-F.; Seneviratne, C.J.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wong, C.-H.; Pang, K.Y.; Wong, C.W.; Wat, E.; Jin, L. Synergistic antibacterial effects of nanoparticles encapsulated with Scutellaria baicalensis and pure chlorhexidine on oral bacterial biofilms. Nanomaterials 2016, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, F.S. Anti-microbial properties of Scutellaria baicalensis and Coptis chinensis, two traditional Chinese medicines. Biosci. Horizon. 2011, 4, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Rong, X.; Jiang, L.; Qu, M.; ul Hassan, S.S.; Liu, Z. Enhancing therapeutic efficacy of donepezil by combined therapy: A comprehensive review. Curr. Pharm. Des. 2020, 27, 332–344. [Google Scholar] [CrossRef]
- Shrestha, D.; Sharma, P.; Adhikari, A.; Mandal, A.K.; Verma, A. A Review on Nepalese medicinal plants used traditionally as alpha-amylase and alpha-glucosidase inhibitors against diabetes mellitus. Curr. Trad. Med. 2021, 7, 63–72. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Bungau, S.; Jhanji, R.; Kumar, A.; Mehta, V.; Zengin, G.; Brata, R.; ul Hassan, S.S.; Fratila, O. Distinctive evidence involved in the role of endocannabinoid signalling in parkinson’s disease: A Perspective on associated therapeutic interventions. Int. J. Mol. Sci. 2020, 21, 6235. [Google Scholar] [CrossRef]
- Wei, L.; Dai, Q.; Zhou, Y.; Zou, M.; Li, Z.; Lu, N.; Guo, Q. Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: C-Src and hexokinase II. Biochim. Biophy. Acta 2013, 1830, 3835–3845. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, W.; Xu, Y.; Zhuo, Y.; Li, M.; He, Y.; Wang, X.; Guo, Q.; Zhao, L. Oroxylin A reverses hypoxia-induced cisplatin resistance through inhibiting HIF-1α mediated XPC transcription. Oncogene 2020, 39, 6893–6905. [Google Scholar] [CrossRef] [PubMed]
- Joshee, N.; Mentreddy, S.; Yadav, A.K. Mycorrhizal fungi and growth and development of micropropagated Scutellaria integrifolia plants. Indus. Crops Prod. 2007, 25, 169–177. [Google Scholar] [CrossRef]
- Cole, M.D.; Bridge, P.D.; Dellar, J.E.; Fellows, L.E.; Cornish, M.C.; Anderson, J.C. Antifungal activity of neo-clerodane diterpenoids from Scutellaria. Phytochemistry 1991, 30, 1125–1127. [Google Scholar] [CrossRef]
- Al-Alwan, L.A.; Chang, Y.; Baglole, C.J.; Risse, P.-A.; Halayko, A.J.; Martin, J.G.; Eidelman, D.H.; Hamid, Q. Autocrine-regulated airway smooth muscle cell migration is dependent on IL-17–induced growth-related oncogenes. J. Allergy Clin. Immunol. 2012, 130, 977–985. [Google Scholar] [CrossRef]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef] [Green Version]
- Katzung, B.G.; Parmley, W.W. Drugs used in heart failure. Basic Clin. Pharmacol. 2010, 24, 212–227. [Google Scholar]
- Ji, X.; Li, J.; Xu, L.; Wang, W.; Luo, M.; Luo, S.; Ma, L.; Li, K.; Gong, S.; He, L. IL4 and IL-17A provide a Th2/Th17-polarized inflammatory milieu in favor of TGF-β1 to induce bronchial epithelial-mesenchymal transition (EMT). Int. J. Clin. Exp. Pathol. 2013, 6, 1481. [Google Scholar]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Xie, Y.G.; Zhao, X.C.; ul Hassan, S.S.; Zhen, X.Y.; Muhammad, I.; Yan, S.K.; Yuan, X.; Li, H.L.; Jin, H.Z. One new sesquiterpene and one new iridoid derivative from Valeriana amurensis. Phytochem. Lett. 2019, 32, 6–9. [Google Scholar] [CrossRef]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Scutellaria: Debates on the anticancer property. Biomed. Pharmacother. 2018, 105, 1299–1310. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, baicalein and baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med. 2009, 75, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.O.; Leem, K.; Park, J.; Lee, P.; Ahn, D.-K.; Lee, B.C.; Park, H.K.; Suk, K.; Kim, S.Y.; Kim, H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001, 77, 183–188. [Google Scholar] [CrossRef]
- Chen, C.-C.; Kao, C.-P.; Chiu, M.-M.; Wang, S.-H. The anti-cancer effects and mechanisms of Scutellaria barbata D. Don on CL1–5 lung cancer cells. Oncotarget 2017, 8, 109340. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Kim, D.H.; Hossain, M.A.; Kang, Y.J.; Jang, J.Y.; Lee, Y.J.; Im, E.; Yoon, J.-H.; Kim, H.S.; Chung, H.Y.; Kim, N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013, 43, 1652–1658. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-L.; Kao, T.-H.; Shiau, C.-Y.; Chen, B.-H. Functional components in Scutellaria barbata D. Don with anti-inflammatory activity on RAW 264.7 cells. J. Food Drug Anal. 2018, 26, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-H.; Lee, A.-R.; Yang, C.-H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis Georgi. Biosci. Biotech. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Dogan, Z.; Telli, G.; Tel, B.C.; Saracoglu, I. Scutellaria brevibracteata Stapf and active principles with anti-inflammatory effects through regulation of NF-κB/COX-2/iNOS pathways. Fitoterapia 2022, 158, 105159. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Joshee, N.; Chinni, S.; Rimando, A.; Mittal, S.; Sethi, S.; Yadav, A. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-κB signaling. J. Neurooncol. 2011, 101, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Mamadalieva, N.Z.; Ovidi, E.; Triggiani, D.; Yuldasheva, N.K.; Ul’chenko, N.T.; Glushenkova, A.I.; Tiezzi, A. Lipid fraction from Scutellaria ramosissima tested on the microtubular array of cancer cell models. Arch. Biomed. Sci. 2014, 2, 11–17. [Google Scholar]
- Jia, X.; Zhang, C.; Bao, J.; Wang, K.; Tu, Y.; Wan, J.-B.; He, C. Flavonoids from Rhynchosia minima root exerts anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 cells via MAPK/NF-κB signaling pathway. Inflammopharmacology 2020, 28, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Han, Q.-T.; Zhang, L.; Dai, S.-J. Two new flavanone glycosides from Scutellaria galericulata with anti-inflammatory activities. Phytochem. Lett. 2017, 20, 151–154. [Google Scholar] [CrossRef]
- Han, Q.-T.; Ren, Y.; Li, G.-S.; Xiang, K.-L.; Dai, S.-J. Flavonoid alkaloids from Scutellaria moniliorrhiza with anti-inflammatory activities and inhibitory activities against aldose reductase. Phytochemistry 2018, 152, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agricul. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The use of Chinese Skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals 2021, 11, 1039. [Google Scholar] [CrossRef]
- Yimam, M.; Zhao, Y.; Ma, W.; Jia, Q.; Do, S.-G.; Shin, J.-H. 90-day oral toxicity study of UP446, a combination of defined extracts of Scutellaria baicalensis and Acacia catechu, in rats. Food Chem. Toxicol. 2010, 48, 1202–1209. [Google Scholar] [CrossRef]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet. Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Burnett, B.; Jia, Q.; Zhao, Y.; Levy, R. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshee, N.; Parajuli, P.; Medina-Bolivar, F.; Rimando, A.M.; Yadav, A.K. Scutellaria biotechnology: Achievements and future prospects. In Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca; Academic Press: Clug-Napoca, Romania, 2010; pp. 50–71. [Google Scholar]
- Li, C.-Y.; Wang, Q.; Wang, X.; Li, G.; Shen, S.; Wei, X. Scutellarin inhibits the invasive potential of malignant melanoma cells through the suppression of epithelial-mesenchymal transition and angiogenesis via the PI3K/Akt/mTOR signaling pathway. Eur. J. Pharmacol. 2019, 858, 172463. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Saleem, A.; Hamid, I.; Alsharif, K.F.; Abdel-Daim, M.M.; Rahman, M.H.; Shah, M.; Sohail, K.; Javaid, Z. Contemporary uses of old folks: The immunomodulatory and toxic potential of fenbufen. Cell. Mol. Biol. 2021, 67, 27–37. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Qaiser, M. Efforts on conservation and sustainable use of medicinal plants of Pakistan. Pak. J. Bot 2011, 43, 5–10. [Google Scholar]
- Zaffini, R.; Gotte, G.; Menegazzi, M. Asthma and poly (ADP-ribose) polymerase inhibition: A new therapeutic approach. Drug Des. Devel. Ther. 2018, 12, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Saradna, A.; Ratan, R.; Ke, X.; Tu, W.; Do, D.C.; Hu, C.; Gao, P. RhoA/Rho-kinases in asthma: From pathogenesis to therapeutic targets. Clin. Transl. Immunol. 2020, 9, 1134. [Google Scholar] [CrossRef]

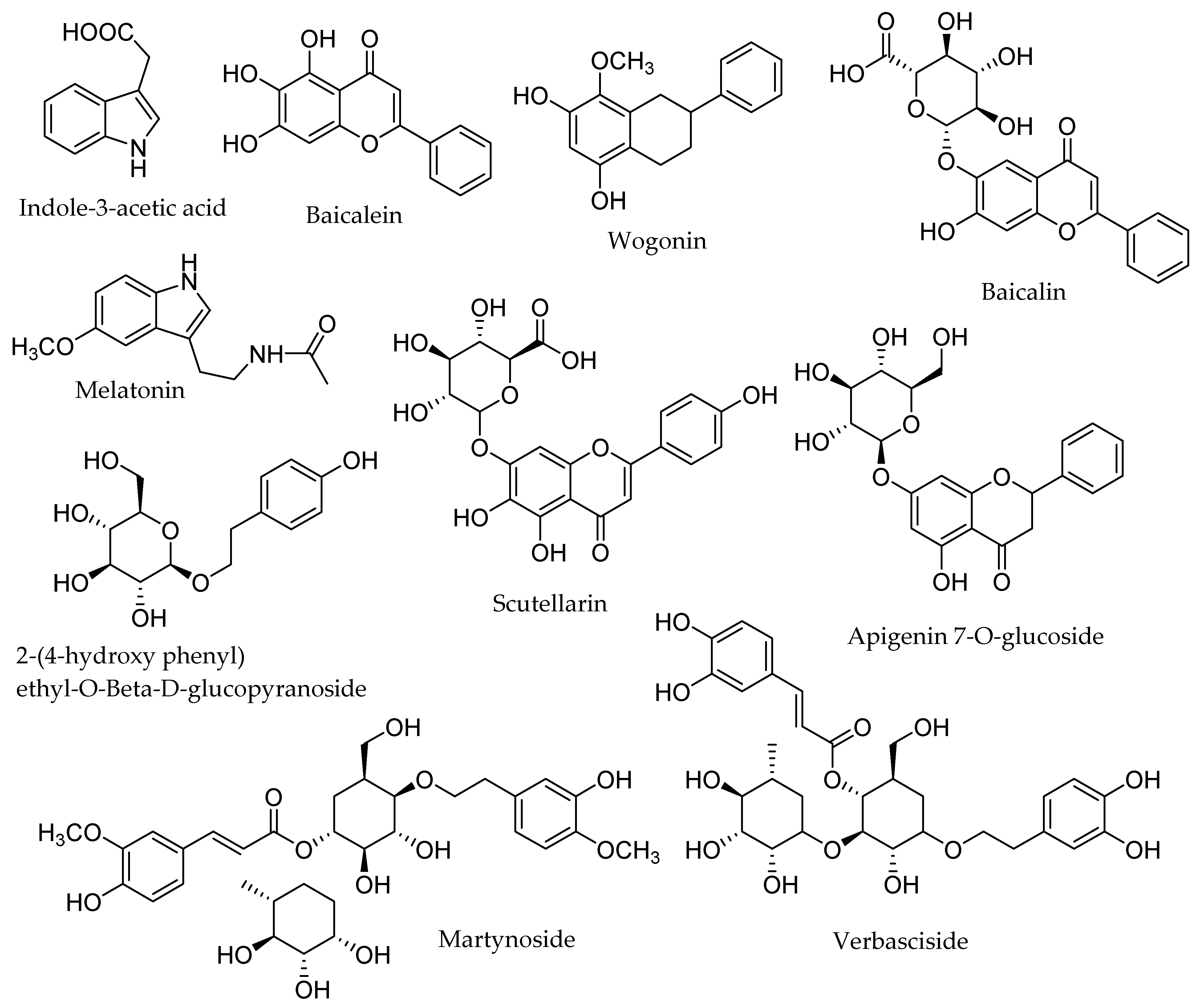


| Compounds | Classification | Species | Biological Activities |
|---|---|---|---|
| Aurantiamide acetate | Alkaloid | S. rivularis [21] | Anti-tumor, anti-stress, hypo-glycemic [22] |
| Acetoside | Glycoside | S. albida [23] | Antioxidant, cytotoxic [24] |
| Ajugapitin | Terpenoid | S. lateriflora [25] | Antiproliferative [26] |
| Barbatin A | Flavonoid | S. barbata [27] | Antimicrobial [28] |
| Dihydrocatalpol | Terpenoid | S. albida [29] | Antioxidant, anti-inflammatory [30] |
| Galloyl-O-glucose | Flavonoid | S. patonii [31] | Anticancer, antidiabetic [32] |
| Gallocatechin | Flavonoid | S. tomentosa [31] | Anti-inflammatory, anti-diabetic, antioxidant [33] |
| Gardoside | Glycoside | S. albida [23] | Antioxidant, antifungal, anti-inflammatory [34] |
| Kaempferol-3,7-di-O-rhamnoside | Flavonoid | S. condensata [31] | Antiproliferative, antiangiogenic [35] |
| Lupulin B | Terpenoid | S. linearis [36] | Antimicrobial [37] |
| Leucosceptoside A | Phenol | S. baicalensis [38] | Antimicrobial [39] |
| Lupulin A | Terpenoid | S. linearis [36] | Antioxidant, anti-inflammatory, anticancer [40] |
| Leucosceptoside A | Phenol | S. baicalensis [41] | Antioxidant, antibacterial [39] |
| Myricetin-3’-methyl Ether | Flavonoid | S. patonii [31] | Anti-inflammatory, anticarcinogenic [42] |
| Macfadienoside | Glycoside | S. albida [23] | Anti-inflammatory [43] |
| Melatonin (N-acetyl-5-methoxytryptamine) | ------- | S. leteriflora [44] | Circadian rhythm dysfunction activity [45] |
| Pinocembrin | Flavonoid | S. altissima [46] | Antimicrobial, anti-inflammatory, antioxidant, anticancer [47] |
| Procyanidin B1 | Flavonoid | S. pinnatifida [31] | Antioxidant, antibacterial [8] |
| Quercetin-3-O-rutinoside | Flavonoid | S. patonii [31] | Anti-inflammatory, antimicrobial, antioxidant [48] |
| Scutellaprostin F | Flavonoid | S. prostrata [49] | Antioxidant, antimicrobial [50] |
| Scutellone D | Terpenoid | S. rivularis [51] | Anticancer, anti-inflammation [52] |
| Scutalpin C | Terpenoid | S. alpina [53] | Antibacterial, antiviral [54] |
| Scutellarein-7-O- neohesperidoside | Flavonoid | S. multicaulis [31] | Antioxidant, antimicrobial [55] |
| Scuteamoenin | Flavonoid | S. amoena [56] | Antioxidant, anti-inflammatory [57] |
| Skullcap flavone I | Flavonoid | S.baicalensis [58] | Antioxidant, anti-microbial, anti-inflammatory [9] |
| Scuteamoenoside | Flavonoid | S. amoena [56] | Anti-inflammatory [59] |
| Saponarin | Flavonoid | S. condensata [31] | Anticonvulsant [60] |
| Scutellaprostin A | Flavonoid | S. prostrata [61] | Antiinflammation, anti-cancer [62] |
| Scutebarbatine B | Terpenoid | S. barbata [63] | Antioxidant, antimicrobial, cytotoxic [27] |
| Scutecolumnin A | Terpenoid | S. albida [29] | Anti-inflammatory [64] |
| Scuteamoenin | Flavonoid | S. amoena [56] | Antioxidant, anti-inflammatory [57] |
| Scutecyprol B | Terpenoid | S. rubicunda [65] | Anti-proliferative [66] |
| Scutalpin M | Terpenoid | S. alpina [67] | Antimicrobial [68] |
| Tenaxin-I | Flavonoid | S. baicalensis [69] | Antimicrobial, anti-inflammatory [70] |
| Tenaxin-I | Flavonoid | S. baicalensis [69] | Anti-inflammatory, anti-bacterial [71] |
| Wogonin | Flavonoid | S. linearis [36] | Anti-inflammatory, antioxidant [72] |
| 6-Hydroxy-4-stigmasten -3-one | Steroid | S. strigillosa [54] | Antibacterial, anticancer [73] |
| 5,7,2,6-Tetrahydroxy flavone | Flavonoid | S. baicalensis [74] | Antimicrobial, anti-angiogenic [75] |
| Compound/Extraction | Cell Lines/Animal | Dose/MTC |
|---|---|---|
| EESB | Mice | LD50 = 39.60 g/kg |
| Baicalin | Embryonic stem cell | IC50 = 135.9 mg/L |
| EESB | Rat | 2500 mg/kg |
| Wogonin | Mice | LD50 = 286.15 mg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Mubin, S.; Hassan, S.S.u.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach. Biomolecules 2022, 12, 936. https://doi.org/10.3390/biom12070936
Shah M, Mubin S, Hassan SSu, Tagde P, Ullah O, Rahman MH, Al-Harrasi A, Rehman NU, Murad W. Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach. Biomolecules. 2022; 12(7):936. https://doi.org/10.3390/biom12070936
Chicago/Turabian StyleShah, Muddaser, Sidra Mubin, Syed Shams ul Hassan, Priti Tagde, Obaid Ullah, Md. Habibur Rahman, Ahmed Al-Harrasi, Najeeb Ur Rehman, and Waheed Murad. 2022. "Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach" Biomolecules 12, no. 7: 936. https://doi.org/10.3390/biom12070936








