How Can Ice Emerge at 0 °C?
Abstract
1. Introduction
2. The Formation of Ice: Theory
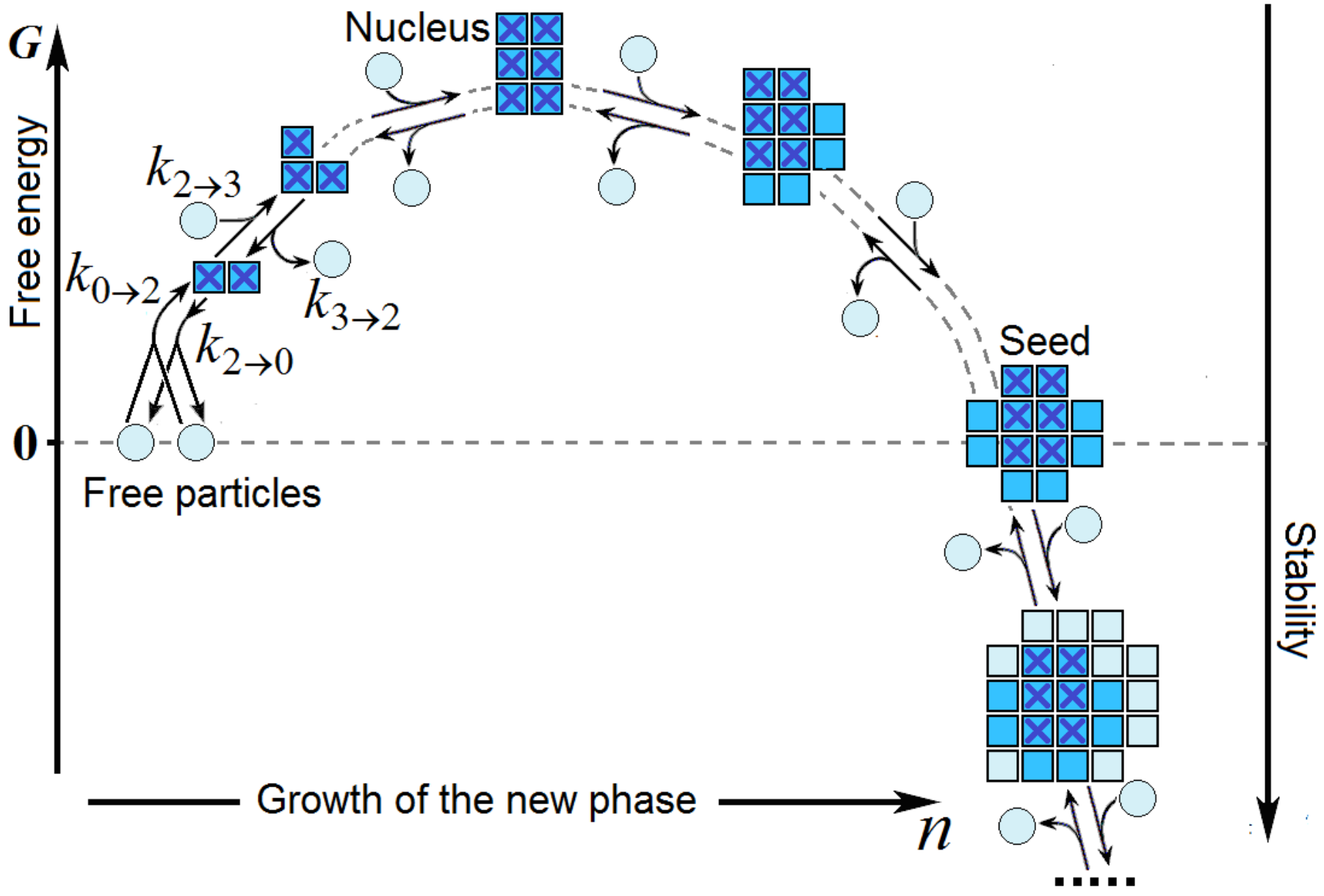
2.1. The Time Required for the Nucleation of a New Phase
2.2. The Homogeneous Nucleation of Ice: Ice Formation in Bulk Water
2.3. The Time of Ice Growth after the Nucleation
2.4. The Heterogeneous Nucleation of Ice: Ice Formation at a Surface
3. The Ice-Nucleating Surfaces
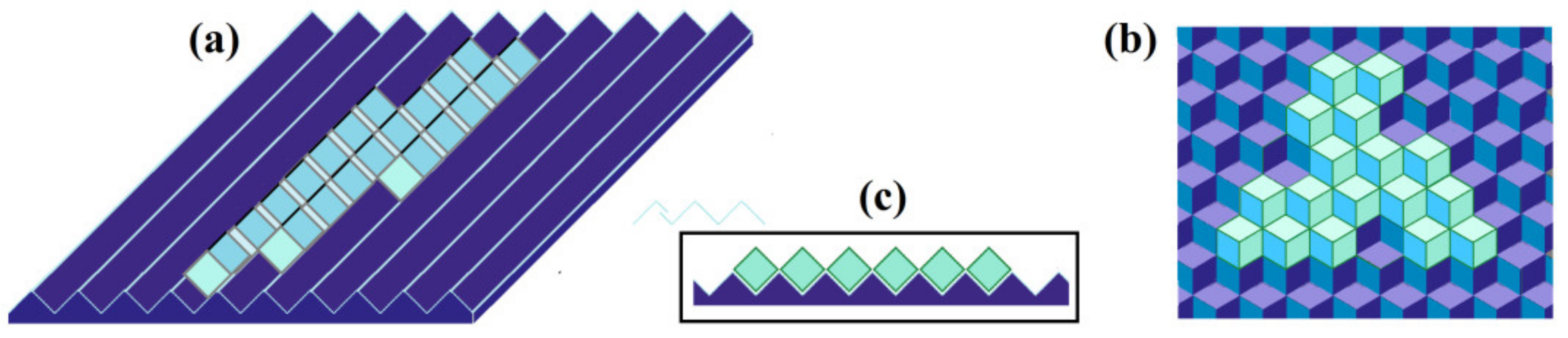
3.1. The Simplest Mechanistic Model
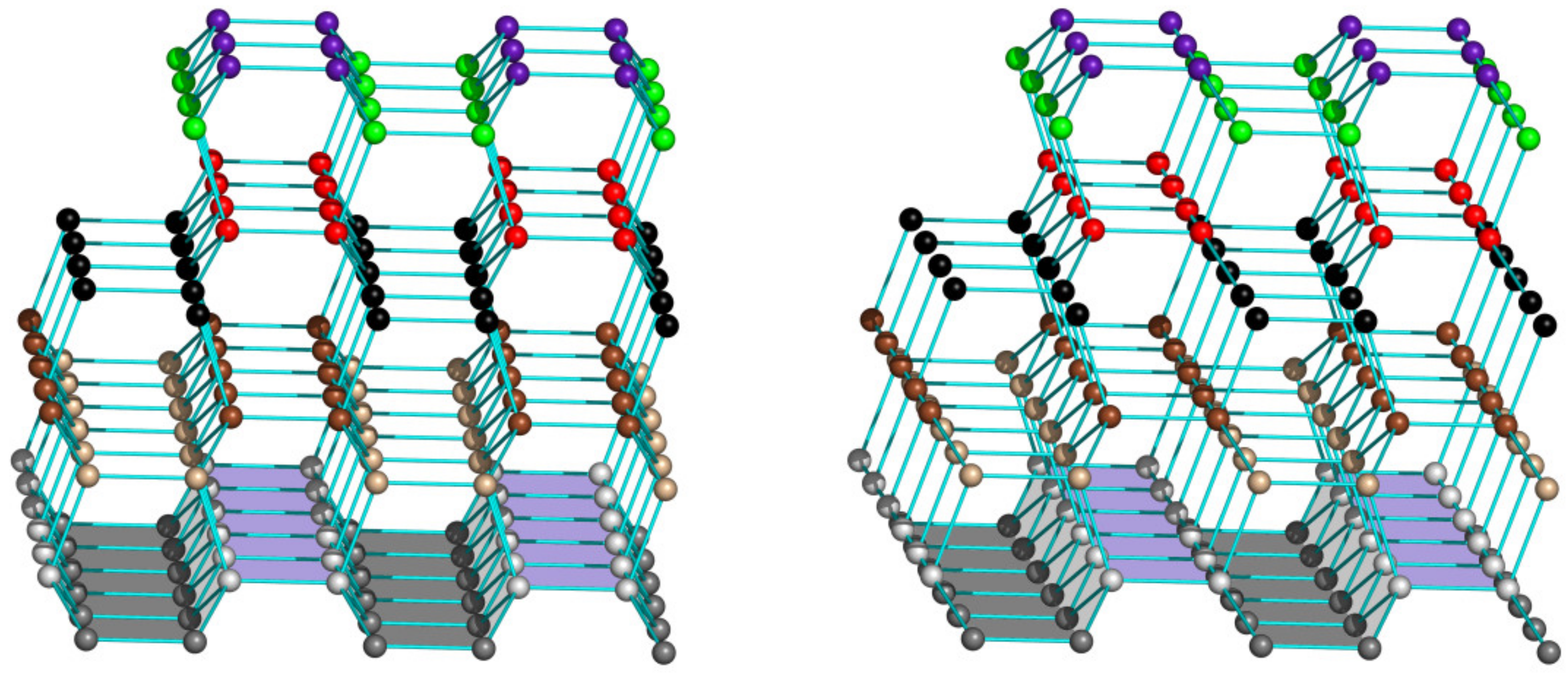
3.2. The Atomic Structure of Ice Has Different Ways of Attachment to Different Ice-Binding Surfaces
4. A Brief Overview of Experiments on Ice Nucleation at High Subzero Temperatures
| Basic Liquid | Added Nucleator | Ice-Binding (Antifreeze) Protein | Freezing (Ice-Nucleating) Temperature | Reference |
|---|---|---|---|---|
| Sodium phosphate buffer 20 mM pH 7, volumes 0.5 and 1.0 mL | - | - | −8–−10 °C | Our experiment |
| - | mIBP83 | −8–−9 °C | Our experiment | |
| AgI | - | −3–−3.5 °C | [31,32] | |
| AgI | No experiment | No experiment | - | |
| CuO | - | −5–−7 °C | Our experiment | |
| CuO | mIBP83 | −9–−11 °C | Our experiment | |
| P. syringae | - | −1–−2 °C | [41], our experiment | |
| P. syringae | mIBP83 | −4–−7 °C | Our experiment |
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ubbelohde, A.R. Melting and Crystal Structure, 1st ed.; Clarendon Press: Oxford, UK, 1965; Chapter 14. [Google Scholar]
- Chernov, A.A. Modern Crystallography III: Crystal Growth; Springer: Berlin/Heidelberg, Germany, 1984; Part 1, Chapters 3–6; p. 8. [Google Scholar] [CrossRef]
- Frenkel, J. Kinetic Theory of Liquids; Dover Publications: Dover, UK, 1955; Chapters 6 and 7. [Google Scholar]
- Slezov, V.V. Kinetics of First Order Phase Transitions, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009; Chapter 3. [Google Scholar]
- Strandburg, K.J. Two-dimensional melting. Rev. Mod. Phys. 1988, 60, 161–207. [Google Scholar] [CrossRef]
- Eyring, H.J. The activated complex in chemical reactions. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Emanuel, N.M.; Knorre, D.G. Chemical Kinetics: Homogeneous Reactions, 2nd ed.; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Ruckenstein, E.; Berim, G. Kinetic Theory of Nucleation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Zeldovich, J.B. Theory of the formation of a new phase. Cavitation. J. Exp. Theor. Phys. 1942, 12, 525–538. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics on CD; CRC Press: Boca Raton, FL, USA, 2005; Chapter 2; p. 6. [Google Scholar]
- Finkelstein, A.V. Time to overcome the high, long and bumpy free-energy barrier in a multi-stage process: The generalized steady-state approach. J. Phys. Chem. B 2015, 119, 158–163. [Google Scholar] [CrossRef]
- Hillig, W.B. Measurement of interfacial free energy for ice/water system. J. Cryst. Growth 1998, 183, 463–468. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W., Jr. Enthalpies of sublimation of organic and organometallic compounds. 1910–2001. J. Phys. Chem. Ref. Data 2002, 31, 537–698. [Google Scholar] [CrossRef]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Bull, S.J.; Wills, R.H.; Christenson, H.K.; Murray, E.J. Kinetics of the homogeneous freezing of water. Phys. Chem. Chem. Phys. 2010, 12, 10380–10387. [Google Scholar] [CrossRef]
- Finkelstein, A.V. Some peculiarities of water freezing at small sub-zero temperatures. arXiv 2020, arXiv:2008.13682. [Google Scholar]
- Melnik, B.S.; Glukhova, K.A.; Sokolova, E.A.; Balalaeva, I.V.; Finkelstein, A.V. A novel view on the mechanism of biological activity of antifreeze proteins. BioRxiv 2021. [Google Scholar] [CrossRef]
- Melnik, B.S.; Finkelstein, A.V. Physical basis of functioning of antifreeze protein. Mol. Biol. 2022, 56, 297–305. [Google Scholar] [CrossRef]
- Koop, T.; Murray, B.J. A physically constrained classical description of the homogeneous nucleation of ice in water. J. Chem. Phys. 2016, 145, 211915. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ishikawa, M.; Toyomasu, T.; Aoki, T.; Price, W.S. Ice nucleation activity in various tissues of Rhododendron flower buds: Their relevance to extraorgan freezing. Front. Plant Sci. 2015, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Yamazaki, H.; Saruwatari, A.; Murakawa, H.; Sekozawa, Y.; Kuchitsu, K.; Price, W.S.; Ishikawa, M. High ice nucleation activity located in blueberry stem bark is linked to primary freeze initiation and adaptive freezing behaviour of the bark. AoB PLANTS 2014, 6, plu044. [Google Scholar] [CrossRef] [PubMed]
- Smit, W.J.; Bakker, H.J. The surface of ice is like supercooled liquid water. Angew. Chem. Int. Ed. 2017, 56, 15540–15544. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, W.M.; Hobbs, P.V. An experimental determination of the surface energies of ice. Phil. Mag. 1969, 19, 1161–1173. [Google Scholar] [CrossRef]
- Pallas, N.R.; Harrison, Y. An automated drop shape apparatus and the surface tension of pure water. Colloids Surf. 1990, 43, 169–194. [Google Scholar] [CrossRef]
- Rottger, K.; Endriss, A.; Ihringer, J.; Doyle, S.; Kuhs, W.F. Lattice constants and thermal expansion of H2O and D2O ice Ih between 10 and 265 K. Acta Cryst. B 1994, 50, 644–648. [Google Scholar] [CrossRef]
- Lin, C.; Corem, G.; Godsi, O.; Alexandrowicz, G.; Darling, G.R.; Hodgson, A. Ice nucleation on a corrugated surface. J. Am. Chem. Soc. 2018, 140, 15804–15811. [Google Scholar] [CrossRef]
- Wood, L.A. The use of dew-point temperature in humidity calculations. J. Res. Natl. Bur. Stand. 1970, 74, 117–122. [Google Scholar] [CrossRef]
- Site “Dew Point”. Available online: https://en.wikipedia.org/wiki/Dew_point (accessed on 7 May 2022).
- Lawrence, M.G. The relationship between relative humidity and the dewpoint temperature in moist air: A simple conversion and applications. Bull. Amer. Meteor. Soc. 2005, 86, 225–233. [Google Scholar] [CrossRef]
- Faraday, M. Experimental Researches in Chemistry and Physics; Taylor and Francis: London, UK, 1859; pp. 372–382. [Google Scholar]
- Vonnegut, K. Cat’s Cradle; Dell Publishing: New York, NY, USA, 1963. [Google Scholar]
- Vonnegut, B. The nucleation of ice formation by silver iodide. J. Appl. Phys. 1947, 18, 593–595. [Google Scholar] [CrossRef]
- Marcolli, C.; Nagare, B.; Welti, A.; Lohmann, U. Ice nucleation efficiency of AgI: Review and new insights. Atmos. Chem. Phys. 2016, 16, 8915–8937. [Google Scholar] [CrossRef]
- Chong, E.; Marak, K.F.; Li, Y.; Freedman, M.A. Ice nucleation activity of iron oxides via immersion freezing and an examination of the high ice nucleation activity of FeO. Phys. Chem. Chem. Phys. 2021, 23, 3565–3573. [Google Scholar] [CrossRef]
- Head, R. Steroids as ice nucleators. Nature 1961, 191, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Power, B.; Power, R. Some amino-acids as ice nucleators. Nature 1962, 194, 1170–1171. [Google Scholar] [CrossRef]
- Popovitz-Biro, R.; Wang, J.L.; Majewski, J.; Shavit, E.; Leiserowitz, L.; Lahav, M. Induced freezing of supercooled water into ice by self-assembled crystalline monolayers of amphiphilic alcohols at the air-water interface. J. Am. Chem. Soc. 1994, 116, 1179–1191. [Google Scholar] [CrossRef]
- Knopf, D.A.; Alpert, P.A.; Wang, B. The role of organic aerosol in atmospheric ice nucleation: A review. ACS Earth Space Chem. 2018, 2, 168–202. [Google Scholar] [CrossRef]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice nucleation induced by Pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar] [CrossRef]
- Gurian-Sherman, D.; Lindow, S.E. Bacterial ice nucleation: Significance and molecular basis. FASEB J. 1993, 7, 1338–1343. [Google Scholar] [CrossRef]
- Gute, E.; Abbatt, J.P.D. Ice nucleating behavior of different tree pollen in the immersion mode. Atmos. Environ. 2020, 231, 117488. [Google Scholar] [CrossRef]
- Majorina, M.A.; Veselova, V.R.; Melnik, B.S. The influence of Pseudomonas syringae on water freezing and ice melting. PLoS ONE 2022, 17, e0265683. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Schwidetzky, R.; Eufemio, R.J.; Bonn, M.; Meister, K. Toward understanding bacterial ice nucleation. J. Phys. Chem. B 2022, 126, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Jorov, A.; Zhorov, B.S.; Yang, D.S.C. Theoretical study of interaction of winter flounder antifreeze protein with ice. Protein Sci. 2004, 13, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Surís-Valls, R.; Voets, I.K. The impact of salts on the ice recrystallization inhibition activity of antifreeze (glyco)proteins. Biomolecules 2019, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Nagae, I.; Obata, H. purification and characterization of a new anti-nucleating protein isolated from Acinetobacter calcoaceticus KINI-1. Biocontrol Sci. 1996, 1, 11–17. [Google Scholar] [CrossRef][Green Version]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef]
- Raymond, J.A.; Christner, B.C.; Schuster, S.C. A bacterial ice-binding protein from the Vostok ice core. Extremophiles 2008, 12, 713–717. [Google Scholar] [CrossRef]
- Hoshino, T.; Kiriaki, M.; Ohgiya, S.; Fujiwara, M.; Kondo, H.; Nishimiya, Y.; Yumoto, I.; Tsuda, S. Antifreeze proteins from snow mold fungi. Can. J. Bot. 2003, 81, 1175–1181. [Google Scholar] [CrossRef]
- Griffith, M.; Ala, P.; Yang, D.S.; Hon, W.C.; Moffatt, B.A. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef]
- Duman, J.G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef]
- Leinala, E.K.; Davies, P.L.; Doucet, D.; Tyshenko, M.G.; Walker, V.K.; Jia, Z. A β-helical antifreeze protein isoform with increased activity. J. Biol. Chem. 2002, 277, 33349–33352. [Google Scholar] [CrossRef] [PubMed]
- Leinala, E.K.; Davies, P.L.; Jia, Z. Crystal structure of β-helical antifreeze protein points to a general ice binding model. Structure 2002, 10, 619–627. [Google Scholar] [CrossRef]
- Doucet, D.; Tyshenko, M.G.; Kuiper, M.J.; Graether, S.P.; Sykes, B.D.; Daugulis, A.J.; Davies, P.L.; Walker, V.K. Structure-function relationships in spruce budworm antifreeze protein revealed by isoform diversity. Eur. J. Biochem. 2000, 267, 6082–6088. [Google Scholar] [CrossRef]
- Glukhova, K.A.; Okulova, J.D.; Melnik, B.S. Designing and studying a mutant form of the ice-binding protein from Choristoneura fumiferana. BioRxiv 2020. [Google Scholar] [CrossRef]
- Deeva, A.A.; Glukhova, K.A.; Isoyan, L.S.; Okulova, Y.D.; Uversky, V.N.; Melnik, B.S. Design and analysis of a mutant form of the ice-binding protein from Choristoneura fumiferana. Protein J. 2022, 41, 304–314. [Google Scholar] [CrossRef]
- Testa, B.; Hill, T.C.J.; Marsden, N.A.; Barry, K.R.; Hume, C.C.; Bian, Q.; Uetake, J.; Hare, H.; Perkins, R.J.; Möhler, O.; et al. Ice nucleating particle connections to regional Argentinian land surface emissions and weather during the Cloud, Aerosol, and Complex Terrain Interactions experiment. J. Geophys. Res. Atmos. 2021, 126, e2021JD035186. [Google Scholar] [CrossRef]
- Kumar, A.; Marcolli, C.; Peter, T. Ice nucleation activity of silicates and aluminosilicates in pure water and aqueous solutions—Part 2: Quartz and amorphous silica. Atmos. Chem. Phys. 2019, 19, 6035–6058. [Google Scholar] [CrossRef]
- Veselova, V.R.; Majorina, M.A.; Melnik, B.S. An experimental technique for accurate measurement of the freezing point of solutions and ice melting in the presence of biological objects on the examples of P. syringae and E. coli. Opera Med. Physiol. 2022, 9, 19–30. [Google Scholar] [CrossRef]


| Volume (V) | Water Molecules in the Volume, NV | |||
|---|---|---|---|---|
| −35 °C: ∆T = 35° | −40 °C: ∆T = 40° | −50 °C: * ∆T = 50° | ||
| A lake 30,000 m3 | 1033 | 100 years | 0.01 s | 10−16 s |
| A glass 30 cm3 | 1024 | 1011 years | 1 year | 10−6 s |
| An eukaryotic cell or a small water droplet [14] (≈30 μm)3 | 1015 | 1020 years | 109 years | 10 min |
| Volume (V) | Number of Water Molecules | |||
|---|---|---|---|---|
| In the Volume, NV | On the Surface, NS | −3 °C †: ∆T = 3 ° | −5 °C †: ∆T = 5 ° | |
| A lake 30,000 m3 | 1033 | NS_max = 1030 NS_min ≈ 1022 | 0.3 s 1 year | 3 × 10−16 s * 3 × 10−8 s |
| A glass 30 cm3 | 1024 | NS_max = 1021 NS_min ≈ 1016 | 10 years 106 years | 3 × 10−7 s * 0.03 s |
| A test tube 1 cm3 | 3⋅1022 | NS_max = 3 × 1019 NS_min ≈ 1015 | 300 years 107 years | 3 × 10−5 s * 1 s |
| An eukaryotic cell (30 μm)3 | 1015 | NS_max = 1012 NS_min ≈ 1010 | 1010 years 1012 years | 5 min 500 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finkelstein, A.V.; Garbuzynskiy, S.O.; Melnik, B.S. How Can Ice Emerge at 0 °C? Biomolecules 2022, 12, 981. https://doi.org/10.3390/biom12070981
Finkelstein AV, Garbuzynskiy SO, Melnik BS. How Can Ice Emerge at 0 °C? Biomolecules. 2022; 12(7):981. https://doi.org/10.3390/biom12070981
Chicago/Turabian StyleFinkelstein, Alexei V., Sergiy O. Garbuzynskiy, and Bogdan S. Melnik. 2022. "How Can Ice Emerge at 0 °C?" Biomolecules 12, no. 7: 981. https://doi.org/10.3390/biom12070981
APA StyleFinkelstein, A. V., Garbuzynskiy, S. O., & Melnik, B. S. (2022). How Can Ice Emerge at 0 °C? Biomolecules, 12(7), 981. https://doi.org/10.3390/biom12070981







