Targeting Oxidative Stress Involved in Endometriosis and Its Pain
Abstract
:1. Introduction
2. Endometriosis and Oxidative Stress
2.1. General Oxidative Stress
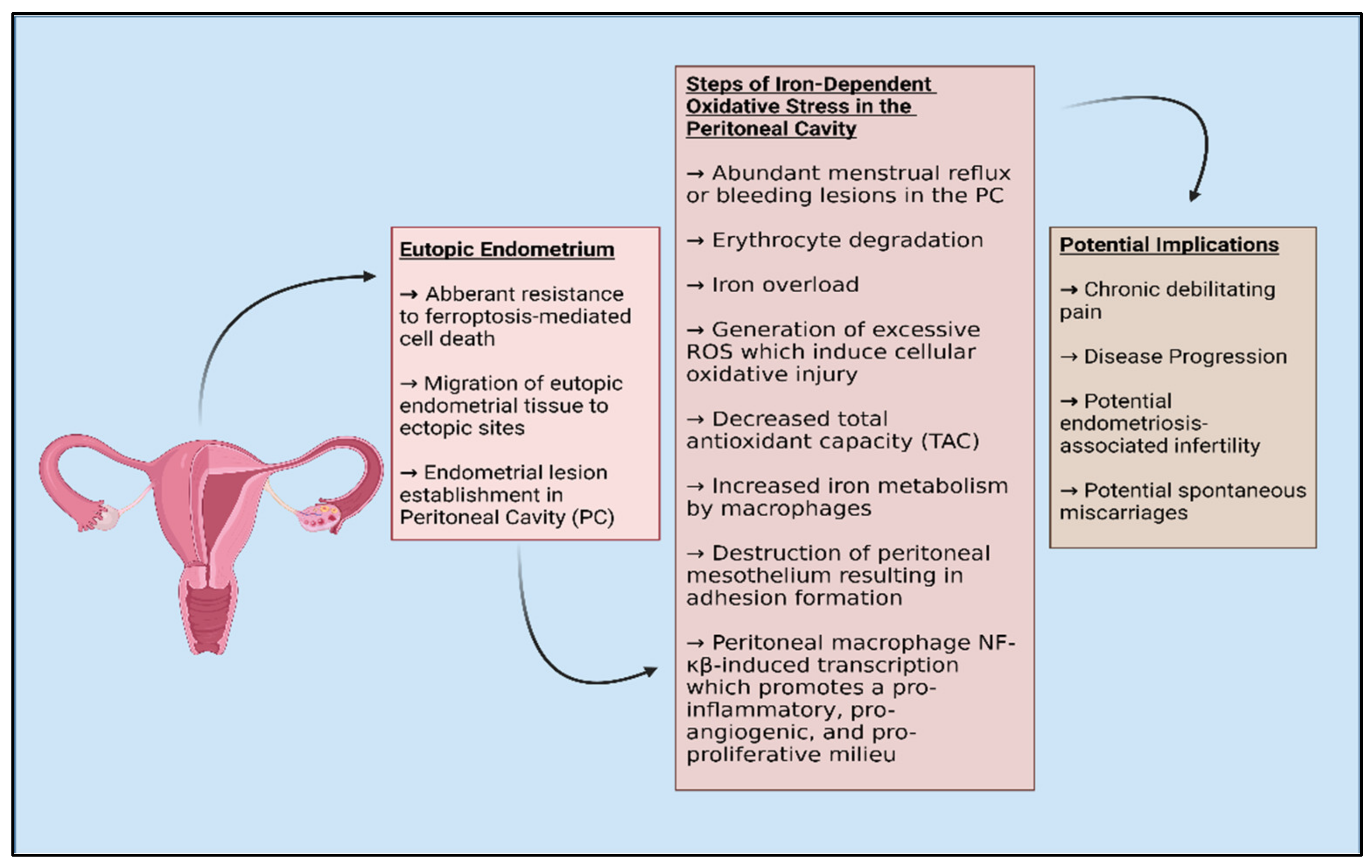
2.2. Endometriosis and Oxidative Stress
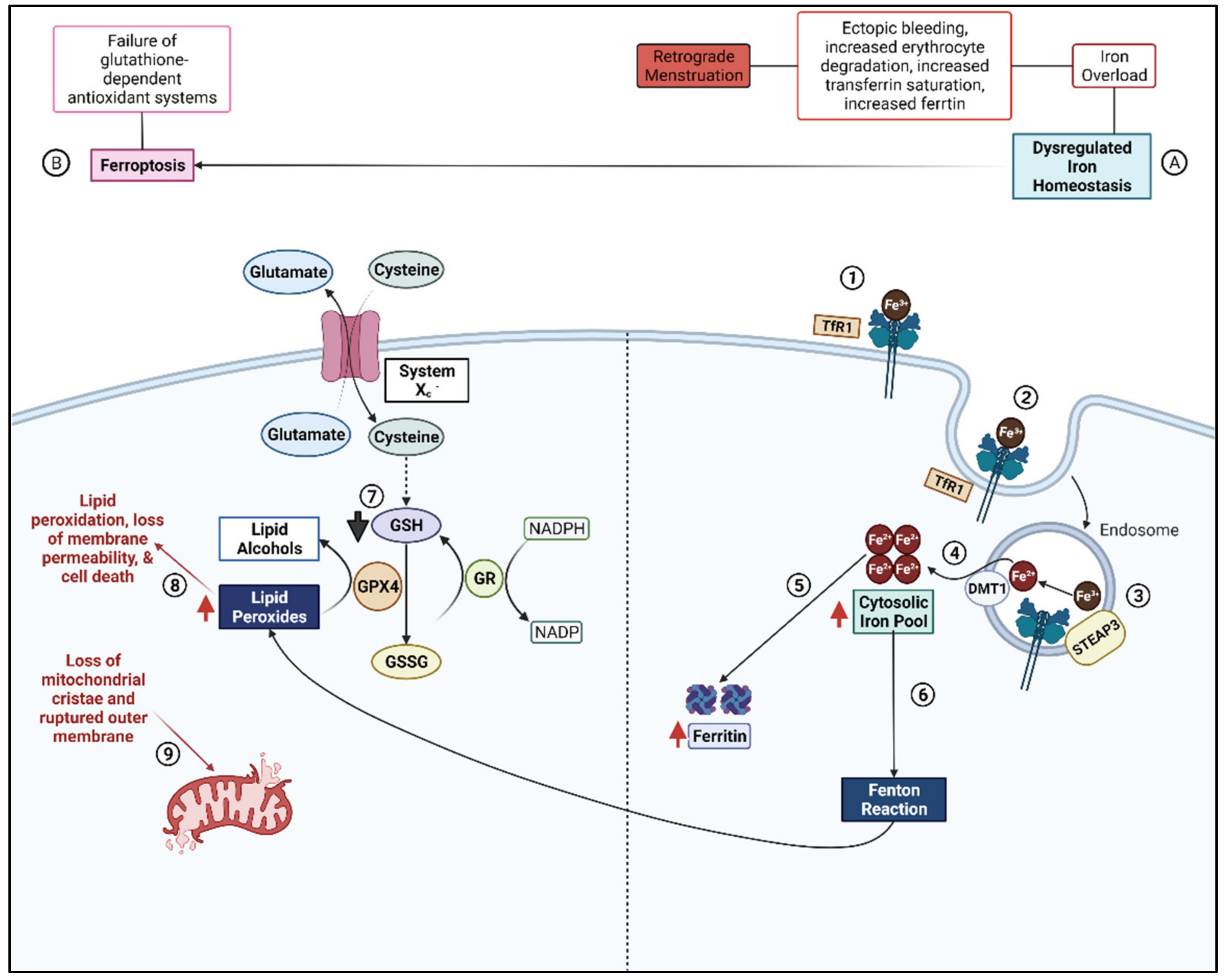
2.3. Ferroptosis
3. Endometriosis and Pain
3.1. Mechanisms of Pain
3.1.1. Macrophages
3.1.2. Neurogenic Inflammation
3.1.3. Lipid Peroxides and Pain-Inducing Prostaglandins
4. Treatments for Oxidative Stress
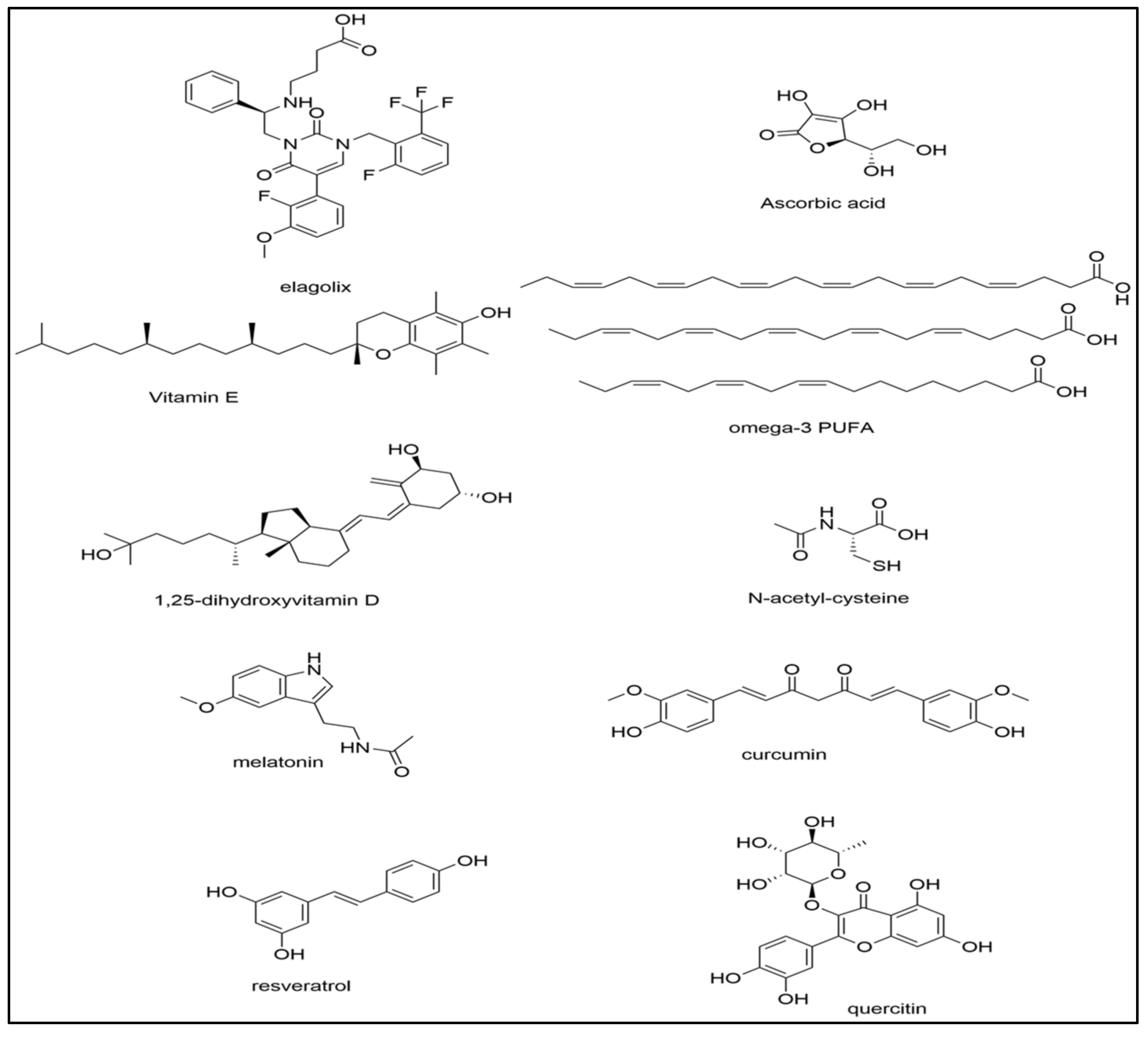
4.1. Vitamins C and E
4.2. Vitamin D and Omega-3 Fatty Acids
4.3. N-Acetylcysteine
4.4. Melatonin
4.5. Curcumin
4.6. Alternative Treatment Options
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mechsner, S. Endometriosis, an Ongoing Pain-Step-by-Step Treatment. J. Clin. Med. 2022, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Members of the Endometriosis Guideline Core Group; Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Tsamantioti, E.S.; Mahdy, H. Endometriosis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Martel, E.; Missmer, S.A.; Clauw, D.J.; Harte, S.E.; As-Sanie, S.; Sieberg, C.B. Pelvic floor, abdominal and uterine tenderness in relation to pressure pain sensitivity among women with endometriosis and chronic pelvic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 247–253. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2021, 23, 333–355. [Google Scholar] [CrossRef]
- Greene, A.D.; Lang, S.A.; Kendziorski, J.A.; Sroga-Rios, J.M.; Herzog, T.J.; Burns, K.A. Endometriosis: Where are we and where are we going? Reproduction 2016, 152, R63–R78. [Google Scholar] [CrossRef] [Green Version]
- Duffy, J.; Hirsch, M.; Vercoe, M.; Abbott, J.; Barker, C.; Collura, B.; Drake, R.; Evers, J.; Hickey, M.; Horne, A.W.; et al. A core outcome set for future endometriosis research: An international consensus development study. BJOG 2020, 127, 967–974. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, M.I.; Romano, M.; Basarali, M.K.; Elzagallaai, A.; Karaman, M.; Demir, Z.; Demir, M.F.; Akcay, F.; Seyrek, M.; Haksever, N.; et al. The Effect of Corrected Inflammation, Oxidative Stress and Endothelial Dysfunction on Fmd Levels in Patients with Selected Chronic Diseases: A Quasi-Experimental Study. Sci. Rep. 2020, 10, 9018. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? Jpn. Med. Assoc. J. 2002, 45, 271–276. [Google Scholar]
- Showell, M.G.; Brown, J.; Clarke, J.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2013, 8, CD007807. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Wilson, C.; Munoz-Palma, E.; Gonzalez-Billault, C. From birth to death: A role for reactive oxygen species in neuronal development. Semin. Cell Dev. Biol. 2018, 80, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Meilhac, O.; Ramachandran, S.; Chiang, K.; Santanam, N.; Parthasarathy, S. Role of arterial wall antioxidant defense in beneficial effects of exercise on atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1681–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavinato, L.; Genise, E.; Luly, F.R.; Di Domenico, E.G.; Del Porto, P.; Ascenzioni, F. Escaping the Phagocytic Oxidative Burst: The Role of SODB in the Survival of Pseudomonas aeruginosa Within Macrophages. Front. Microbiol. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, J.; Lai, H.; Lai, Y.; Meng, G.; Wang, Z.; Zhou, Z.; Chen, H.; Yu, Z.; Li, S.; et al. Vagus Nerve Stimulation Ameliorates Renal Ischemia-Reperfusion Injury through Inhibiting NF-kappaB Activation and iNOS Protein Expression. Oxid. Med. Cell Longev. 2020, 2020, 7106525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- El-Benna, J.; Dang, P.M.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Braut-Boucher, F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: Structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009, 41, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Lingappan, K. NF-kappaB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX Activation by Subunit Interaction and Underlying Mechanisms in Disease. Front. Cell Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.; Boloor, K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra, E.O.T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Status of oxidative stress in rheumatoid arthritis. Int. J. Rheum. Dis. 2009, 12, 29–33. [Google Scholar] [CrossRef]
- Wojcik, P.; Gegotek, A.; Zarkovic, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Bolner, A.; Pilleri, M.; De Riva, V.; Nordera, G.P. Plasma and urinary HPLC-ED determination of the ratio of 8-OHdG/2-dG in Parkinson’s disease. Clin. Lab. 2011, 57, 859–866. [Google Scholar]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Murphy, A.A.; Santanam, N.; Parthasarathy, S. Endometriosis: A disease of oxidative stress? Semin. Reprod. Endocrinol. 1998, 16, 263–273. [Google Scholar] [CrossRef]
- Shanti, A.; Santanam, N.; Morales, A.J.; Parthasarathy, S.; Murphy, A.A. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil. Steril. 1999, 71, 1115–1118. [Google Scholar] [CrossRef]
- Santanam, N.; Murphy, A.A.; Parthasarathy, S. Macrophages, oxidation, and endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 183–198; discussion 119–200, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Song, M.; Rong, R.; Murphy, A.A.; Parthasarathy, S. Atherosclerosis, oxidation and endometriosis. Free Radic. Res. 2002, 36, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Song, M.; Dominguez, C.E.; Walter, M.F.; Santanam, N.; Parthasarathy, S.; Murphy, A.A. Glycodelin mediates the increase in vascular endothelial growth factor in response to oxidative stress in the endometrium. Am. J. Obstet. Gynecol. 2006, 195, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, K.; Fahrmann, J.; Mitchell, B.; Paul, D.; King, H.; Crain, C.; Cook, C.; Golovko, M.; Brose, S.; Golovko, S.; et al. Oxidation-sensitive nociception involved in endometriosis-associated pain. Pain 2015, 156, 528–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, K.R.; Mitchell, B.; Santanam, N. Redox regulation of microRNAs in endometriosis-associated pain. Redox Biol. 2017, 12, 956–966. [Google Scholar] [CrossRef]
- Brunty, S.; Santanam, N. Current assessment of the (dys)function of macrophages in endometriosis and its associated pain. Ann. Transl. Med. 2019, 7, S381. [Google Scholar] [CrossRef]
- Ito, F.; Yamada, Y.; Shigemitsu, A.; Akinishi, M.; Kaniwa, H.; Miyake, R.; Yamanaka, S.; Kobayashi, H. Role of Oxidative Stress in Epigenetic Modification in Endometriosis. Reprod. Sci. 2017, 24, 1493–1502. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Dutta, M.; Joshi, M.; Srivastava, S.; Chakravarty, B.; Chaudhury, K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. BioMed Res. Int. 2013, 2013, 329058. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, S.W. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 198–203. [Google Scholar] [CrossRef]
- Wingfield, M.; Macpherson, A.; Healy, D.L.; Rogers, P.A. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril. 1995, 64, 340–346. [Google Scholar] [CrossRef]
- Song, M.; Karabina, S.A.; Kavtaradze, N.; Murphy, A.A.; Parthasarathy, S. Presence of endometrial epithelial cells in the peritoneal cavity and the mesothelial inflammatory response. Fertil. Steril. 2003, 79 (Suppl. 1), 789–794. [Google Scholar] [CrossRef]
- Jackson, L.W.; Schisterman, E.F.; Dey-Rao, R.; Browne, R.; Armstrong, D. Oxidative stress and endometriosis. Hum. Reprod. 2005, 20, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of Oxidative Stress and Severity of Endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative Stress Statues in Serum and Follicular Fluid of Women with Endometriosis. Cell J. 2017, 18, 582–587. [Google Scholar] [CrossRef]
- Murphy, A.A.; Santanam, N.; Morales, A.J.; Parthasarathy, S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J. Clin. Endocrinol. Metab. 1998, 83, 2110–2113. [Google Scholar] [CrossRef]
- Mori, M.; Ito, F.; Shi, L.; Wang, Y.; Ishida, C.; Hattori, Y.; Niwa, M.; Hirayama, T.; Nagasawa, H.; Iwase, A.; et al. Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron. Redox Biol. 2015, 6, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.H.; Choi, Y.S.; Choi, J.H. Iron-Storage Protein Ferritin Is Upregulated in Endometriosis and Iron Overload Contributes to a Migratory Phenotype. Biomedicines 2020, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Duan, H.; Wang, S.; Li, Y. Ferroptosis resistance mechanisms in endometriosis for diagnostic model establishment. Reprod. Biomed. Online 2021, 43, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, S.G.; Taylor, H.S.; Norwitz, E.R. Endometriosis: The Role of Iron Overload and Ferroptosis. Reprod. Sci. 2020, 27, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Rosa e Silva, J.C.; do Amara, V.F.; Mendonca, J.L.; Rosa e Silva, A.C.; Nakao, L.S.; Poli Neto, O.B.; Ferriani, R.A. Serum markers of oxidative stress and endometriosis. Clin. Exp. Obstet. Gynecol. 2014, 41, 371–374. [Google Scholar] [CrossRef]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.E.; Batteux, F.; Borderie, D.; Chapron, C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.J.; Twal, W.O.; Soodavar, F.; Virella, G.; Lopes-Virella, M.F.; Hammad, S.M. Heat shock protein 70B’ (HSP70B’) expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. J. Biol. Chem. 2010, 285, 15985–15993. [Google Scholar] [CrossRef] [Green Version]
- Lambrinoudaki, I.V.; Augoulea, A.; Christodoulakos, G.E.; Economou, E.V.; Kaparos, G.; Kontoravdis, A.; Papadias, C.; Creatsas, G. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil. Steril. 2009, 91, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010, 93, 2431–2432. [Google Scholar] [CrossRef]
- Nanda, A.; Thangapandi, K.; Banerjee, P.; Dutta, M.; Wangdi, T.; Sharma, P.; Chaudhury, K.; Jana, S.K. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann. Lab. Med. 2020, 40, 390–397. [Google Scholar] [CrossRef]
- Qiu, X.M.; Lai, Z.Z.; Ha, S.Y.; Yang, H.L.; Liu, L.B.; Wang, Y.; Shi, J.W.; Ruan, L.Y.; Ye, J.F.; Wu, J.N.; et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-gamma and IL-10 in endometriosis. Reproduction 2020, 159, 251–260. [Google Scholar] [CrossRef] [PubMed]
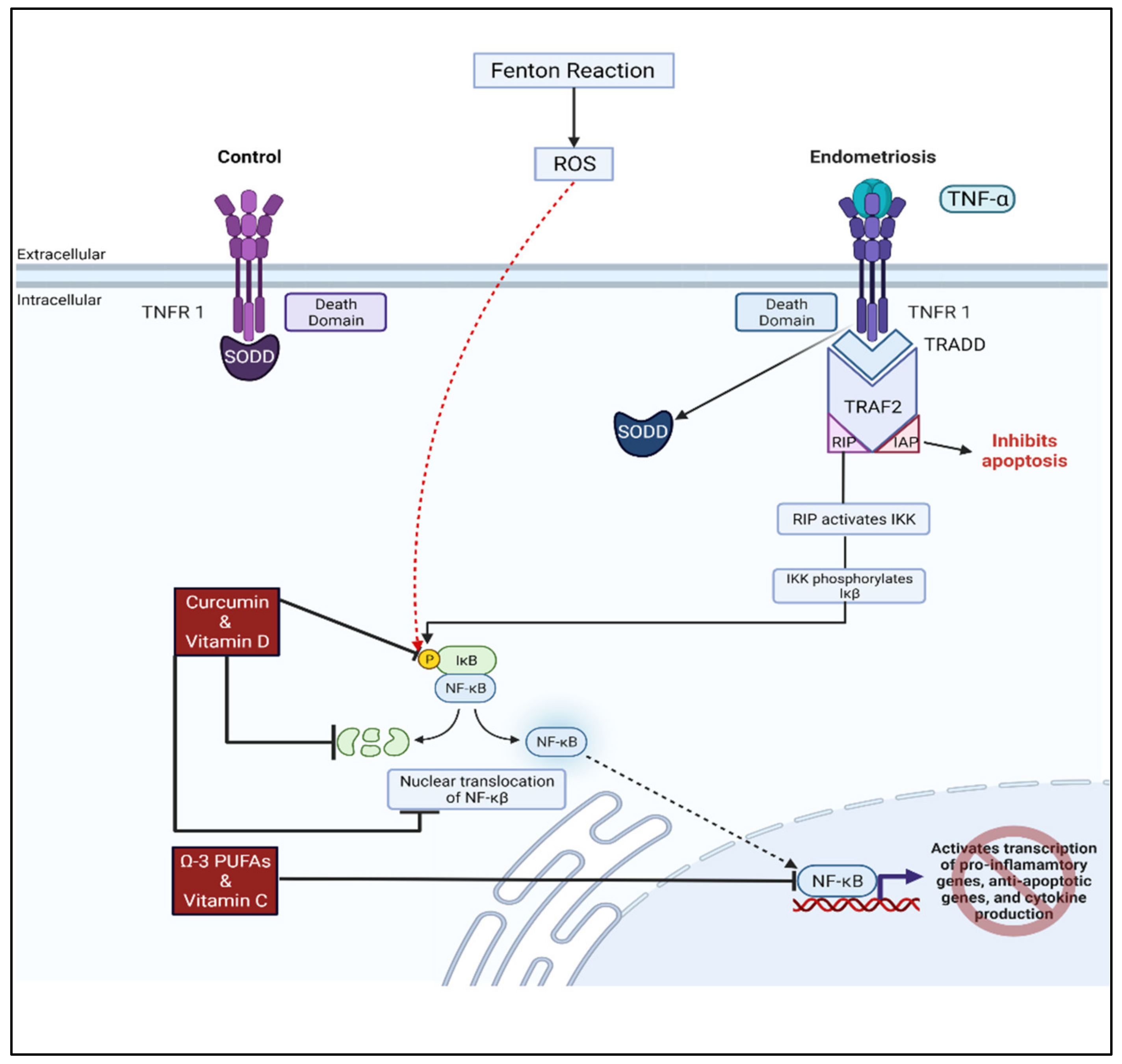
- Gonzalez-Ramos, R.; Defrere, S.; Devoto, L. Nuclear factor-kappaB: A main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil. Steril. 2012, 98, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Vallee, A.; Lecarpentier, Y. Curcumin and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Fusco, R.; et al. The Methyl Ester of 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic Acid Reduces Endometrial Lesions Development by Modulating the NFkB and Nrf2 Pathways. Int. J. Mol. Sci. 2021, 22, 3991. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-kappaB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef] [Green Version]
- Ngo, C.; Chereau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Rong, R.; Ramachandran, S.; Santanam, N.; Murphy, A.A.; Parthasarathy, S. Induction of monocyte chemotactic protein-1 in peritoneal mesothelial and endometrial cells by oxidized low-density lipoprotein and peritoneal fluid from women with endometriosis. Fertil. Steril. 2002, 78, 843–848. [Google Scholar] [CrossRef]
- Gill, K.; Kirma, N.; Gunna, V.S.; Santanam, N.; Parthasarathy, S.; Tekmal, R.R. Regulation of colony stimulating factor-1 (CSF-1) in endometrial cells: Glucocorticoids and oxidative stress regulate the expression of CSF-1 and its receptor c-fms in endometrial cells. Fertil. Steril. 2001, 76, 1005–1011. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2021, 36, 951–964. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Ralph, S.J. Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 2022, 62, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Yasui, H.; Higashikawa, K.; Miyamoto, N.; Kuge, Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS ONE 2019, 14, e0225931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Liang, Y.; Lin, H.; Dai, Y.; Yao, S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J. Neuroinflamm. 2020, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Freschi, L.; Wenger, J.M.; Streuli, I. Innovations in classical hormonal targets for endometriosis. Expert Rev. Clin. Pharmacol. 2016, 9, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.L.; Mitchell, B.L.; Santanam, N. Power over pain: A brief review of current and novel interventions for endometriosis-associated pain. J. Endometr. Pelvic Pain Disord. 2014, 6, 163–173. [Google Scholar] [CrossRef]
- Siegenthaler, F.; Knabben, L.; Mohr, S.; Nirgianakis, K.; Imboden, S.; Mueller, M.D. Visualization of endometriosis with laparoscopy and near-infrared optics with indocyanine green. Acta Obstet. Gynecol. Scand. 2020, 99, 591–597. [Google Scholar] [CrossRef]
- Abrao, M.S.; Surrey, E.; Gordon, K.; Snabes, M.C.; Wang, H.; Ijacu, H.; Taylor, H.S. Reductions in endometriosis-associated pain among women treated with elagolix are consistent across a range of baseline characteristics reflective of real-world patients. BMC Womens Health 2021, 21, 246. [Google Scholar] [CrossRef]
- Shebley, M.; Polepally, A.R.; Nader, A.; Ng, J.W.; Winzenborg, I.; Klein, C.E.; Noertersheuser, P.; Gibbs, M.A.; Mostafa, N.M. Clinical Pharmacology of Elagolix: An Oral Gonadotropin-Releasing Hormone Receptor Antagonist for Endometriosis. Clin. Pharmacokinet. 2020, 59, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.; Donnelly, C.R.; Ji, R.R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25. [Google Scholar] [CrossRef]
- Jeljeli, M.; Riccio, L.G.C.; Chouzenoux, S.; Moresi, F.; Toullec, L.; Doridot, L.; Nicco, C.; Bourdon, M.; Marcellin, L.; Santulli, P.; et al. Macrophage Immune Memory Controls Endometriosis in Mice and Humans. Cell Rep. 2020, 33, 108325. [Google Scholar] [CrossRef]
- Maddern, J.; Grundy, L.; Castro, J.; Brierley, S.M. Pain in Endometriosis. Front. Cell Neurosci. 2020, 14, 590823. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persoons, E.; Hennes, A.; De Clercq, K.; Van Bree, R.; Vriens, G.; O, D.F.; Peterse, D.; Vanhie, A.; Meuleman, C.; Voets, T.; et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int. J. Mol. Sci. 2018, 19, 2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Zhang, L.; Westlund, K.N. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol. Pain 2009, 5, 1744–8069, 1731. [Google Scholar] [CrossRef] [Green Version]
- Cunha, F.Q.; Poole, S.; Lorenzetti, B.B.; Ferreira, S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660–664. [Google Scholar] [CrossRef]
- Machairiotis, N.; Vasilakaki, S.; Thomakos, N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021, 9, 54. [Google Scholar] [CrossRef]
- Rather, L.J. Disturbance of function (functio laesa): The legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull. N. Y. Acad. Med. 1971, 47, 303–322. [Google Scholar]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, A.; Conceicao Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Liu, X.; Rong, P.; Li, S.; Guo, S.W. Reduced vagal tone in women with endometriosis and auricular vagus nerve stimulation as a potential therapeutic approach. Sci. Rep. 2021, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sharma, A.V.; Mahapatra, P.D.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.S.; Nagi, S.S.; Mahns, D.A. Minocycline reduces experimental muscle hyperalgesia induced by repeated nerve growth factor injections in humans: A placebo-controlled double-blind drug-crossover study. Eur. J. Pain 2020, 24, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Qiu, C.; Sun, Y.; Li, W.; Jiang, J.; Zhang, J.M. Fractalkine/CX3CR1 Contributes to Endometriosis-Induced Neuropathic Pain and Mechanical Hypersensitivity in Rats. Front. Cell Neurosci. 2018, 12, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Fu, Y.; Xue, S.; Ai, A.; Chen, H.; Lyu, Q.; Kuang, Y. The M2 polarization of macrophage induced by fractalkine in the endometriotic milieu enhances invasiveness of endometrial stromal cells. Int. J. Clin. Exp. Pathol. 2014, 7, 194–203. [Google Scholar]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar]
- Waiyaput, W.; Pumipichet, S.; Weerakiet, S.; Rattanasiri, S.; Sophonsritsuk, A. Effect of simvastatin on monocyte chemoattractant protein-1 expression in endometriosis patients: A randomized controlled trial. BMC Womens Health 2017, 17, 89. [Google Scholar] [CrossRef] [Green Version]
- Kunori, S.; Matsumura, S.; Okuda-Ashitaka, E.; Katano, T.; Audoly, L.P.; Urade, Y.; Ito, S. A novel role of prostaglandin E2 in neuropathic pain: Blockade of microglial migration in the spinal cord. Glia 2011, 59, 208–218. [Google Scholar] [CrossRef]
- Greaves, E.; Horne, A.W.; Jerina, H.; Mikolajczak, M.; Hilferty, L.; Mitchell, R.; Fleetwood-Walker, S.M.; Saunders, P.T. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci. Rep. 2017, 7, 44169. [Google Scholar] [CrossRef] [Green Version]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Mendez, S.; Jimenez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernandez-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: A randomized controlled study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhu, Y.; Zhang, J.; Li, Y.; Peng, Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: Study protocol for a multicentre randomised controlled trial. BMJ Open 2020, 10, e039519. [Google Scholar] [CrossRef] [PubMed]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [Green Version]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef]
- Huang, J.; Hodis, H.N.; Weinstein, S.J.; Mack, W.J.; Sampson, J.N.; Mondul, A.M.; Albanes, D. Serum Metabolomic Response to Low- and High-Dose Vitamin E Supplementation in Two Randomized Controlled Trials. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005, 293, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhang, Y.; Lou, H.; Ou, Z.; Liu, J.; Duan, W.; Wang, H.; Ge, Y.; Min, J.; Wang, F.; et al. GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis. 2021, 12, 706. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Huang, Y.; Wang, C. 1,25(OH)2D3 Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-kappaB-hepcidin Axis. Int. J. Mol. Sci. 2021, 22, 11334. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef]
- Almassinokiani, F.; Khodaverdi, S.; Solaymani-Dodaran, M.; Akbari, P.; Pazouki, A. Effects of Vitamin D on Endometriosis-Related Pain: A Double-Blind Clinical Trial. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4960–4966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nodler, J.L.; DiVasta, A.D.; Vitonis, A.F.; Karevicius, S.; Malsch, M.; Sarda, V.; Fadayomi, A.; Harris, H.R.; Missmer, S.A. Supplementation with vitamin D or omega-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2020, 112, 229–236. [Google Scholar] [CrossRef]
- Lasco, A.; Catalano, A.; Benvenga, S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: Results of a randomized, double-blind, placebo-controlled study. Arch. Intern. Med. 2012, 172, 366–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Alin, L.; Chen, Y.; Brand, D.; Bourassa, M.W.; Dietrich, K.; Wilkinson, C.M.; Nadeau, C.A.; Kumar, A.; Perry, S.; et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 2018, 84, 854–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porpora, M.G.; Brunelli, R.; Costa, G.; Imperiale, L.; Krasnowska, E.K.; Lundeberg, T.; Nofroni, I.; Piccioni, M.G.; Pittaluga, E.; Ticino, A.; et al. A promise in the treatment of endometriosis: An observational cohort study on ovarian endometrioma reduction by N-acetylcysteine. Evid. Based Complement Altern. Med. 2013, 2013, 240702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, A.C.; Kupershmidt, I.; Edlundh-Rose, E.; Greco, G.; Serafino, A.; Krasnowska, E.K.; Lundeberg, T.; Bracci-Laudiero, L.; Romano, M.C.; Parasassi, T.; et al. Global gene expression analysis in time series following N-acetyl L-cysteine induced epithelial differentiation of human normal and cancer cells in vitro. BMC Cancer 2005, 5, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittaluga, E.; Costa, G.; Krasnowska, E.; Brunelli, R.; Lundeberg, T.; Porpora, M.G.; Santucci, D.; Parasassi, T. More than antioxidant: N-acetyl-L-cysteine in a murine model of endometriosis. Fertil. Steril. 2010, 94, 2905–2908. [Google Scholar] [CrossRef] [Green Version]
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Guohua, F.; Tieyuan, Z.; Xinping, M.; Juan, X. Melatonin protects against PM2.5-induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2-dependent manner. Ecotoxicol. Environ. Saf. 2021, 223, 112588. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Alleviates Acute Sleep Deprivation-Induced Memory Loss in Mice by Suppressing Hippocampal Ferroptosis. Front. Pharmacol. 2021, 12, 708645. [Google Scholar] [CrossRef]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.K.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef]
- Kizilay, G.; Uz, Y.H.; Seren, G.; Ulucam, E.; Yilmaz, A.; Cukur, Z.; Kayisli, U.A. In vivo effects of curcumin and deferoxamine in experimental endometriosis. Adv. Clin. Exp. Med. 2017, 26, 207–213. [Google Scholar] [CrossRef]
- Chen, T.C.; Chuang, J.Y.; Ko, C.Y.; Kao, T.J.; Yang, P.Y.; Yu, C.H.; Liu, M.S.; Hu, S.L.; Tsai, Y.T.; Chan, H.; et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. 2020, 30, 101413. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-kappaB signaling pathway. J. Cell Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.; Mokhtar, M.H. Potential Health Benefits of Curcumin on Female Reproductive Disorders: A Review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorile, P.G.; Viceconte, R.; Baldi, A. Novel dietary supplement association reduces symptoms in endometriosis patients. J. Cell Physiol. 2018, 233, 5920–5925. [Google Scholar] [CrossRef] [PubMed]
- Jelodar, G.; Azimifar, A. Evaluation of serum cancer antigen 125, resistin, leptin, homocysteine, and total antioxidant capacity in rat model of endometriosis treated with Curcumin. Physiol. Rep. 2019, 7, e14016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.Y.; Zhang, C.J. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran. J. Reprod. Med. 2013, 11, 415–422. [Google Scholar]
- Taniguchi, F.; Kaponis, A.; Izawa, M.; Kiyama, T.; Deura, I.; Ito, M.; Iwabe, T.; Adonakis, G.; Terakawa, N.; Harada, T. Apoptosis and endometriosis. Front. Biosci. 2011, 3, 648–662. [Google Scholar] [CrossRef] [Green Version]
- Duggan, C.; Tapsoba, J.D.; Wang, C.Y.; Campbell, K.L.; Foster-Schubert, K.; Gross, M.D.; McTiernan, A. Dietary Weight Loss, Exercise, and Oxidative Stress in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev. Res. 2016, 9, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arikawa, A.Y.; Thomas, W.; Gross, M.; Smith, A.; Phipps, W.R.; Kurzer, M.S.; Schmitz, K.H. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp. Clin. Trials 2013, 34, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Jaiswal, K.S.; Gupta, B. Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Nirgianakis, K.; Egger, K.; Kalaitzopoulos, D.R.; Lanz, S.; Bally, L.; Mueller, M.D. Effectiveness of Dietary Interventions in the Treatment of Endometriosis: A Systematic Review. Reprod. Sci. 2022, 29, 26–42. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Kolahdouz Mohammadi, R.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kohama, T.; Herai, K.; Inoue, M. Effect of French maritime pine bark extract on endometriosis as compared with leuprorelin acetate. J. Reprod. Med. 2007, 52, 703–708. [Google Scholar]
- Maia, H., Jr.; Haddad, C.; Casoy, J. Combining oral contraceptives with a natural nuclear factor-kappa B inhibitor for the treatment of endometriosis-related pain. Int. J. Womens Health 2013, 6, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations Between Endometriosis and Gut Microbiota. Reprod. Sci. 2021, 28, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. The gut microbiota: A puppet master in the pathogenesis of endometriosis? Am. J. Obstet. Gynecol. 2016, 215, 68.e1–68.e4. [Google Scholar] [CrossRef] [PubMed]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef] [PubMed]
- Chadchan, S.B.; Popli, P.; Ambati, C.R.; Tycksen, E.; Han, S.J.; Bulun, S.E.; Putluri, N.; Biest, S.W.; Kommagani, R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 2021, 4, e202101224. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, J.; Weis, S.; Schafer, K.H.; Menger, M.D.; Kohl, M.; Egert, M.; Laschke, M.W. Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS ONE 2019, 14, e0226835. [Google Scholar] [CrossRef] [Green Version]
- Le, N.; Cregger, M.; Fazleabas, A.; Braundmeier-Fleming, A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci. Rep. 2022, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. https://doi.org/10.3390/biom12081055
Clower L, Fleshman T, Geldenhuys WJ, Santanam N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules. 2022; 12(8):1055. https://doi.org/10.3390/biom12081055
Chicago/Turabian StyleClower, Lauren, Taylor Fleshman, Werner J. Geldenhuys, and Nalini Santanam. 2022. "Targeting Oxidative Stress Involved in Endometriosis and Its Pain" Biomolecules 12, no. 8: 1055. https://doi.org/10.3390/biom12081055





