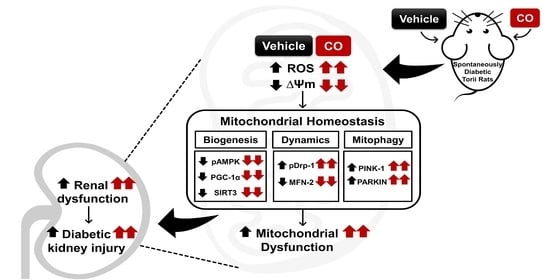
Crocodile Oil Disrupts Mitochondrial Homeostasis and Exacerbates Diabetic Kidney Injury in Spontaneously Diabetic Torii Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crocodile Oil Preparation
2.2. Animals and Study Protocol
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Biochemical Analysis of Renal Function
2.5. Measurement of Mitochondrial Function
2.5.1. Preparation of Renal Mitochondrial Fractions
2.5.2. Determination of Mitochondrial Reactive Oxygen Species (ROS)
2.5.3. Determination of Mitochondrial Membrane Potential Change (∆Ψm)
2.6. Light Microscopic Studies
2.7. Electron Microscopic Studies
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Gas Chromatography Reveals Omega 9 Oleic Acid as a Major Constituent of CO
3.2. SDT Rats Proceed to Pre-Diabetic Stage at the Age of 12 Weeks
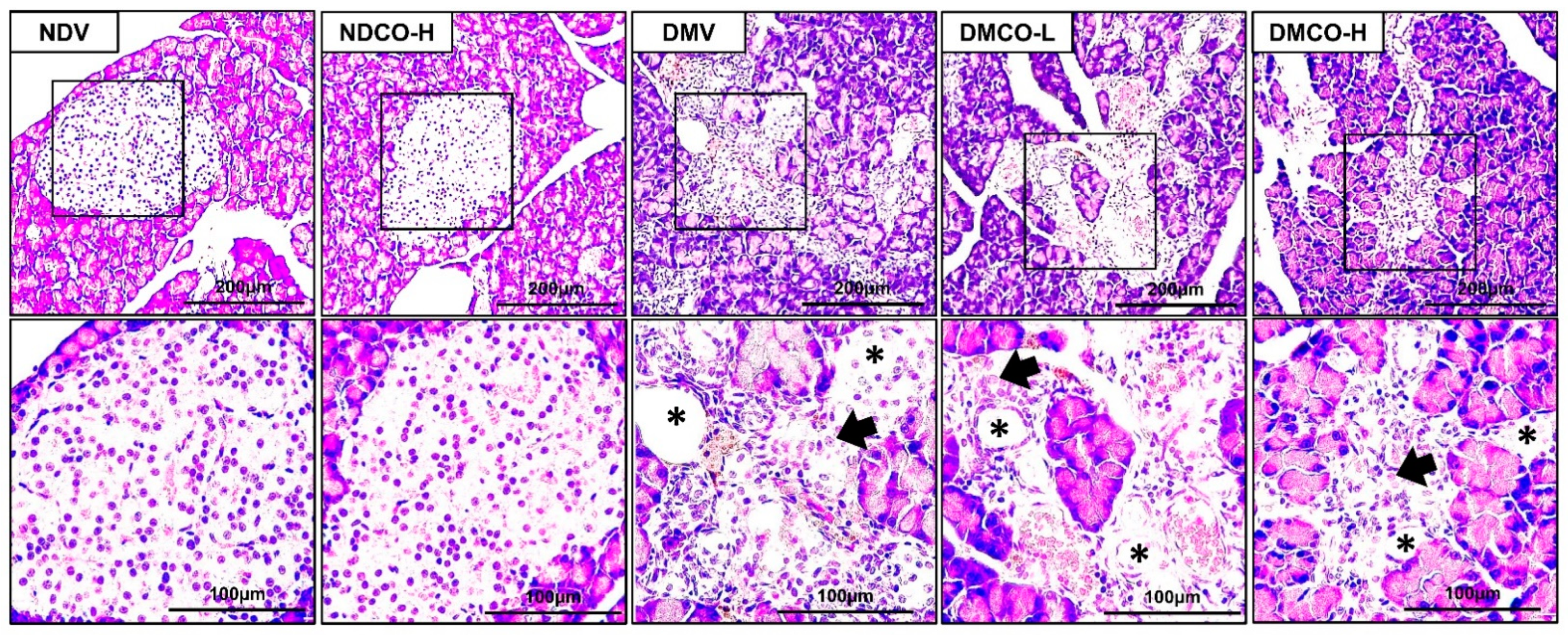
3.3. CO Does Not Attenuate Diabetes-Induced Metabolic Impairments and Abnormal Pancreatic Islet Histopathology
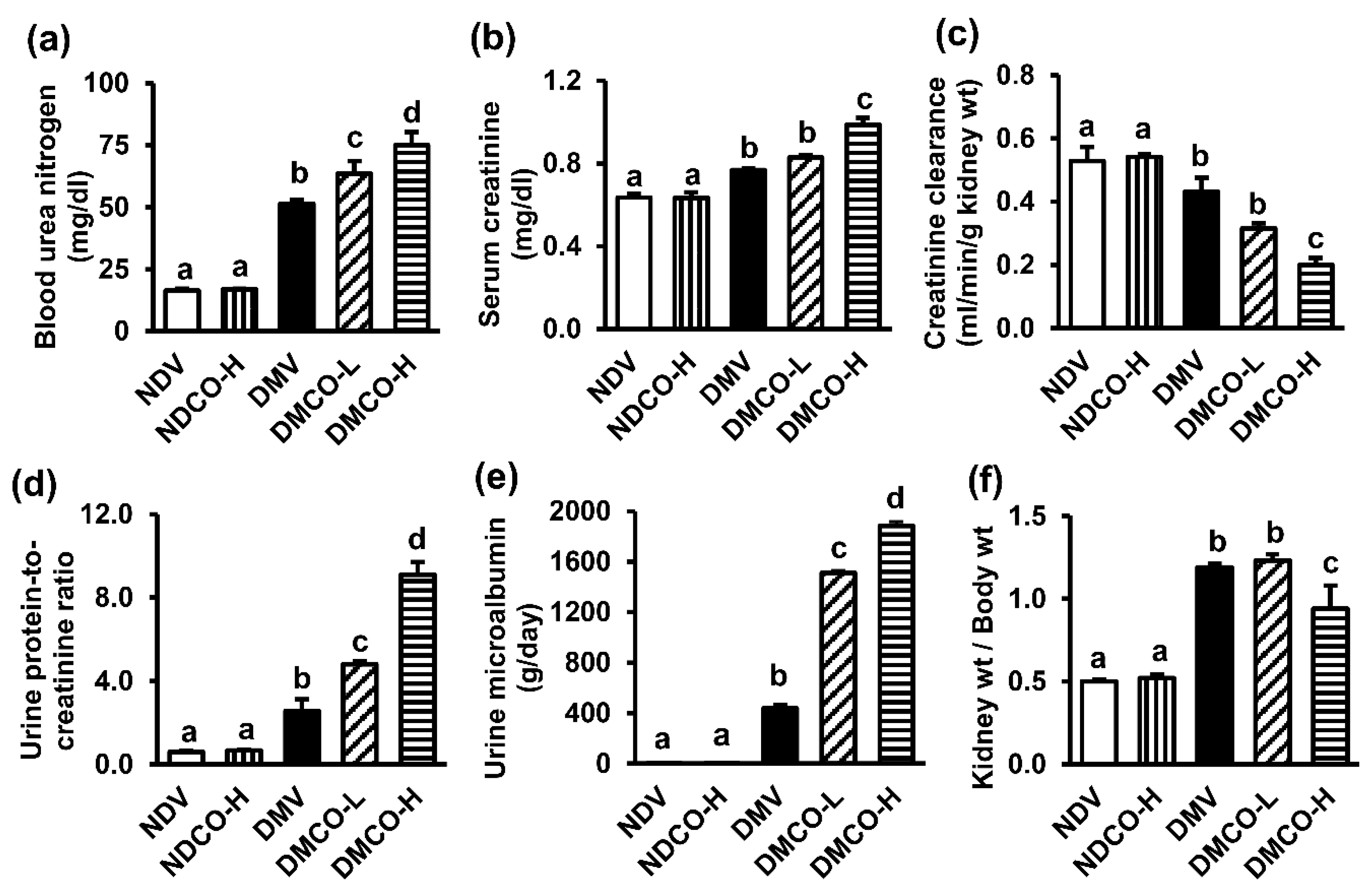
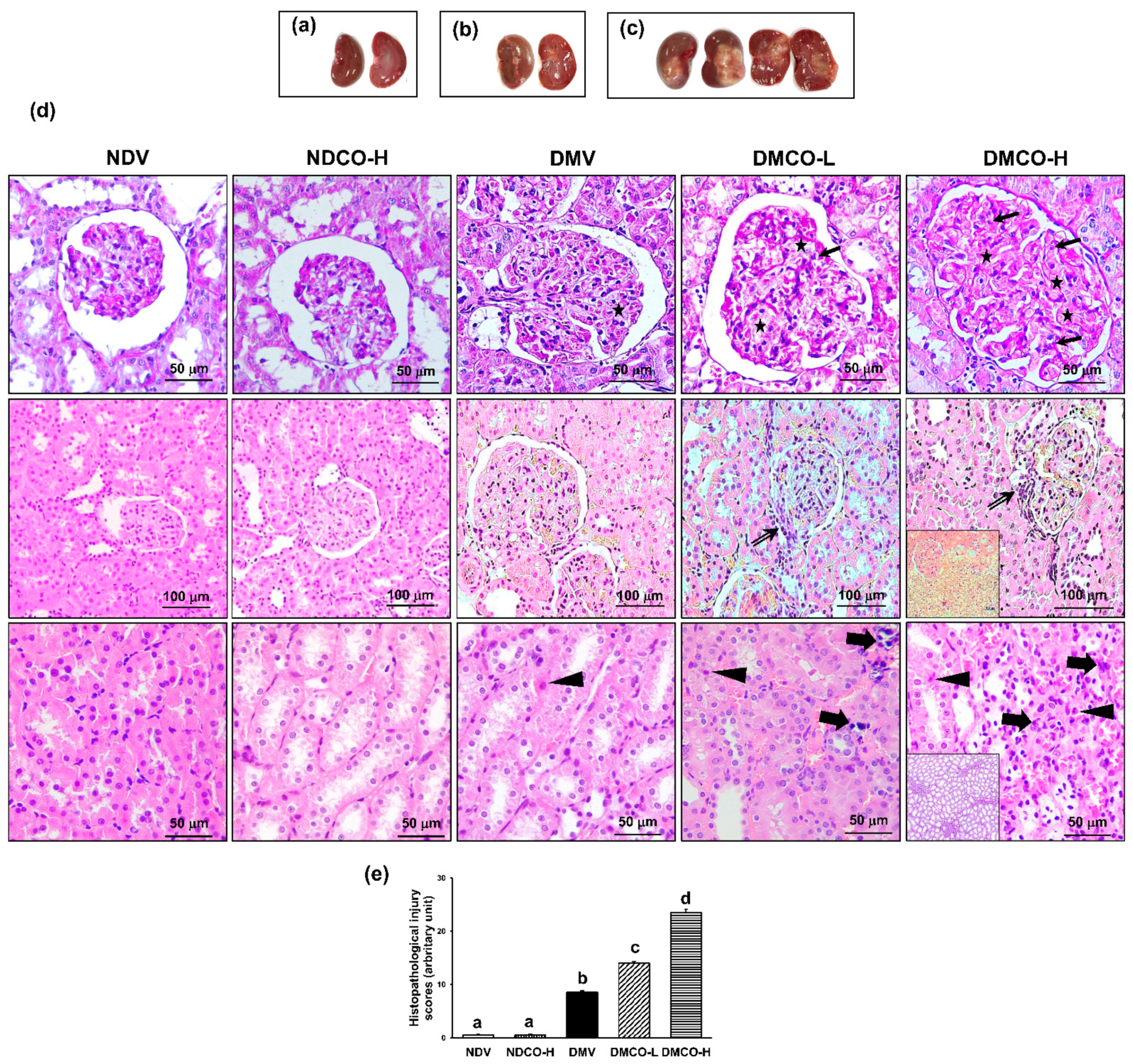
3.4. CO Supplementation Aggravates Renal Functional and Structural Damage in Diabetic Kidneys
3.5. CO Worsens Mitochondrial Dysfunction and Ultrastructural Abnormalities in Diabetic Kidneys
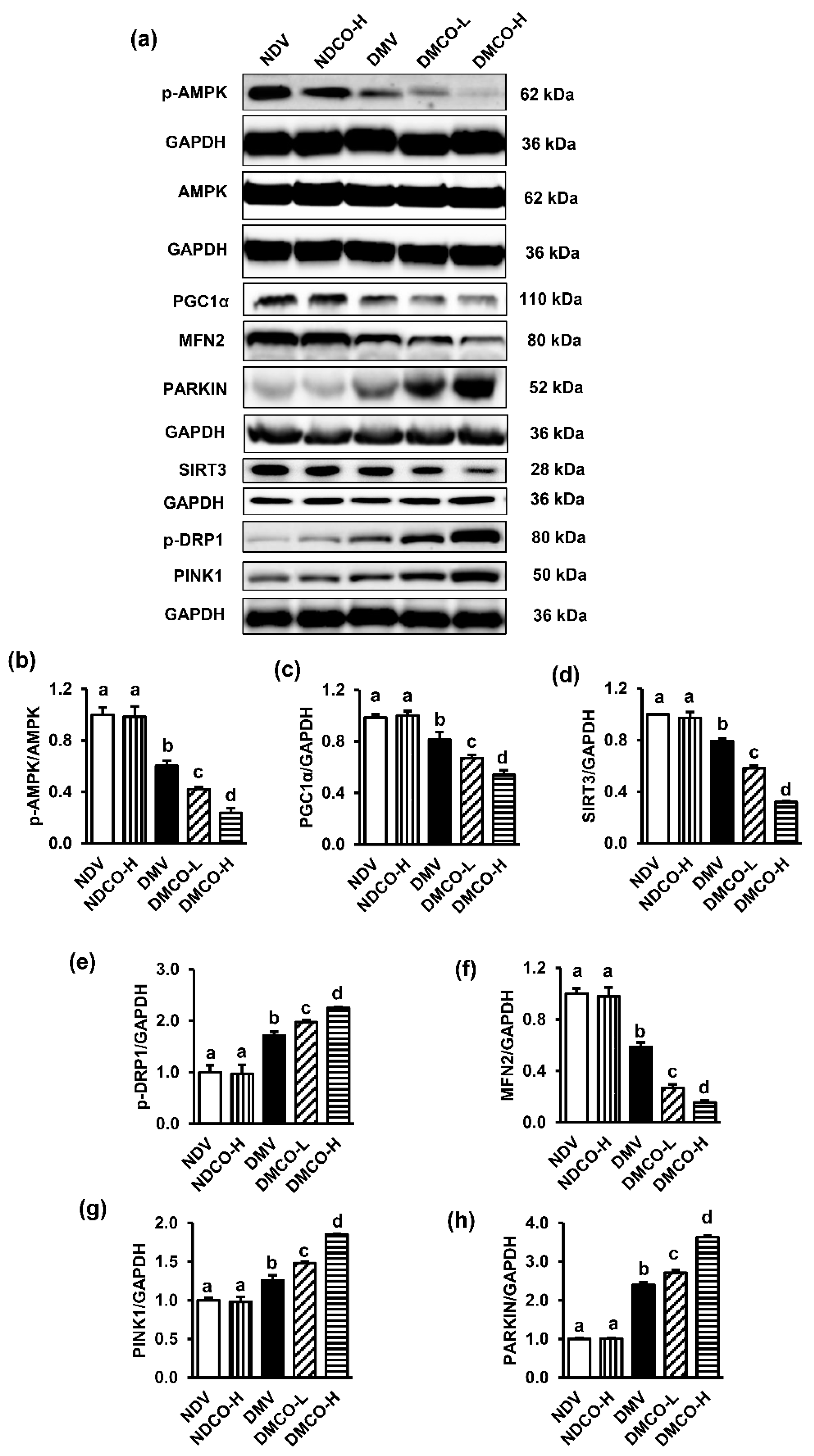
3.6. CO Disrupts Signal Transduction Pathway Involved in Mitochondria Homeostasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.B.; Cheung, W.L.; Li, P.K. Renal registry in Hong Kong-the first 20 years. Kidney Int. Suppl. 2015, 5, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, F.A.; Pflueger, A. Role of oxidative stress in diabetic kidney disease. Med. Sci. Monit. 2010, 16, Ra37–Ra48. [Google Scholar]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Nguyen, T.V.; Penfold, S.A.; Higgins, G.C.; Thallas-Bonke, V.; Tan, S.M.; Van Bergen, N.J.; Sourris, K.C.; Harcourt, B.E.; Thorburn, D.R.; et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin. Sci. 2016, 130, 711–720. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Higgins, G.C.; Nguyen, T.V.; Penfold, S.A.; Thallas-Bonke, V.; Tan, S.M.; Ramm, G.; Van Bergen, N.J.; Henstridge, D.C.; Sourris, K.C.; et al. Deficiency in Apoptosis-Inducing Factor Recapitulates Chronic Kidney Disease via Aberrant Mitochondrial Homeostasis. Diabetes 2016, 65, 1085–1098. [Google Scholar] [CrossRef] [Green Version]
- Parchwani, D.; Upadhyah, A. Diabetic Nephropathy: Progression and Pathophysiology. Int. J. Med. Sci. Public Health 2012, 1, 59–70. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Salas-Salvadó, J.; Martínez-González, M.A.; Sun, Q.; Willett, W.C.; Hu, F.B. Olive oil consumption and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Rattanaphot, T.; Mengumphan, K.; Tongsiri, S.; Srimaroeng, C.; Amornlerdpison, D. Enhancement of oxidative defense and growth performance of Nile tilapia by omega-9-rich freshwater fish oil. Maejo Int. J. Sci. Technol. 2018, 12, 79–88. [Google Scholar]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39 (Suppl. 1), 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y.; Poynter, M.E.; Stevens, R.; Bain, J.; Ikayeva, O.; Ukagawa, N.K.; Champagne, C.M.; Crain, K.I.; Koves, T.R.; et al. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes 2013, 62, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzefovych, L.; Wilson, G.; Rachek, L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: Role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1096–E1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarbolouki, S.; Javanbakht, M.H.; Derakhshanian, H.; Hosseinzadeh, P.; Zareei, M.; Hashemi, S.B.; Dorosty, A.R.; Eshraghian, M.R.; Djalali, M. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: A double-blind randomised clinical trial. Singap. Med. J. 2013, 54, 387–390. [Google Scholar] [CrossRef]
- Ogawa, S.; Abe, T.; Nako, K.; Okamura, M.; Senda, M.; Sakamoto, T.; Ito, S. Eicosapentaenoic acid improves glycemic control in elderly bedridden patients with type 2 diabetes. Tohoku J. Exp. Med. 2013, 231, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirtori, C.R.; Crepaldi, G.; Manzato, E.; Mancini, M.; Rivellese, A.; Paoletti, R.; Pazzucconi, F.; Pamparana, F.; Stragliotto, E. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: Reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis 1998, 137, 419–427. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Sámano-García, C.A.; Calderón-Cortés, E.; Pérez-Hernández, I.H.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar] [CrossRef]
- Li, H.L.; Chen, L.P.; Hu, Y.H.; Qin, Y.; Liang, G.; Xiong, Y.X.; Chen, Q.X. Crocodile oil enhances cutaneous burn wound healing and reduces scar formation in rats. Acad. Emerg. Med. 2012, 19, 265–273. [Google Scholar] [CrossRef]
- Buthelezi, S.; Southway, C.; Govinden, U.; Bodenstein, J.; du Toit, K. An investigation of the antimicrobial and anti-inflammatory activities of crocodile oil. J. Ethnopharmacol. 2012, 143, 325–330. [Google Scholar] [CrossRef]
- Sasase, T.; Ohta, T.; Masuyama, T.; Yokoi, N.; Kakehashi, A.; Shinohara, M. The spontaneously diabetic torii rat: An animal model of nonobese type 2 diabetes with severe diabetic complications. J. Diabetes Res. 2013, 2013, 976209. [Google Scholar] [CrossRef] [Green Version]
- Masuyama, T.; Komeda, K.; Hara, A.; Noda, M.; Shinohara, M.; Oikawa, T.; Kanazawa, Y.; Taniguchi, K. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem. Biophys. Res. Commun. 2004, 314, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Bisphenol A aggravates renal ischemia-reperfusion injury by disrupting mitochondrial homeostasis and N-acetylcysteine mitigates the injurious outcomes. IUBMB Life 2020, 72, 758–770. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, A.L.; Figueira, R.L.; Gonçalves, F.L.; Mitidiero, L.F.; Silva, O.C.; Peiró, J.L.; Sbragia, L. Temporal profile of intestinal tissue expression of intestinal fatty acid-binding protein in a rat model of necrotizing enterocolitis. Clinics 2016, 71, 412–419. [Google Scholar] [CrossRef]
- Shetty, S.S.; Kumari, N.S.; Shetty, P.K. ω-6/ω-3 fatty acid ratio as an essential predictive biomarker in the management of type 2 diabetes mellitus. Nutrition 2020, 79–80, 110968. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, J.; Kim, C.S.; Jo, K.; Kim, J.S. Osteomeles schwerinae Extract Prevents Diabetes-Induced Renal Injury in Spontaneously Diabetic Torii Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 6824215. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Wada, J.; Nakatsuka, A. Mitochondrial Dynamics and Mitochondrial Dysfunction in Diabetes. Acta. Med. Okayama 2016, 70, 151–158. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, H.; Han, Y.; Li, L.; Xiong, S.; Zeng, L.; Xiao, Y.; Wei, L.; Xiong, X.; Gao, P.; et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020, 53, e12909. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Y.; Wang, J.; Zhu, L.; Gao, L. Dysregulation in the Unfolded Protein Response in the FGR Rat Pancreas. Int. J. Endocrinol. 2020, 2020, 5759182. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Y.; Kelly, D.J.; Tan, C.Y.; Gill, A.; Cheng, D.; Braet, F.; Park, J.S.; Sue, C.M.; Pollock, C.A.; et al. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 2016, 6, 29196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Praduptong, A.; Srimangkornkaew, P.; Siruntawineti, J.; Chaeychomsri, S.; Chaeychomsri, W. Acute Oral Toxicity of Mixed Crocodylus siamensis Oil and Kaempferia parviflora Wall. Ex. Baker in Wistar Rats. Bull. Med. Sci. 2020, 62, 297–304. [Google Scholar]
- Henique, C.; Mansouri, A.; Fumey, G.; Lenoir, V.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; Cohen, I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 2010, 285, 36818–36827. [Google Scholar] [CrossRef] [Green Version]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; McAllan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Avila, O.; Figueroa-García, M.D.; García-Berumen, C.I.; Calderón-Cortés, E.; Mejía-Barajas, J.A.; Rodriguez-Orozco, A.R.; Mejía-Zepeda, R.; Saavedra-Molina, A.; Cortés-Rojo, C. Avocado oil induces long-term alleviation of oxidative damage in kidney mitochondria from type 2 diabetic rats by improving glutathione status. J. Bioenerg. Biomembr. 2017, 49, 205–214. [Google Scholar] [CrossRef]
- Arany, I.; Clark, J.S.; Reed, D.K.; Juncos, L.A.; Dixit, M. Role of p66shc in renal toxicity of oleic acid. Am. J. Nephrol. 2013, 38, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Zhang, H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: A meta-analysis. Clinics 2017, 72, 58–64. [Google Scholar] [CrossRef]
- Iwase, Y.; Kamei, N.; Takeda-Morishita, M. Antidiabetic Effects of Omega-3 Polyunsaturated Fatty Acids: From Mechanism to Therapeutic Possibilities. Pharmacol. Pharm. 2015, 6, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Coelho, O.G.L.; da Silva, B.P.; Rocha, D.; Lopes, L.L.; Alfenas, R.C.G. Polyunsaturated fatty acids and type 2 diabetes: Impact on the glycemic control mechanism. Crit. Rev. Food Sci. Nutr. 2017, 57, 3614–3619. [Google Scholar] [CrossRef]
- Lands, W.E. Dietary fat and health: The evidence and the politics of prevention: Careful use of dietary fats can improve life and prevent disease. Ann. N. Y. Acad. Sci. 2005, 1055, 179–192. [Google Scholar] [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 2016, 65, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shon, E.; Kim, C.S.; Kim, J.S. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp. Diabetes Res. 2012, 2012, 210821. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Fatty Acid Compositions | Quantity |
|---|---|
| Saturated fatty acids (SFAs) | 28.63 ± 0.08 |
| Palmitic acid (C16:0) | 21.98 ± 0.75 |
| Stearic acid (C18:0) | 5.35 ± 0.21 |
| Monounsaturated fatty acids (MUFAs) | 46.02 ± 1.79 |
| Palmitoleic acid (C16:1n7) | 4.43 ± 0.27 |
| Oleic acid (C18:1n9c) | 40.87 ± 1.62 |
| Polyunsaturated fatty acids (PUFAs) | 25.35 ± 1.74 |
| Linoleic acid, LA (C18:2n6) | 23.29 ± 1.56 |
| α-linolenic acid, ALA (C18:3n3) | 1.05 ± 0.16 |
| Eicosapentenoic acid, EPA (C20:5n3) | 0.04 ± 0.00 |
| Docosahexaenoic acid, DHA (C22:6n3) | 0.17 ± 0.01 |
| Age 8 Weeks | Age 12 Weeks | |||
|---|---|---|---|---|
| ND | SDT | ND | SDT | |
| Metabolic parameters | ||||
| Body weight (g) | 207.00 ± 1.53 a | 199.67 ± 3.22 a | 491.50 ± 5.43 b | 394.33 ± 8.81 b |
| Food intake (g/day) | 23.60 ± 0.36 a | 23.10 ± 0.27 a | 28.70 ± 0.56 b | 25.73 ± 0.61 b |
| Non-fasting plasma glucose (mg/dL) | 124.70 ± 1.16 a | 126.67 ± 1.03 a | 144.60 ± 2.57 b | 149.27 ± 2.26 b |
| Oral glucose tolerance test (OGTT) | ||||
| Fasting plasma glucose (mg/dL) | - | - | 124.63 ± 5.56 a | 102.97 ± 9.96 a |
| Fasting plasma insulin (ng/mL) | - | - | 0.24 ± 0.00 a | 1.38 ± 0.09 b |
| TAUC (mg/dL/h) | - | - | 234.26 ± 15.01 a | 459.10 ± 40.71 b |
| Renal function parameters | ||||
| Serum creatinine (mg/dL) | 0.45 ± 0.02 a | 0.46 ± 0.01 a | 0.46 ± 0.01 a | 0.45 ± 0.01 a |
| Urine protein-to-creatinine ratio (UPCR) | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a |
| NDV | NDCO-H | DMV | DMCO-L | DMCO-H | |
|---|---|---|---|---|---|
| Bodyweight (g) | 760.50 ± 27.64 a | 788.75 ± 22.16 a | 352.50 ± 3.35 b | 353.75 ± 11.77 b | 345.00 ± 6.71 b |
| Food intake (g/day) | 26.50 ±1.12 a | 29.00 ± 1.22 a | 53.50 ± 1.60 b | 52.00 ± 0.77 b | 53.00 ± 3.13 b |
| Non-fasting plasma glucose (mg/dL) | 120.12 ± 5.74 a | 117.67 ± 4.17 a | 483.89 ± 5.10 b | 487.63 ± 3.39 b | 486.74 ± 29.81 b |
| Plasma insulin (ng/mL) | 1.09 ± 0.05 a | 0.73 ± 0.13 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.09 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wai Linn, T.; Kobroob, A.; Ngernjan, M.; Amornlerdpison, D.; Lailerd, N.; Wongmekiat, O. Crocodile Oil Disrupts Mitochondrial Homeostasis and Exacerbates Diabetic Kidney Injury in Spontaneously Diabetic Torii Rats. Biomolecules 2022, 12, 1068. https://doi.org/10.3390/biom12081068
Wai Linn T, Kobroob A, Ngernjan M, Amornlerdpison D, Lailerd N, Wongmekiat O. Crocodile Oil Disrupts Mitochondrial Homeostasis and Exacerbates Diabetic Kidney Injury in Spontaneously Diabetic Torii Rats. Biomolecules. 2022; 12(8):1068. https://doi.org/10.3390/biom12081068
Chicago/Turabian StyleWai Linn, Thiri, Anongporn Kobroob, Metas Ngernjan, Doungporn Amornlerdpison, Narissara Lailerd, and Orawan Wongmekiat. 2022. "Crocodile Oil Disrupts Mitochondrial Homeostasis and Exacerbates Diabetic Kidney Injury in Spontaneously Diabetic Torii Rats" Biomolecules 12, no. 8: 1068. https://doi.org/10.3390/biom12081068
APA StyleWai Linn, T., Kobroob, A., Ngernjan, M., Amornlerdpison, D., Lailerd, N., & Wongmekiat, O. (2022). Crocodile Oil Disrupts Mitochondrial Homeostasis and Exacerbates Diabetic Kidney Injury in Spontaneously Diabetic Torii Rats. Biomolecules, 12(8), 1068. https://doi.org/10.3390/biom12081068