Role of Arginase-II in Podocyte Injury under Hypoxic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Generation of Recombinant Adenovirus (rAd)
2.3. Podocyte Cell Culture
2.4. Generation of Arg-ii Knockout Cell Line Using CRISPR/Cas9 Technologies
2.5. Immunoblotting
2.6. Cytoskeleton Staining
2.7. Mitochondrial ROS
2.8. Animal Experiments
2.9. Ex Vivo Experiments with Isolated Kidneys
2.10. In Vivo Hypoxia Experiments with Animals
2.11. Measurement of Urinary Creatinine and Albuminuria
2.12. Confocal Immunofluorescence Staining of Arg-II, Synaptopodin, and ACE1
2.13. Statistics
3. Results
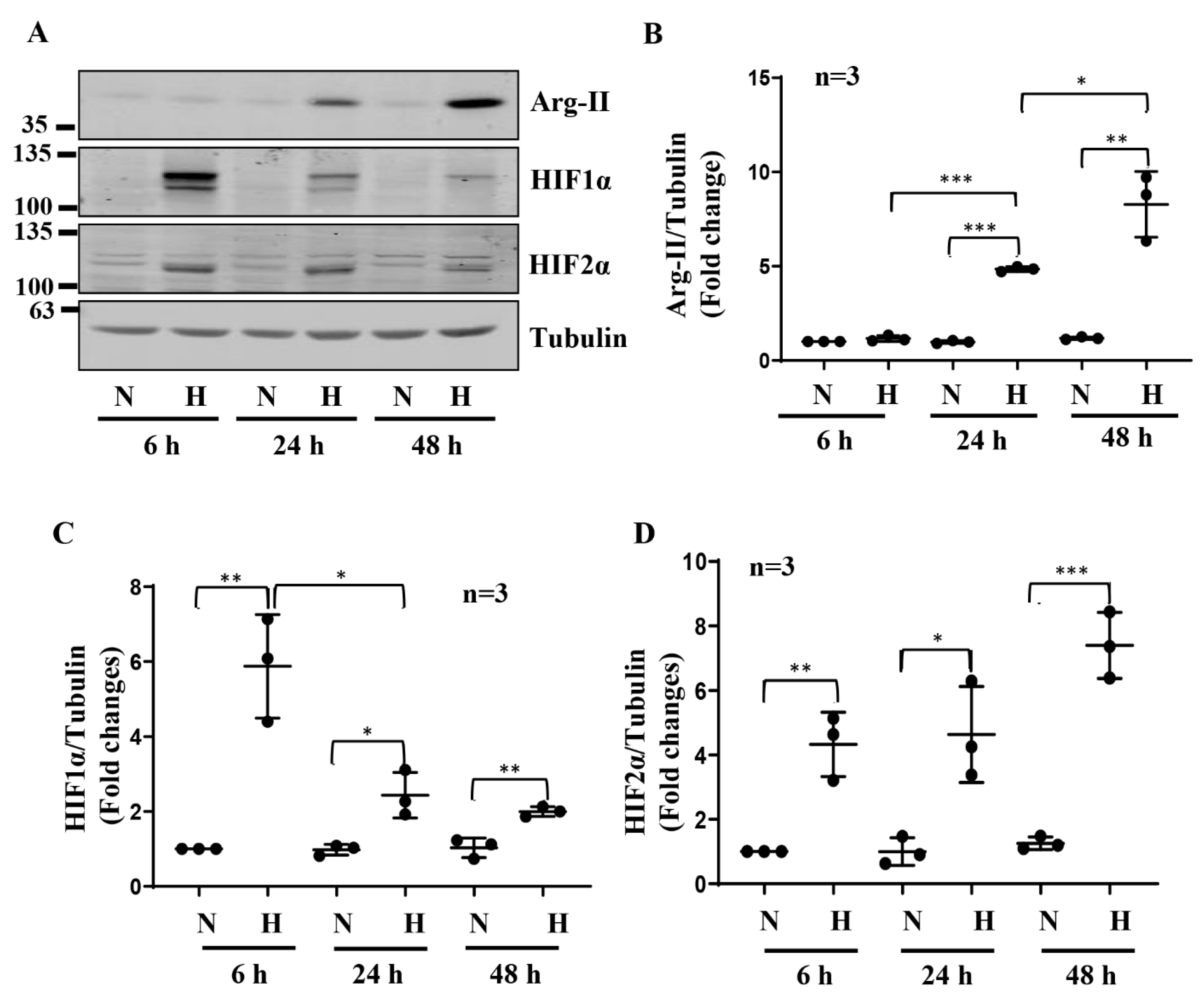
3.1. Hypoxia Enhances Arg-II Levels in Human Podocytes through HIF1α
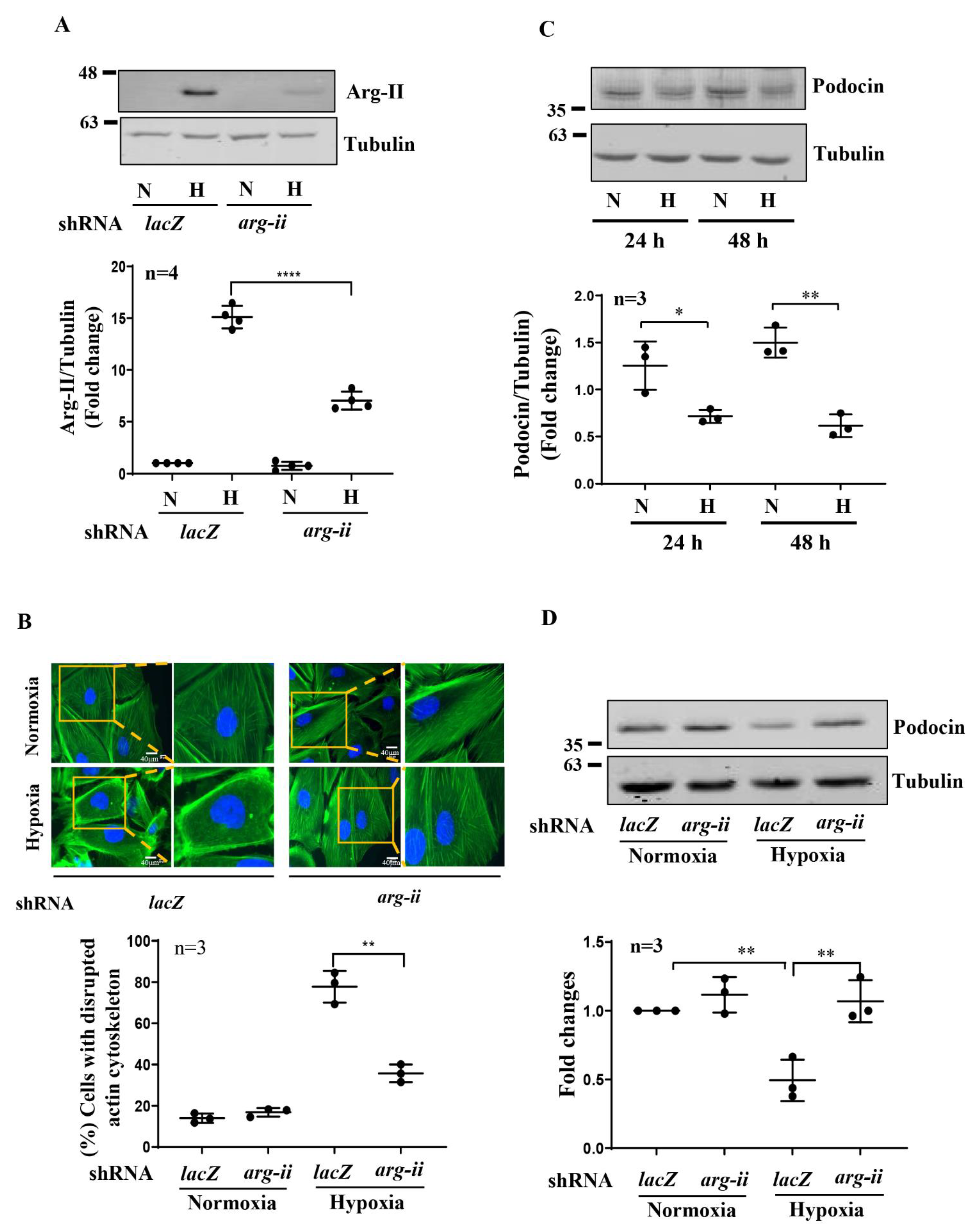
3.2. Silencing Arg-II Reduces Hypoxia-Induced Cytoskeleton Filament Derangement
3.3. Silencing Arg-II Prevents Hypoxia-Mediated Decrease in Podocin Levels
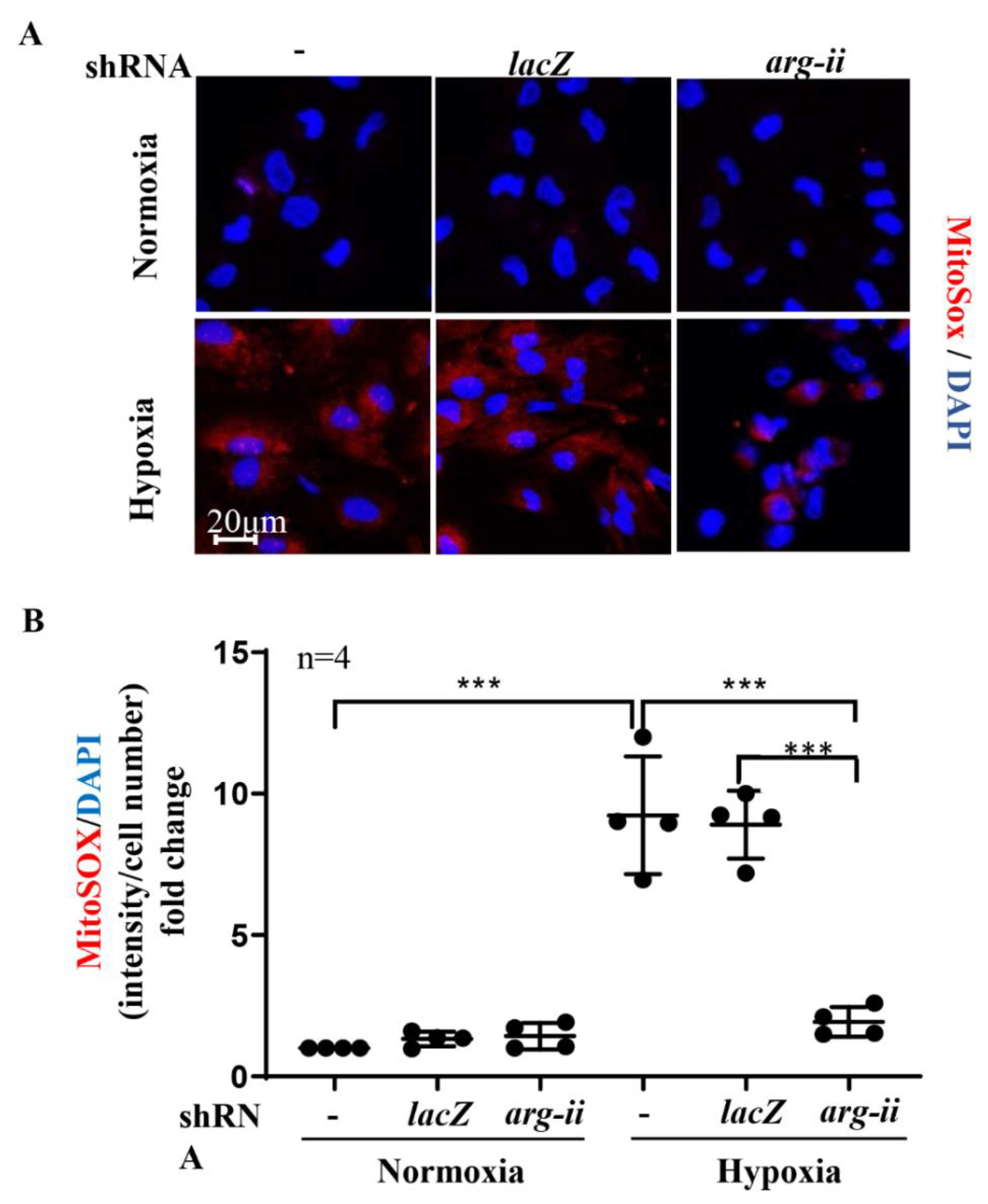
3.4. Silencing Arg-II Prevents Mitochondrial ROS Production
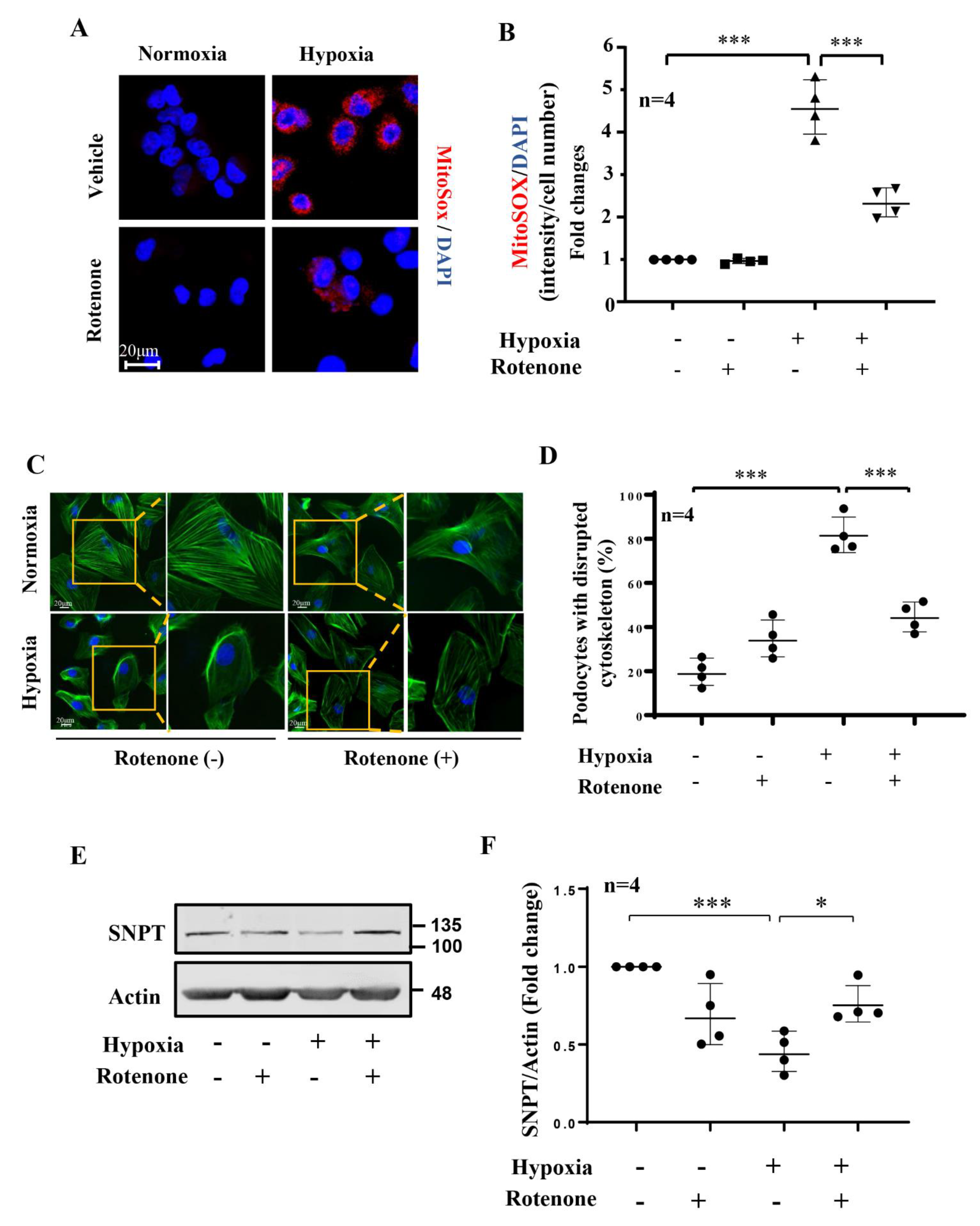
3.5. Role of mtROS in Hypoxia-Induced Podocyte Injury
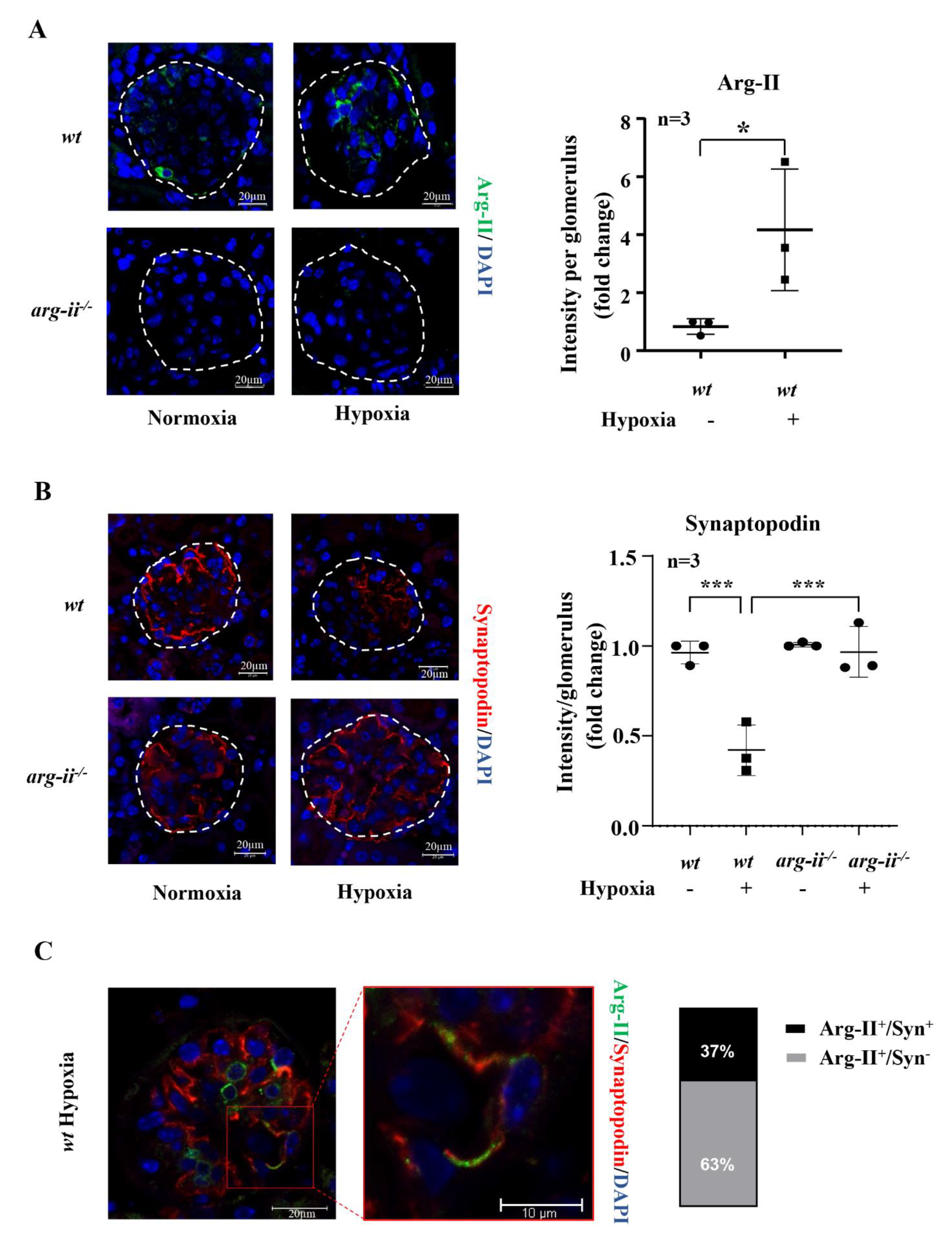
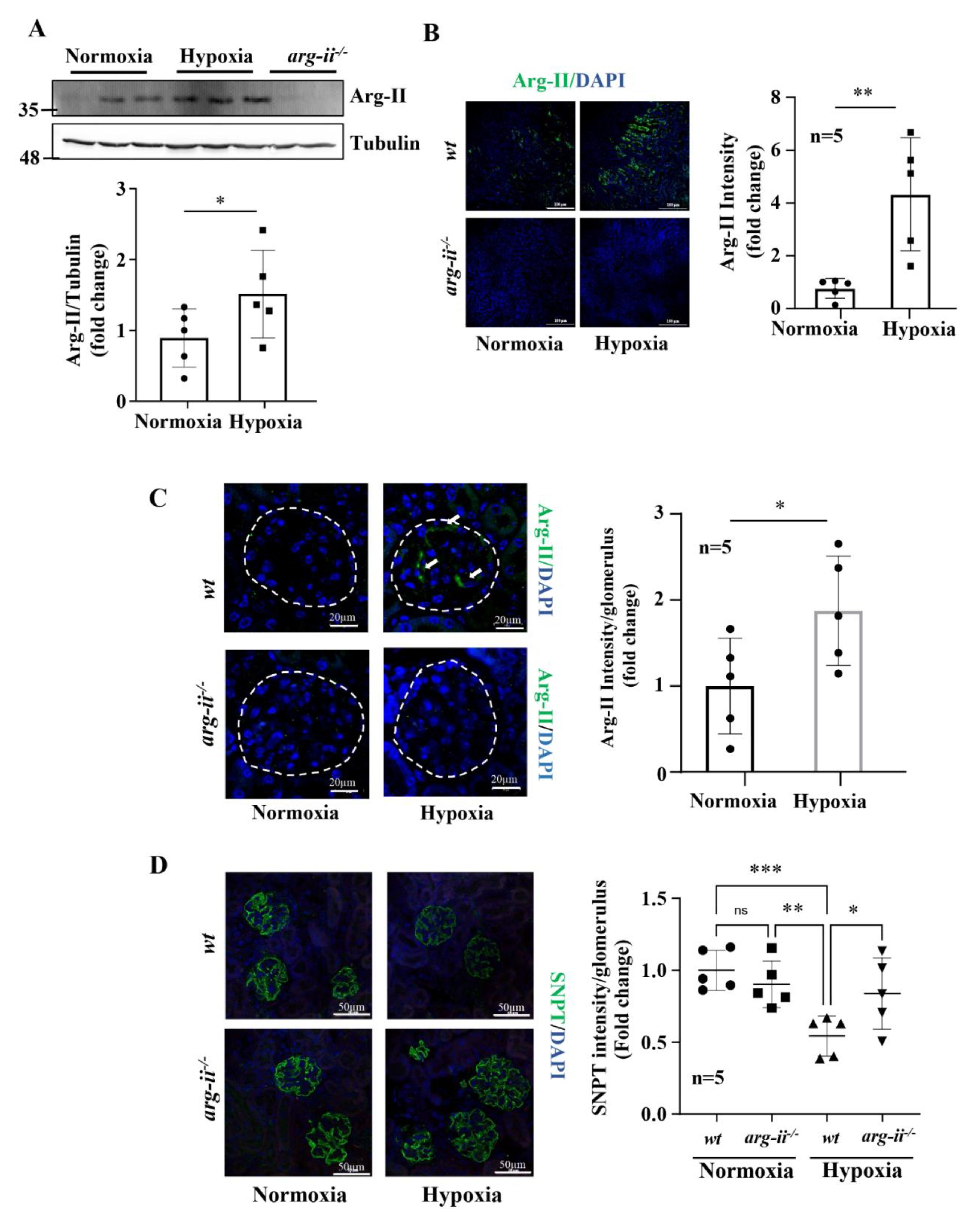
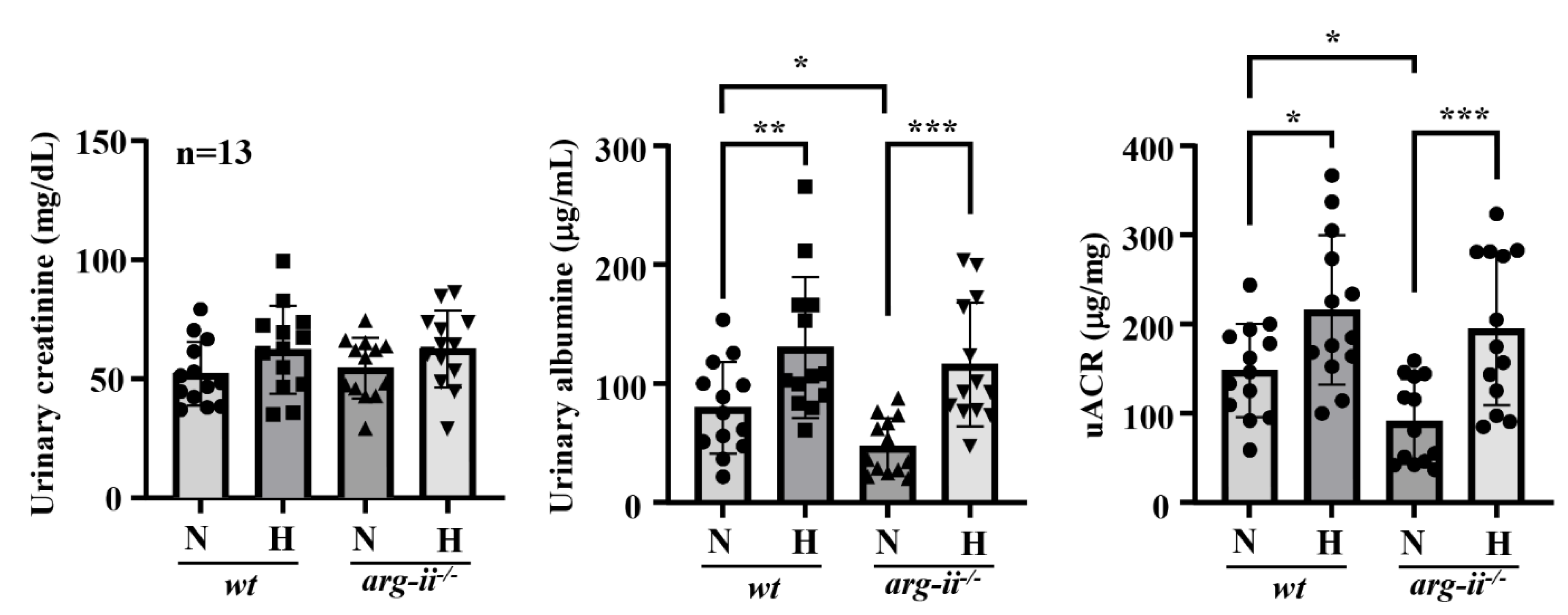
3.6. Hypoxia Causes Podocyte Injury in Mouse Kidneys Ex Vivo and In Vivo: Prevention by Arg-II Deficiency
4. Discussion
Study Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J.; Wang, Y.; Shaw, A.S.; Vaughan, J.C.; Pippin, J.W.; Wessely, O. Podocyte Aging: Why and How Getting Old Matters. J. Am. Soc. Nephrol. 2021, 32, 2697–2713. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M. Renal oxygen delivery: Matching delivery to metabolic demand. Clin. Exp. Pharmacol. Physiol. 2006, 33, 961–967. [Google Scholar] [CrossRef]
- Faivre, A.; Scholz, C.C.; de Seigneux, S. Hypoxia in chronic kidney disease: Towards a paradigm shift? Nephrol. Dial. Transplant. 2020, 36, 1782–1790. [Google Scholar] [CrossRef]
- Nangaku, M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006, 17, 17–25. [Google Scholar] [CrossRef]
- Heyman, S.N.; Khamaisi, M.; Zorbavel, D.; Rosen, S.; Abassi, Z. Role of Hypoxia in Renal Failure Caused by Nephrotoxins and Hypertonic Solutions. Semin. Nephrol. 2019, 39, 530–542. [Google Scholar] [CrossRef]
- Lu, H.; Kapur, G.; Mattoo, T.K.; Lyman, W.D. Hypoxia decreases podocyte expression of slit diaphragm proteins. Int. J. Nephrol. Renovasc. Dis. 2012, 5, 101–107. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, S.I.; Oh, S.Y.; Na, W.; Kang, Y.H. Tangeretin Ameliorates Glucose-Induced Podocyte Injury through Blocking Epithelial to Mesenchymal Transition Caused by Oxidative Stress and Hypoxia. Int. J. Mol. Sci. 2020, 21, 8577. [Google Scholar] [CrossRef]
- Singh, A.K.; Kolligundla, L.P.; Francis, J.; Pasupulati, A.K. Detrimental effects of hypoxia on glomerular podocytes. J. Physiol. Biochem. 2021, 77, 193–203. [Google Scholar] [CrossRef]
- Shved, N.; Warsow, G.; Eichinger, F.; Hoogewijs, D.; Brandt, S.; Wild, P.; Kretzler, M.; Cohen, C.D.; Lindenmeyer, M.T. Transcriptome-based network analysis reveals renal cell type-specific dysregulation of hypoxia-associated transcripts. Sci. Rep. 2017, 7, 8576. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, Q. Understanding the Oxygen-Sensing Pathway and Its Therapeutic Implications in Diseases. Am. J. Pathol. 2020, 190, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Wenger, R.H.; Hoogewijs, D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am. J. Physiol. Renal. Physiol. 2010, 298, F1287–F1296. [Google Scholar] [CrossRef]
- Ivan, M.; Kaelin, W.G., Jr. The EGLN-HIF O2-Sensing System: Multiple Inputs and Feedbacks. Mol. Cell 2017, 66, 772–779. [Google Scholar] [CrossRef]
- Nakuluri, K.; Mukhi, D.; Mungamuri, S.K.; Pasupulati, A.K. Stabilization of hypoxia-inducible factor 1alpha by cobalt chloride impairs podocyte morphology and slit-diaphragm function. J. Cell. Biochem. 2018, 120, 7667–7678. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. Arginase: The emerging therapeutic target for vascular oxidative stress and inflammation. Front. Immunol. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.P.; Grody, W.W.; Cederbaum, S.D. Comparative properties of arginases. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 107–132. [Google Scholar] [CrossRef]
- Huang, J.; Liang, X.; Ladeiras, D.; Fellay, B.; Ming, X.F.; Yang, Z. Role of tubular epithelial arginase-II in renal inflammaging. NPJ Aging Mech. Dis. 2021, 7, 5. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem.J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, D.M.; Morris, S.M., Jr.; Ash, D.E. Expression, purification, and characterization of human type II arginase. Arch. Biochem. Biophys. 2001, 389, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Mimura, I.; Nangaku, M.; Kanki, Y.; Tsutsumi, S.; Inoue, T.; Kohro, T.; Yamamoto, S.; Fujita, T.; Shimamura, T.; Suehiro, J.; et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol. Cell. Biol. 2012, 32, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Potenza, D.M.; Brenna, A.; Ma, Y.; Ren, Z.; Cheng, X.; Ming, X.F.; Yang, Z. Hypoxia Induces Renal Epithelial Injury and Activates Fibrotic Signaling Through Up-Regulation of Arginase-II. Front. Physiol. 2021, 12, 773719. [Google Scholar] [CrossRef]
- Huang, J.; Rajapakse, A.; Xiong, Y.; Montani, J.-P.; Verrey, F.; Ming, X.-F.; Yang, Z. Genetic Targeting of Arginase-II in Mouse Prevents Renal Oxidative Stress and Inflammation in Diet-Induced Obesity. Front. Physiol. 2016, 7, 560. [Google Scholar] [CrossRef]
- Huang, J.; Montani, J.P.; Verrey, F.; Feraille, E.; Ming, X.F.; Yang, Z. Arginase-II negatively regulates renal aquaporin-2 and water reabsorption. FASEB J. 2018, 32, 5520–5531. [Google Scholar] [CrossRef]
- Li, L.; Long, J.; Mise, K.; Galvan, D.L.; Overbeek, P.A.; Tan, L.; Kumar, S.V.; Chan, W.K.; Lorenzi, P.L.; Chang, B.H.; et al. PGC1alpha is required for the renoprotective effect of lncRNA Tug1 in vivo and links Tug1 with urea cycle metabolites. Cell Rep. 2021, 36, 109510. [Google Scholar] [CrossRef]
- Yepuri, G.; Velagapudi, S.; Xiong, Y.Y.; Rajapakse, A.G.; Montani, J.P.; Ming, X.F.; Yang, Z.H. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 2012, 11, 1005–1016. [Google Scholar] [CrossRef]
- Xiong, Y.; Yu, Y.; Montani, J.P.; Yang, Z.; Ming, X.F. Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: Implications for atherosclerotic plaque vulnerability. J. Am. Heart Assoc. 2013, 2, e000096. [Google Scholar] [CrossRef]
- Randi, E.B.; Vervaet, B.; Tsachaki, M.; Porto, E.; Vermeylen, S.; Lindenmeyer, M.T.; Thuy, L.T.T.; Cohen, C.D.; Devuyst, O.; Kistler, A.D.; et al. The Antioxidative Role of Cytoglobin in Podocytes: Implications for a Role in Chronic Kidney Disease. Antioxid Redox Signal. 2020, 32, 1155–1171. [Google Scholar] [CrossRef]
- Shi, O.; Morris, S.M.; Zoghbi, H.; Porter, C.W.; O’Brien, W.E. Generation of a Mouse Model for Arginase II Deficiency by Targeted Disruption of the Arginase II Gene. Mol. Cell. Biol. 2001, 21, 811–813. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Vergara, V.B.; Mog, S.R.; Kalinich, J.F. Hydrophobic Sand Is a Non-Toxic Method of Urine Collection, Appropriate for Urinary Metal Analysis in the Rat. Toxics 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-S.; Shi, Y.; Chenier, I.; Ghosh, A.; Wu, C.-H.; Cailhier, J.-F.; Ethier, J.; Lattouf, J.-B.; Filep, J.G.; Ingelfinger, J.R.; et al. Heterogeneous Nuclear Ribonucleoprotein F Stimulates Sirtuin-1 Gene Expression and Attenuates Nephropathy Progression in Diabetic Mice. Diabetes 2017, 66, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2018, 47 (Suppl. S1), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Brukamp, K.; Jim, B.; Moeller, M.J.; Haase, V.H. Hypoxia and podocyte-specific Vhlh deletion confer risk of glomerular disease. Am. J. Physiol. Renal. Physiol. 2007, 293, F1397–F1407. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Yoshida, H.; Kimura, H.; Kamiyama, K.; Kurose, T.; Sugimoto, H.; Imura, T.; Yokoi, S.; Mikami, D.; Kasuno, K.; et al. Chronic hypoxia exacerbates diabetic glomerulosclerosis through mesangiolysis and podocyte injury in db/db mice. Nephrol. Dial. Transplant. 2020, 35, 1678–1688. [Google Scholar] [CrossRef]
- Hurtado-Arestegui, A.; Plata-Cornejo, R.; Cornejo, A.; Mas, G.; Carbajal, L.; Sharma, S.; Swenson, E.R.; Johnson, R.J.; Pando, J. Higher prevalence of unrecognized kidney disease at high altitude. J. Nephrol. 2018, 31, 263–269. [Google Scholar] [CrossRef]
- Hochman, M.E.; Watt, J.P.; Reid, R.; O’Brien, K.L. The prevalence and incidence of end-stage renal disease in Native American adults on the Navajo reservation. Kidney Int. 2007, 71, 931–937. [Google Scholar] [CrossRef][Green Version]
- Sayarlioglu, H.; Erkoc, R.; Dogan, E.; Topal, C.; Algun, E.; Erem, C.; Atmaca, H.; Kocak, E.; Yilmaz, R.; Erdol, H.; et al. Nephropathy and retinopathy in type 2 diabetic patients living at moderately high altitude and sea level. Ren. Fail 2005, 27, 67–71. [Google Scholar] [CrossRef]
- Arestegui, A.H.; Fuquay, R.; Sirota, J.; Swenson, E.R.; Schoene, R.B.; Jefferson, J.A.; Chen, W.; Yu, X.Q.; Kelly, J.P.; Johnson, R.J.; et al. High altitude renal syndrome (HARS). J. Am. Soc. Nephrol. 2011, 22, 1963–1968. [Google Scholar] [CrossRef]
- Schell, C.; Huber, T.B. The Evolving Complexity of the Podocyte Cytoskeleton. J. Am. Soc. Nephrol. 2017, 28, 3166–3174. [Google Scholar] [CrossRef]
- Liang, X.; Arullampalam, P.; Yang, Z.; Ming, X.F. Hypoxia Enhances Endothelial Intercellular Adhesion Molecule 1 Protein Level Through Upregulation of Arginase Type II and Mitochondrial Oxidative Stress. Front. Physiol. 2019, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Cowburn, A.S.; Crosby, A.; Macias, D.; Branco, C.; Colaco, R.D.; Southwood, M.; Toshner, M.; Crotty Alexander, L.E.; Morrell, N.W.; Chilvers, E.R.; et al. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2016, 113, 8801–8806. [Google Scholar] [CrossRef]
- Krotova, K.; Patel, J.M.; Block, E.R.; Zharikov, S. Hypoxic upregulation of arginase II in human lung endothelial cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1541–C1548. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Nomura, Y.; Rossberg, M.C.; Hori, D.; Bhatta, A.; Keceli, G.; Leucker, T.; Santhanam, L.; Shimoda, L.A.; Berkowitz, D.; et al. Hypoxia Triggers SENP1 (Sentrin-Specific Protease 1) Modulation of KLF15 (Kruppel-Like Factor 15) and Transcriptional Regulation of Arg2 (Arginase 2) in Pulmonary Endothelium. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 913–926. [Google Scholar] [CrossRef]
- Prieto, C.P.; Krause, B.J.; Quezada, C.; San Martin, R.; Sobrevia, L.; Casanello, P. Hypoxia-reduced nitric oxide synthase activity is partially explained by higher arginase-2 activity and cellular redistribution in human umbilical vein endothelium. Placenta 2011, 32, 932–940. [Google Scholar] [CrossRef]
- Xue, J.; Nelin, L.D.; Chen, B. Hypoxia induces arginase II expression and increases viable human pulmonary artery smooth muscle cell numbers via AMPKalpha1 signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L568–L578. [Google Scholar] [CrossRef]
- Nayak, B.K.; Shanmugasundaram, K.; Friedrichs, W.E.; Cavaglierii, R.C.; Patel, M.; Barnes, J.; Block, K. HIF-1 Mediates Renal Fibrosis in OVE26 Type 1 Diabetic Mice. Diabetes 2016, 65, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Kabei, K.; Tateishi, Y.; Nozaki, M.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nishide, S.; Uchida, J.; Nakatani, T.; Tomita, S.; et al. Role of hypoxia-inducible factor-1 in the development of renal fibrosis in mouse obstructed kidney: Special references to HIF-1 dependent gene expression of profibrogenic molecules. J. Pharmacol. Sci. 2018, 136, 31–38. [Google Scholar] [CrossRef]
- Bessho, R.; Takiyama, Y.; Takiyama, T.; Kitsunai, H.; Takeda, Y.; Sakagami, H.; Ota, T. Hypoxia-inducible factor-1alpha is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci. Rep. 2019, 9, 14754. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, H.; Lu, H.; Yang, Y.; Zhang, Y.; Zhang, K.; Liu, H. Deficiency of hypoxia inducible factor-1alpha promoted progression of diabetic nephropathy with hypertension. Exp. Ther. Med. 2018, 16, 3658–3662. [Google Scholar] [PubMed]
- Nordquist, L.; Friederich-Persson, M.; Fasching, A.; Liss, P.; Shoji, K.; Nangaku, M.; Hansell, P.; Palm, F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Potenza, D.M.; Ma, Y.; Ajalbert, G.; Hoogewijs, D.; Ming, X.-F.; Yang, Z. Role of Arginase-II in Podocyte Injury under Hypoxic Conditions. Biomolecules 2022, 12, 1213. https://doi.org/10.3390/biom12091213
Ren Z, Potenza DM, Ma Y, Ajalbert G, Hoogewijs D, Ming X-F, Yang Z. Role of Arginase-II in Podocyte Injury under Hypoxic Conditions. Biomolecules. 2022; 12(9):1213. https://doi.org/10.3390/biom12091213
Chicago/Turabian StyleRen, Zhilong, Duilio Michele Potenza, Yiqiong Ma, Guillaume Ajalbert, David Hoogewijs, Xiu-Fen Ming, and Zhihong Yang. 2022. "Role of Arginase-II in Podocyte Injury under Hypoxic Conditions" Biomolecules 12, no. 9: 1213. https://doi.org/10.3390/biom12091213
APA StyleRen, Z., Potenza, D. M., Ma, Y., Ajalbert, G., Hoogewijs, D., Ming, X.-F., & Yang, Z. (2022). Role of Arginase-II in Podocyte Injury under Hypoxic Conditions. Biomolecules, 12(9), 1213. https://doi.org/10.3390/biom12091213







