Design, Synthesis and Activity of New N1-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
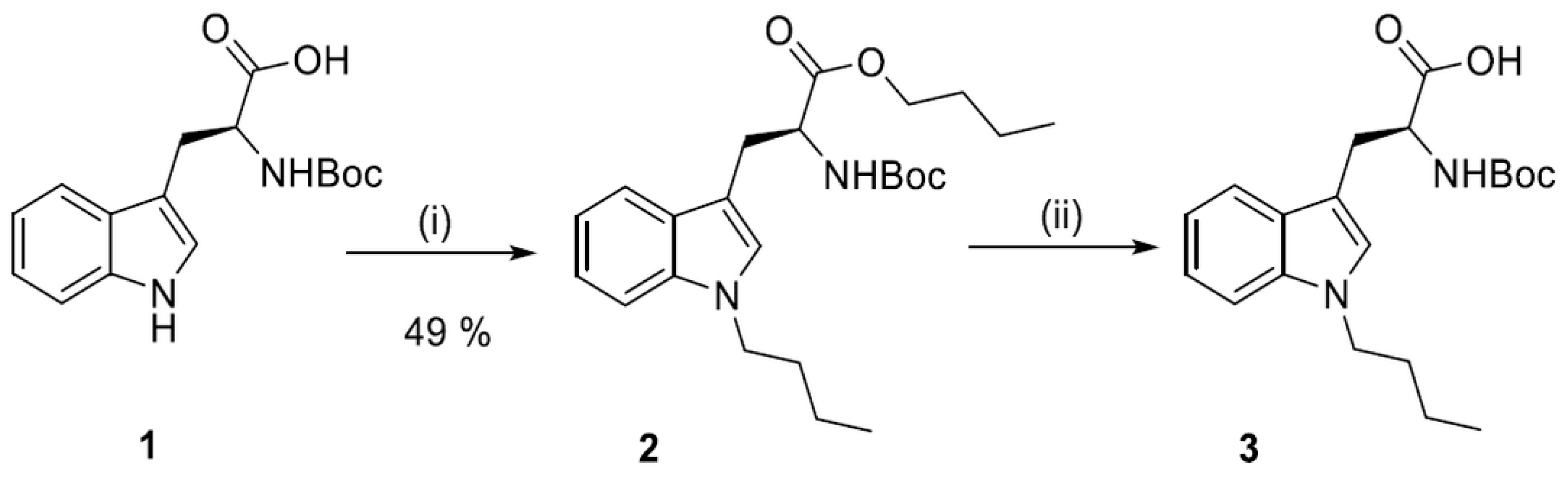
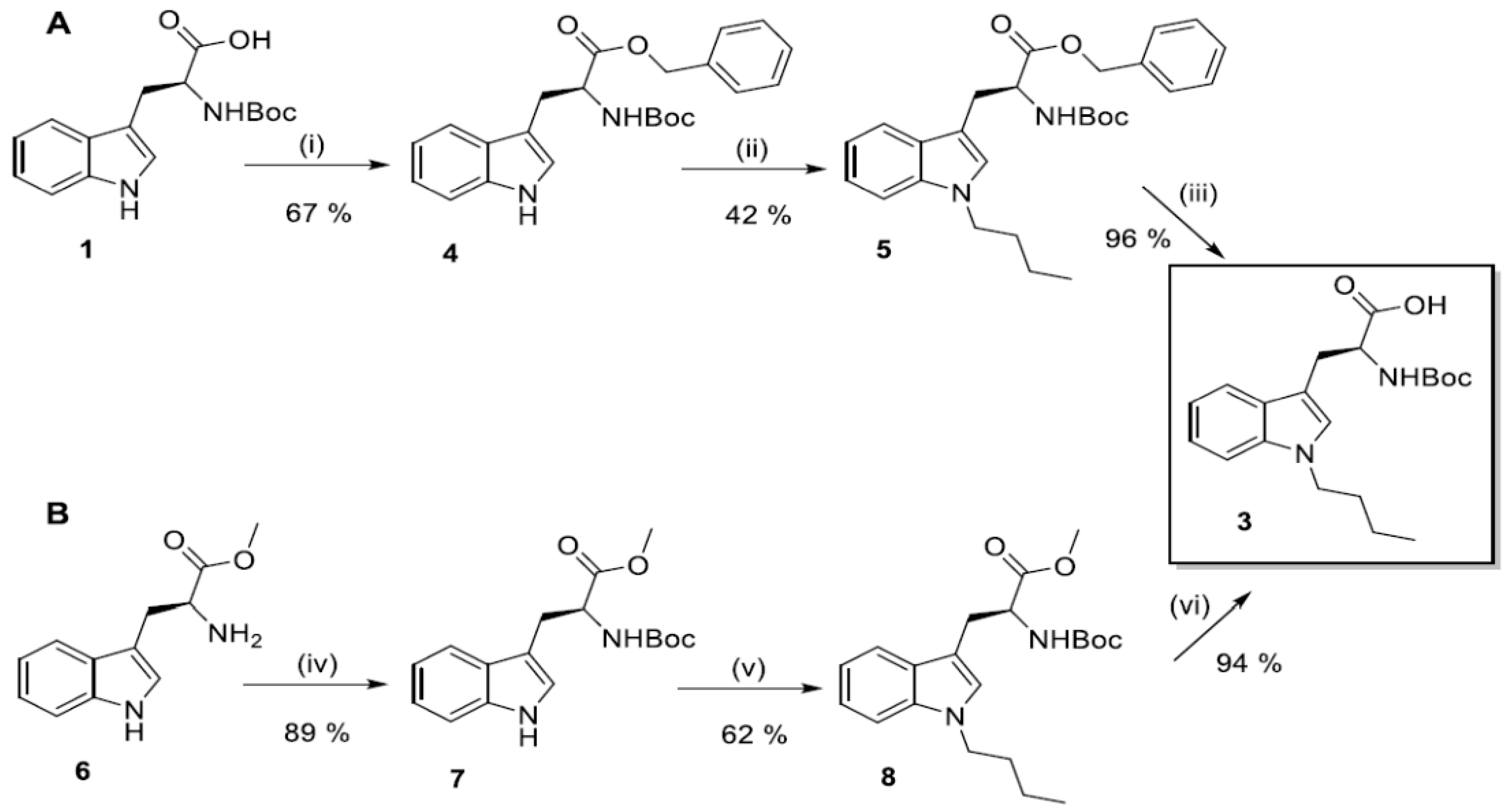
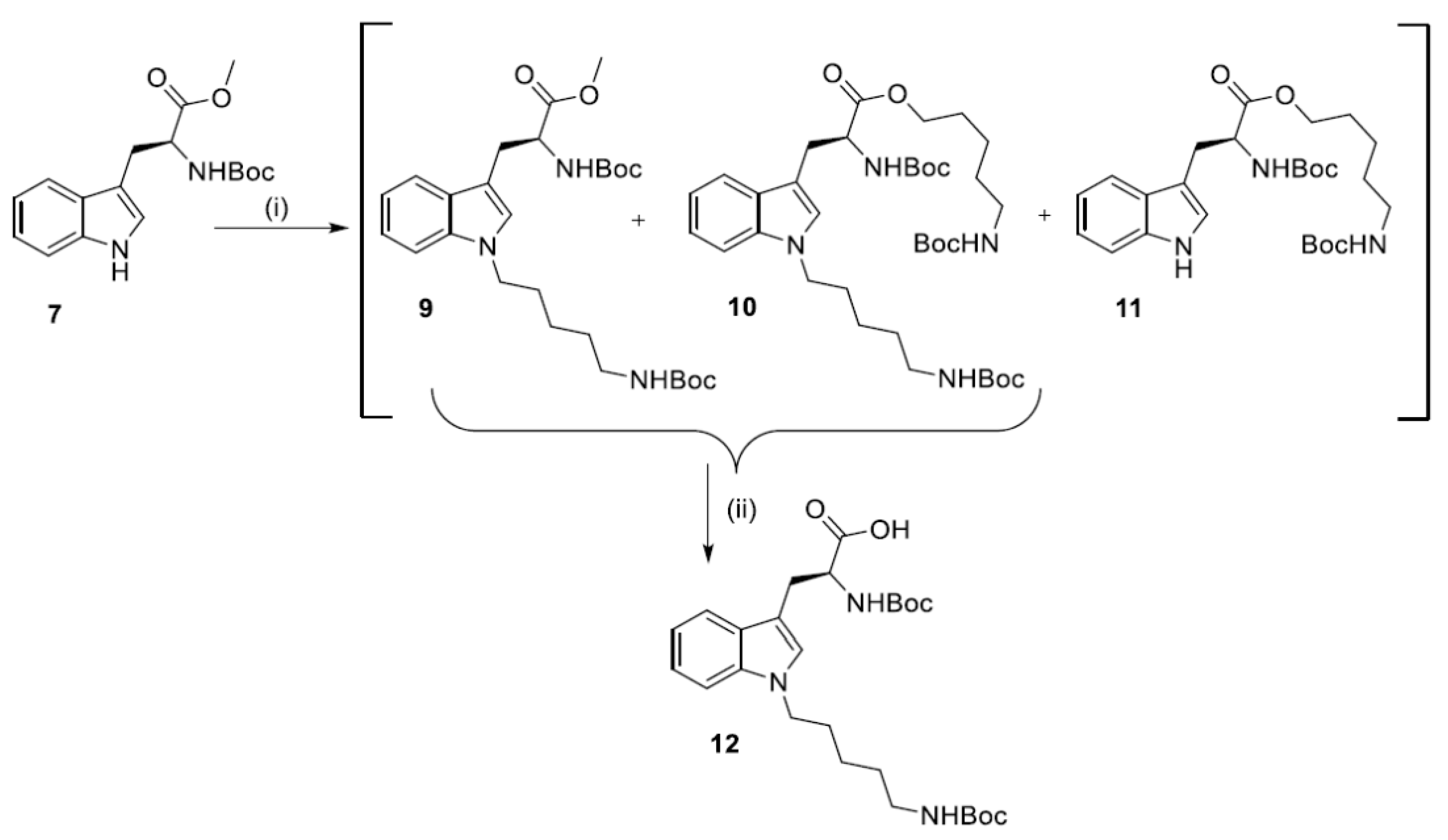
2.2. Synthesis of N1-Susbstituted Tryptophanes
2.3. Synthesis of Dendrimers Functionalized with N1-Alkyl Tryptophanes
2.4. Human GBM Cell Lines and Human Astrocytes Culture
2.5. Cell Survival
2.6. Colony-Forming Assay
2.7. Statistical Analysis
2.8. Antioxidant Assays
2.8.1. Chemicals
2.8.2. Ferric Reducing Antioxidant Power (FRAP)
2.8.3. ABTS Assay
2.8.4. DPPH Assay
3. Results
3.1. Molecular Design
3.2. Susceptibility of Human GBM Cells and Primary Human Astrocytes to Treatment with Dendrimers
3.3. Antioxidant Properties: Radical Scavenging Potency (DPPH and ABTS) and Redox Potential (FRAP) of Dendrimers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| GBM | glioblastoma |
| ROS | reactive oxygen species |
| NHA | normal human astrocytes |
References
- Tewarie, I.A.; Senders, J.T.; Kremer, S.; Devi, S.; Gormley, W.B.; Arnaout, O.; Smith, T.R.; Broekman, M.L.D. Survival prediction of glioblastoma patients—Are we there yet? A systematic review of prognostic modeling for glioblastoma and its clinical potential. Neurosurg. Rev. 2020, 44, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Dymova, M.; Kuligina, E.; Richter, V. Molecular Mechanisms of Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6385. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.A.; Shushanov, S.S.; Rybalkina, E.Y. Problems of glioblastoma multiforme drug resistance. Biochemistry 2016, 81, 91–100. [Google Scholar] [CrossRef]
- Rajaratnam, V.; Islam, M.; Yang, M.; Slaby, R.; Ramirez, H.; Mirza, S. Glioblastoma: Pathogenesis and Current Status of Chemotherapy and Other Novel Treatments. Cancers 2020, 12, 937. [Google Scholar] [CrossRef]
- Burster, T.; Traut, R.; Yermekkyzy, Z.; Mayer, K.; Westhoff, M.A.; Bischof, J.; Knippschild, U. Critical View of Novel Treatment Strategies for Glioblastoma: Failure and Success of Resistance Mechanisms by Glioblastoma Cells. Front. Cell Dev. Biol. 2021, 9, 695325. [Google Scholar] [CrossRef]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neuro-Oncol. 2020, 151, 41–53. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.-L.; Li, Y.-A.; Li, W.-Y.; Wang, S.; Song, C.; Dong, Y.-B. Nanoscale Covalent Organic Framework for Combinatorial Antitumor Photodynamic and Photothermal Therapy. ACS Nano 2019, 13, 13304–13316. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The Role of Branch Cell Symmetry and Other Critical Nanoscale Design Parameters in the Determination of Dendrimer Encapsulation Properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.; Reyna, L.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer Peptide and Protein Dendrimers: A Cross-Sectional Analysis. Chem. Rev. 2019, 119, 11391–11441. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, K.; Wołowiec, S.; Markowicz, J.; Wałajtys-Rode, E.; Uram, Ł. Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines. Cancers 2022, 14, 714. [Google Scholar] [CrossRef]
- Patil, C. Micro and Nanotechnologies, Nanocarriers for Cancer Diagnosis and Targeted Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–128. [Google Scholar]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2019, 101, 43–68. [Google Scholar] [CrossRef]
- Majoros, I.J.; Myc, A.; Thomas, T.; Mehta, C.B.; Baker, J.R. PAMAM Dendrimer-Based Multifunctional Conjugate for Cancer Therapy: Synthesis, Characterization, and Functionality. Biomacromolecules 2006, 7, 572–579. [Google Scholar] [CrossRef]
- Del Olmo, N.S.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; de la Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef]
- Del Olmo, N.S.; Bajo, A.M.; Ionov, M.; Garcia-Gallego, S.; Bryszewska, M.; Gomez, R.; de la Mata, F.J. Cyclopentadienyl ruthenium(II) carbosilane metallodendrimers as a promising treatment against advanced prostate cancer. Eur. J. Med. Chem. 2020, 199, 112414. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Ramirez, R.G.; de la Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47. [Google Scholar] [CrossRef]
- Dufès, C.; Keith, W.N.; Bilsland, A.; Proutski, I.; Uchegbu, I.F.; Schätzlein, A.G. Synthetic Anticancer Gene Medicine Exploits Intrinsic Antitumor Activity of Cationic Vector to Cure Established Tumors. Cancer Res. 2005, 65, 8079–8084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Swieton, J.; Lipkowski, A.W.; Urbanczyk-Lipkowska, Z. Low molecular mass peptide dendrimers that express antimicrobial properties. Bioorg. Med. Chem. Lett. 2003, 13, 3711–3713. [Google Scholar] [CrossRef]
- Touaibia, M.; Roy, R. Glycodendrimers as anti-adhesion drugs against type 1 fimbriated E-coli uropathogenic infections. Mini-Rev. Med. Chem. 2007, 7, 1270–1283. [Google Scholar] [CrossRef]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as Drugs: Discovery and Preclinical and Clinical Development of Dendrimer-Based Microbicides for HIV and STI Prevention. Mol. Pharm. 2005, 2, 312–318. [Google Scholar] [CrossRef]
- Janiszewska, J.; Posadas, I.; Játiva, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second Generation Amphiphilic Poly-Lysine Dendrons Inhibit Glioblastoma Cell Proliferation without Toxicity for Neurons or Astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef]
- Sowinska, M.; Morawiak, M.; Bochyńska-Czyż, M.; Lipkowski, A.W.; Ziemińska, E.; Zabłocka, B.; Urbanczyk-Lipkowska, Z. Molecular Antioxidant Properties and In Vitro Cell Toxicity of the p-Aminobenzoic Acid (PABA) Functionalized Peptide Dendrimers. Biomolecules 2019, 9, 89. [Google Scholar] [CrossRef]
- Sowińska, M.; Szeliga, M.; Morawiak, M.; Ziemińska, E.; Zabłocka, B.; Urbańczyk-Lipkowska, Z. Peptide Dendrimers with Non-Symmetric Bola Structure Exert Long Term Effect on Glioblastoma and Neuroblastoma Cell Lines. Biomolecules 2021, 11, 435. [Google Scholar] [CrossRef]
- Pal, S.; Koeppe, R.E.; Chattopadhyay, A. Membrane electrostatics sensed by tryptophan anchors in hydrophobic model peptides depends on non-aromatic interfacial amino acids: Implications in hydrophobic mismatch. Faraday Discuss. 2020, 232, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Khemaissa, S.; Sagan, S.; Walrant, A. Tryptophan, an Amino-Acid Endowed with Unique Properties and Its Many Roles in Membrane Proteins. Crystals 2021, 11, 1032. [Google Scholar] [CrossRef]
- Holt, A.; Killian, J.A. Orientation and dynamics of transmembrane peptides: The power of simple models. Eur. Biophys. J. 2009, 39, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Zamanakou, M.; Germenis, A.E.; Karanikas, V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol. Lett. 2007, 111, 69–75. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.S.; Auffinger, B.; Lesniak, M.S. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology 2020, 9, 1777625. [Google Scholar] [CrossRef]
- Qian, F.; Villella, J.; Wallace, P.K.; Mhawech-Fauceglia, P.; Tario, J.D.; Andrews, C.; Matsuzaki, J.; Valmori, D.; Ayyoub, M.; Frederick, P.J.; et al. Efficacy of Levo-1-Methyl Tryptophan and Dextro-1-Methyl Tryptophan in Reversing Indoleamine-2,3-Dioxygenase–Mediated Arrest of T-Cell Proliferation in Human Epithelial Ovarian Cancer. Cancer Res. 2009, 69, 5498–5504. [Google Scholar] [CrossRef]
- Lewis, H.C.; Chinnadurai, R.; Bosinger, S.E.; Galipeau, J. The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells. Oncotarget 2017, 8, 91914–91927. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.-L.; Tian, H.; Wang, S.-C.; Cai, J. Synthesis and Biological Evaluation of Novel 1-Alkyltryptophan Analogs as Potential Antitumor Agents. Molecules 2009, 14, 5339–5348. [Google Scholar] [CrossRef]
- Ishii, N.; Maier, D.; Merlo, A.; Tada, M.; Sawamura, Y.; Diserens, A.C.; Van Meir, E.G. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999, 9, 469–479. [Google Scholar] [CrossRef]
- Sowińska, M.; Laskowska, A.; Guśpiel, A.; Solecka, J.; Bochynska-Czyż, M.; Lipkowski, A.W.; Trzeciak, K.; Urbanczyk-Lipkowska, Z. Bioinspired Amphiphilic Peptide Dendrimers as Specific and Effective Compounds against Drug Resistant Clinical Isolates of E. coli. Bioconjugate Chem. 2018, 29, 3571–3585. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Adv. Hyg. Exp. Med. 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- da Silva AM, B.; Silva-Gonçalves, L.C.; Oliveira, F.A.; Arcisio-Miranda, M. Pro-necrotic Activity of Cationic Mastoparan Peptides in Human Glioblastoma Multiforme Cells Via Membranolytic Action. Mol. Neurobiol. 2018, 55, 5490–5504. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Dennison, S.R.; Whittaker, M.; Harris, F.; Phoenix, D.A. Anticancer α-Helical Peptides and Structure/Function Relationships Underpinning Their Interactions with Tumour Cell Membranes. Curr. Protein Pept. Sci. 2006, 7, 487–499. [Google Scholar] [CrossRef]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef]
- Su, B.C.; Wu, T.H.; Hsu, C.H.; Chen, J.Y. Distribution of positively charged amino acid residues in antimicrobial peptide epinecidin-1 is crucial for in vitro glioblastoma cytotoxicity and its underlying mechanisms. Chem. Biol. Interact. 2020, 315, 108904. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Huang, P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Deben, C. Auranofin and Cold Atmospheric Plasma Synergize to Trigger Distinct Cell Death Mechanisms and Immunogenic Responses in Glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Increases Temozolomide Sensitivity of Three-Dimensional Glioblastoma Spheroids via Oxidative Stress-Mediated DNA Damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef]
- Dave, N.; Chow, L.M.; Gudelsky, G.A.; LaSance, K.; Qi, X.; Desai, P.B. Preclinical Pharmacological Evaluation of Letrozole as a Novel Treatment for Gliomas. Mol. Cancer Ther. 2015, 14, 857–864. [Google Scholar] [CrossRef]
- Velazquez, F.N.; Miretti, M.; Baumgartner, M.T.; Caputto, B.L.; Tempesti, T.C.; Prucca, C.G. Effectiveness of ZnPc and of an amine derivative to inactivate Glioblastoma cells by Photodynamic Therapy: An in vitro comparative study. Sci. Rep. 2019, 9, 3010. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Lee, E.; Youk, H.-J.; Kim, S.H.; Lee, H.J.; Park, Y.-G.; Lim, S.-J. Potentiation by alpha-tocopheryl succinate of the etoposide response in multidrug resistance protein 1-expressing glioblastoma cells. Cancer Lett. 2005, 217, 181–190. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Khoei, S.; Shoja, M.; Mostaar, A.; Faeghi, F. Effects of resveratrol and methoxyamine on the radiosensitivity of iododeoxyuridine in U87MG glioblastoma cell line. Exp. Biol. Med. 2016, 241, 1229–1236. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.Y.; Zhang, Z.F.; Brown, J.; Le, A.D. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1α and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol. Cancer Ther. 2005, 4, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Zhang, V.X.; Sze KM, F.; Chan, L.K.; Ho DW, H.; Tsui, Y.M.; Chiu, Y.T.; Ng IO, L. Antioxidant supplements promote tumor formation and growth and confer drug resistance in hepatocellular carcinoma by reducing intracellular ROS and induction of TMBIM1. Cell Biosci. 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Silva, C.; Vacondio, F.; Rivara, M.; Plazzi, P.V.; Franceschinit, D.; et al. Antioxidant and cytoprotective activity of indole derivatives related to melatonin. Adv. Exp. Med. Biol. 2003, 527, 567–575. [Google Scholar] [PubMed]
- Suzen, S.; Cihaner, S.S.; Coban, T. Synthesis and Comparison of Antioxidant Properties of Indole-Based Melatonin Analogue Indole Amino Acid Derivatives. Chem. Biol. Drug Des. 2011, 79, 76–83. [Google Scholar] [CrossRef]
- Nechab, M.; Mondal, S.; Bertrand, M.P. 1,n-Hydrogen-atom transfer (HAT) reactions in which n’5: An updated inventory. Chemistry 2014, 20, 16034–16059. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Lee, E.; Choi, M.-K.; Ku, J.-L.; Kim, S.H.; Park, Y.-G.; Lim, S.-J. Role of reactive oxygen species in the induction of apoptosis by α-tocopheryl succinate. Int. J. Cancer 2004, 112, 385–392. [Google Scholar] [CrossRef]
- Björkblom, B.; Wibom, C.; Jonsson, P.; Mörén, L.; Andersson, U.; Johannesen, T.B.; Langseth, H.; Antti, H.; Melin, B. Metabolomic screening of pre-diagnostic serum samples identifies association between α- and γ-tocopherols and glioblastoma risk. Oncotarget 2016, 7, 37043–37053. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
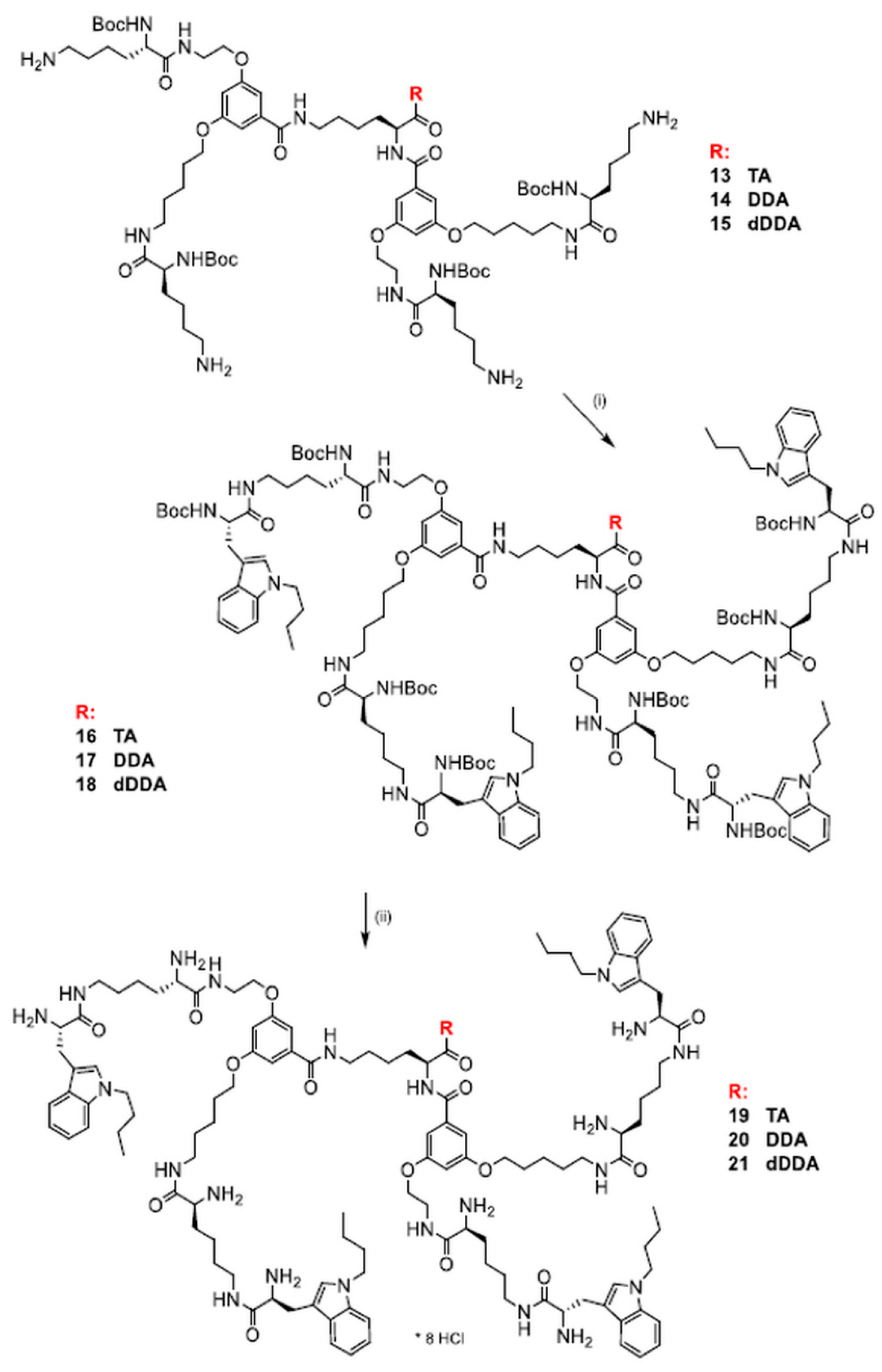
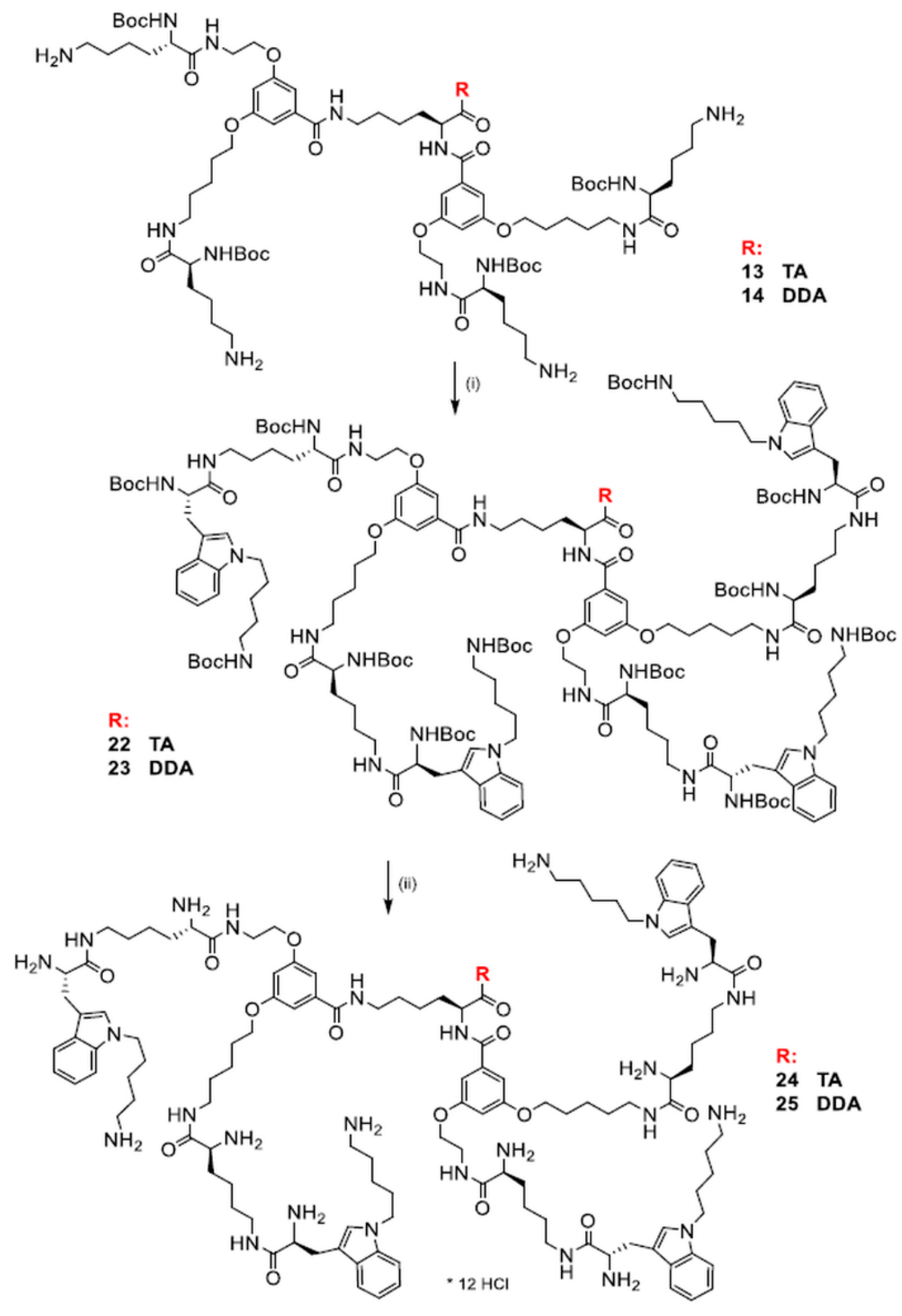

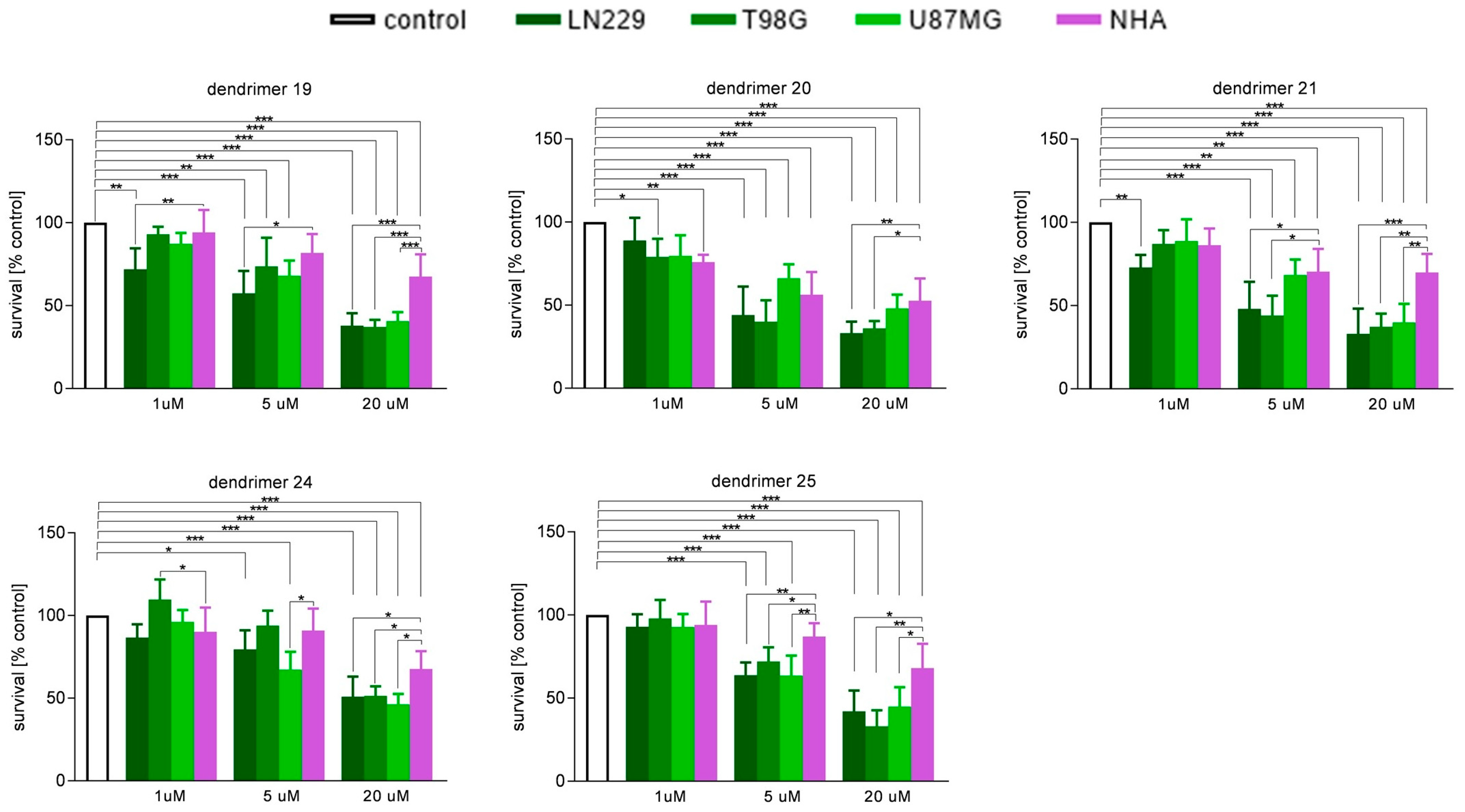
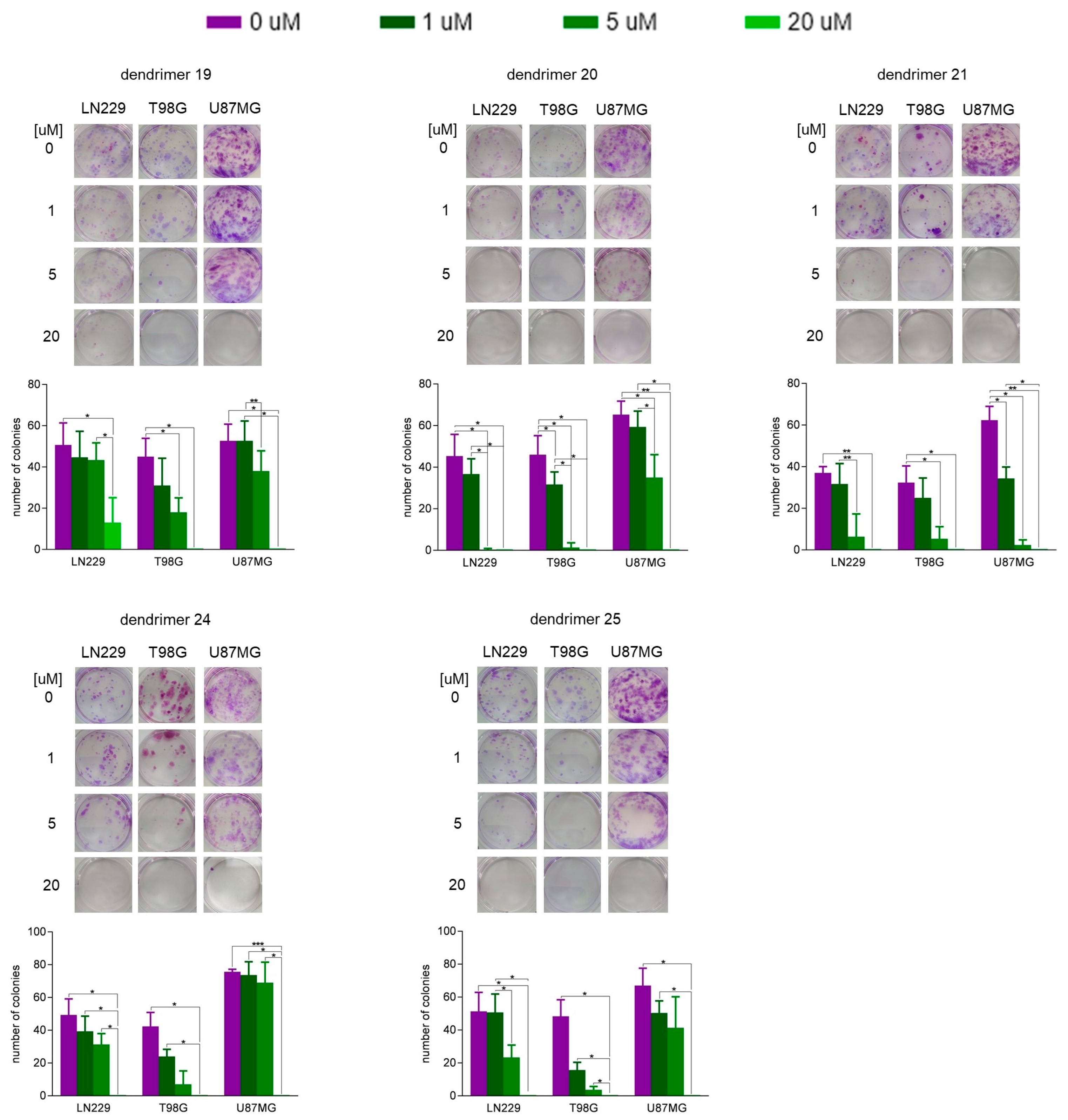


| N1-Protected Dendrimers | Final Dendrimers | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yield (%) | m.p. (°C) | (α)_D25 (c 1, MeOH) | Rf (CHCl3/MeOH 8:1) | No | Yield. (%) | m.p. (°C) | (α)D25 (c 1, MeOH) |
| 16 | 56.6 | 120–122 | −6.5 | 0.46 | 19 | 97.6 | 182–184.5 | +18.0 |
| 17 | 35.8 | 102–104.5 | −5.4 | 0.55 | 20 | 89.8 | 174–177 | +12.3 |
| 18 | 71.4 | 94–97 | −7.1 | 0.60 | 21 | 95.8 | 175–178 | +8.9 |
| 22 | 78.9 | 117–119.5 | −6.5 | 0.44 | 24 | 97.8 | 189–192 | +6.3 |
| 23 | 68.1 | 99–102 | −5.7 | 0.52 | 25 | 89.5 | 184–187 | +6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowińska, M.; Szeliga, M.; Morawiak, M.; Zabłocka, B.; Urbanczyk-Lipkowska, Z. Design, Synthesis and Activity of New N1-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma. Biomolecules 2022, 12, 1116. https://doi.org/10.3390/biom12081116
Sowińska M, Szeliga M, Morawiak M, Zabłocka B, Urbanczyk-Lipkowska Z. Design, Synthesis and Activity of New N1-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma. Biomolecules. 2022; 12(8):1116. https://doi.org/10.3390/biom12081116
Chicago/Turabian StyleSowińska, Marta, Monika Szeliga, Maja Morawiak, Barbara Zabłocka, and Zofia Urbanczyk-Lipkowska. 2022. "Design, Synthesis and Activity of New N1-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma" Biomolecules 12, no. 8: 1116. https://doi.org/10.3390/biom12081116
APA StyleSowińska, M., Szeliga, M., Morawiak, M., Zabłocka, B., & Urbanczyk-Lipkowska, Z. (2022). Design, Synthesis and Activity of New N1-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma. Biomolecules, 12(8), 1116. https://doi.org/10.3390/biom12081116






