Iridium-Functionalized Cellulose Microcrystals as a Novel Luminescent Biomaterial for Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
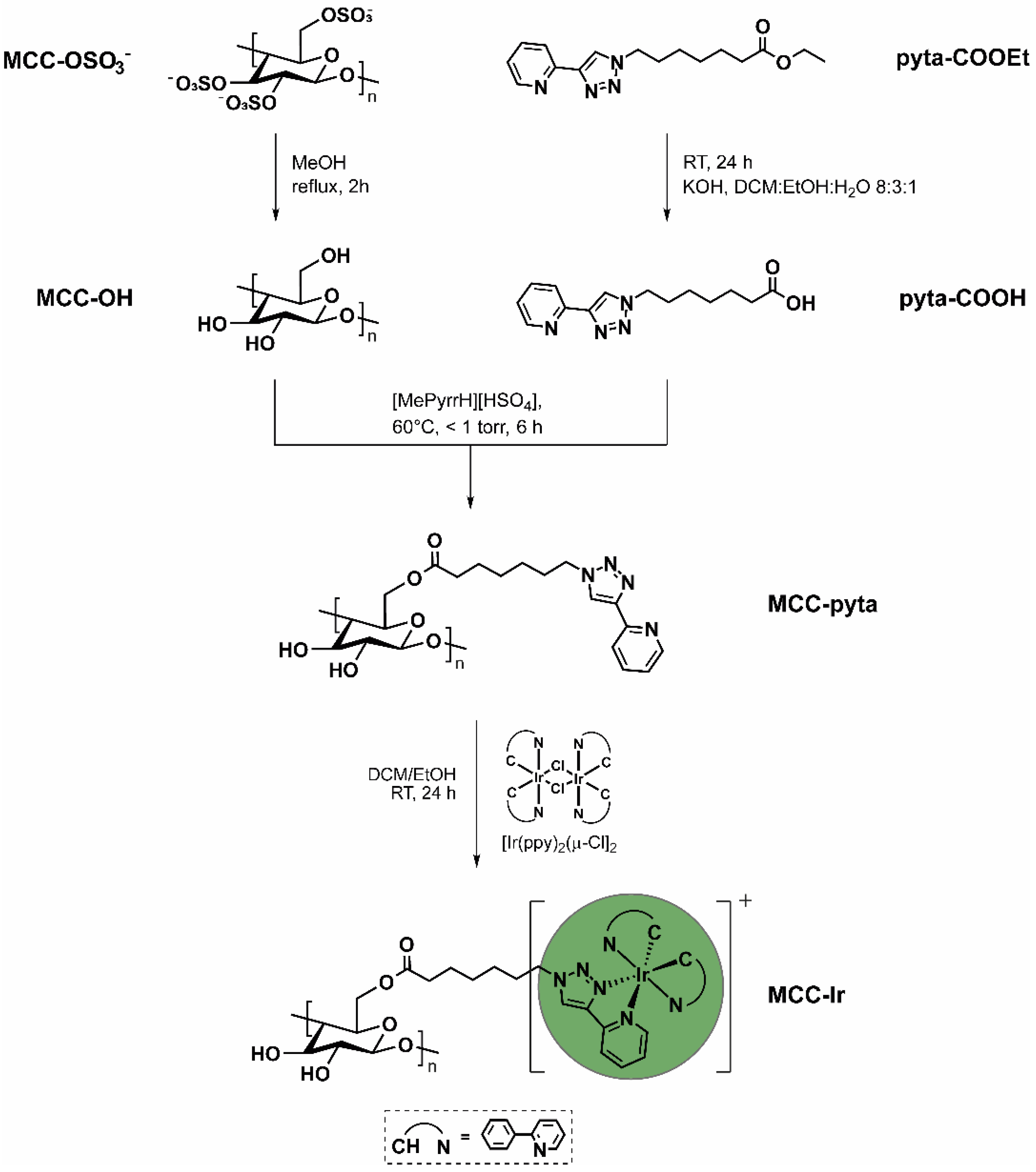
2.1. Desulfonylation of MCC-OSO3−
2.2. Synthesis of N-Methylpyrrolidinium Hydrogensulphate [MePyrrH][HSO4]
2.3. Synthesis of 7-(4-(Pyridin-2-yl)-1H-1,2,3-triazol-1-yl)heptanoic Acid (pyta-COOH) and 2-(1-Nonyl-1H-1,2,3-triazol-4-yl)pyridine (pyta-CH3)
2.4. Esterification of MCC-OH with Pyta-COOH
2.5. Synthesis of MCC-Ir and [Ir(ppy)2(pyta-COOH)]Cl
2.6. Materials Characterization
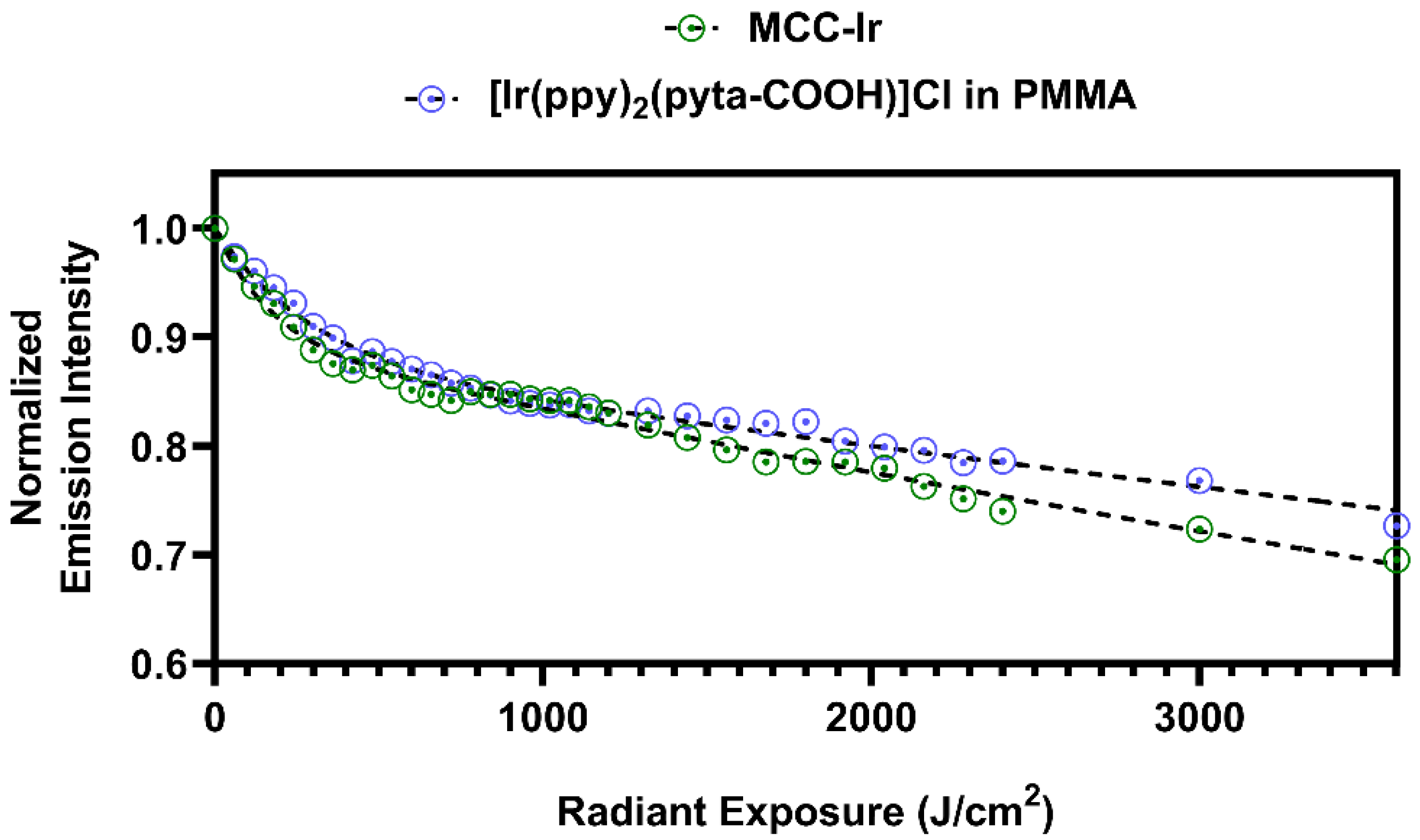
2.7. Photostability of MCC-Ir
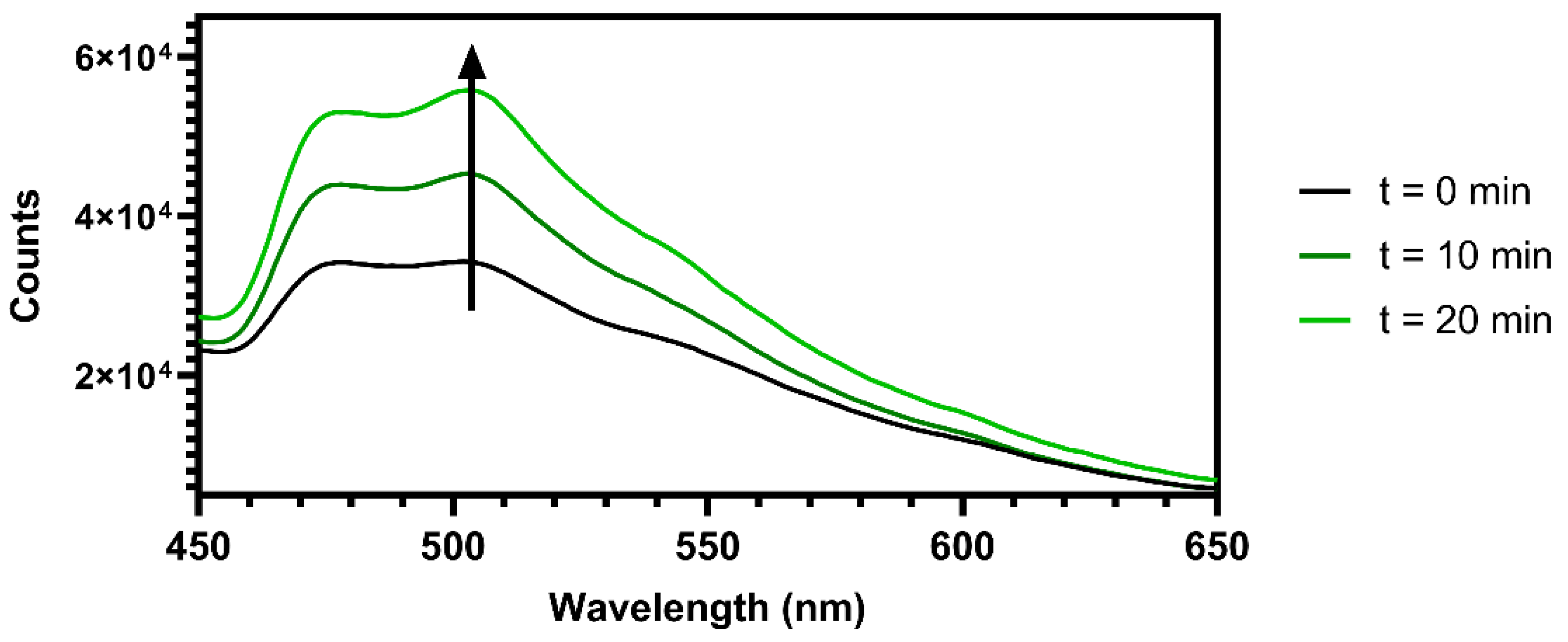
2.8. Oxygen Sensing Capability of MCC-Ir
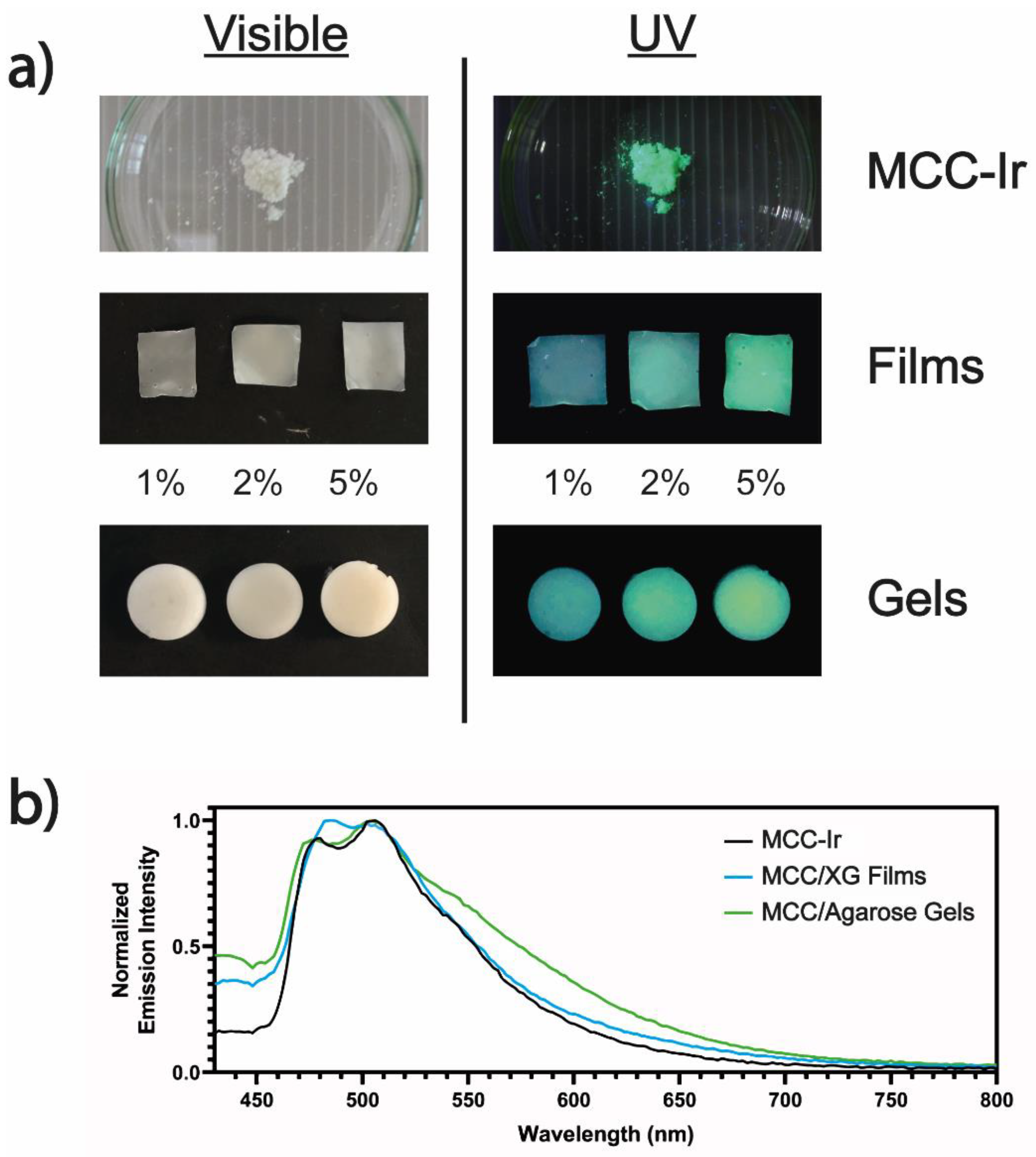
2.9. Formulation of MCC-Ir-Loaded Cellulose/Agarose (MCC/Agarose) Hydrogels
2.10. Solvent-Casting of MCC-Ir-Loaded Cellulose/Xanthan Gum (MCC/XG) Thin Films
3. Results
3.1. Synthesis and Characterization of MCC-Ir
3.2. Photostability of MCC-Ir
3.3. Oxygen Sensing Capability of MCC-Ir
3.4. Formulation of MCC-Ir in Dry and Wet 3D Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef] [PubMed]
- George, J.; SN, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2022, 278, 118956. [Google Scholar] [CrossRef]
- Padzil, F.N.M.; Lee, C.H.; Lee, S.H.; Asa’ari, A.Z.M.; Chin, K.L.; Yasim-Anuar, T.A.T.; Ariffin, H. Nanocellulose composites in the pulp and paper industry. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 375–395. [Google Scholar]
- Gauss, C.; Pickering, K.L.; Muthe, L.P. The use of cellulose in bio-derived formulations for 3D/4D printing: A review. Compos. Part C Open Access 2021, 4, 100113. [Google Scholar] [CrossRef]
- Jin, T.; Moores, A. Nanocellulose in Catalysis: A Renewable Support toward Enhanced Nanocatalysis. In Nanoparticles in Catalysis; Wiley: Hoboken, NJ, USA, 2021; pp. 139–157. [Google Scholar]
- Teodoro, K.B.R.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.M.; Correa, D.S. A Review on the Role and Performance of Cellulose Nanomaterials in Sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef]
- Tortorella, S.; Vetri Buratti, V.; Maturi, M.; Sambri, L.; Comes Franchini, M.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Sulaiman, S.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Sulaiman, A. A Review: Potential Usage of Cellulose Nanofibers (CNF) for Enzyme Immobilization via Covalent Interactions. Appl. Biochem. Biotechnol. 2015, 175, 1817–1842. [Google Scholar] [CrossRef]
- Shang, W.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.; Xie, G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Tortorella, S.; Maturi, M.; Dapporto, F.; Spanu, C.; Sambri, L.; Comes Franchini, M.; Chiariello, M.; Locatelli, E. Surface modification of nanocellulose through carbamate link for a selective release of chemotherapeutics. Cellulose 2020, 27, 8503–8511. [Google Scholar] [CrossRef]
- Peng, S.X.; Chang, H.; Kumar, S.; Moon, R.J.; Youngblood, J.P. A comparative guide to controlled hydrophobization of cellulose nanocrystals via surface esterification. Cellulose 2016, 23, 1825–1846. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Cellante, L.; Costa, R.; Monaco, I.; Cenacchi, G.; Locatelli, E. One-step esterification of nanocellulose in a Brønsted acid ionic liquid for delivery to glioblastoma cancer cells. New J. Chem. 2018, 42, 5237–5242. [Google Scholar] [CrossRef]
- Pan, T.; Liu, S.; Zhang, L.; Xie, W.; Yu, C. A flexible, multifunctional, optoelectronic anticounterfeiting device from high-performance organic light-emitting paper. Light Sci. Appl. 2022, 11, 59. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, K.; Tian, J.; Yin, L.; Sheng, X. Biocompatible and Biodegradable Light-Emitting Materials and Devices. Adv. Mater. Technol. 2022, 7, 2100006. [Google Scholar] [CrossRef]
- Di Girolamo, A.; Monti, F.; Mazzanti, A.; Matteucci, E.; Armaroli, N.; Sambri, L.; Baschieri, A. 4-Phenyl-1,2,3-triazoles as Versatile Ligands for Cationic Cyclometalated Iridium(III) Complexes. Inorg. Chem. 2022, 61, 8509–8520. [Google Scholar] [CrossRef]
- Lo, K.K.-W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Ruseckas, A.; Staton, S.V.; Knights, K.; Cumpstey, N.; Burn, P.L.; Samuel, I.D.W. Phosphorescence quenching of fac-tris(2-phenylpyridyl)iridium(III) complexes in thin films on dielectric surfaces. Phys. Chem. Chem. Phys. 2016, 18, 3575–3580. [Google Scholar] [CrossRef] [Green Version]
- Naddaka, M.; Locatelli, E.; Colecchia, D.; Sambri, L.; Monaco, I.; Baschieri, A.; Sasdelli, F.; Chiariello, M.; Matteucci, E.; Zani, P.; et al. Hybrid cholesterol-based nanocarriers containing phosphorescent Ir complexes: In vitro imaging on glioblastoma cell line. RSC Adv. 2015, 5, 1091–1096. [Google Scholar] [CrossRef]
- Monaco, I.; Maturi, M.; Matteucci, E.; Locatelli, E.; Baschieri, A.; Zani, P.; Armanetti, P.; Menichetti, L.; Sambri, L.; Comes Franchini, M. Phosphorescent iridium-containing nanomicelles: Synthesis, characterization and preliminary applications in nanomedical imaging. RSC Adv. 2018, 8, 34162–34167. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Esker, A.R.; Roman, M. Acid-Catalyzed and Solvolytic Desulfation of H2SO4 -Hydrolyzed Cellulose Nanocrystals. Langmuir 2010, 26, 17919–17925. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Ho, P.-Y.; Ho, C.-L.; Wong, W.-Y. Recent advances of iridium(III) metallophosphors for health-related applications. Coord. Chem. Rev. 2020, 413, 213267. [Google Scholar] [CrossRef]
- Vacha, M.; Koide, Y.; Kotani, M.; Sato, H. Photobleaching and single molecule detection of a phosphorescent organometallic iridium(III) complex. J. Lumin. 2004, 107, 51–56. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef]
- Langari, M.M.; Nikzad, M.; Ghoreyshi, A.A.; Mohammadi, M. Isolation of Nanocellulose from Broomcorn Stalks and Its Application for Nanocellulose/Xanthan Film Preparation. ChemistrySelect 2019, 4, 11987–11994. [Google Scholar] [CrossRef]
- Melo, C.P.B.; Grossmann, M.V.E.; Yamashita, F.; Youssef, E.Y.; Dall’Antônia, L.H.; Mali, S. Effect of Manufacturing Process and Xanthan Gum Addition on the Properties of Cassava Starch Films. J. Polym. Environ. 2011, 19, 739–749. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New Insight into Agarose Gel Mechanical Properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, K.J.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Rheological study of reinforcement of agarose hydrogels by cellulose nanowhiskers. Carbohydr. Polym. 2015, 116, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient Visible Light Photocatalysis of [2+2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Hofbeck, T.; Yersin, H. The Triplet State of fac-Ir(ppy)3. Inorg. Chem. 2010, 49, 9290–9299. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Fulgheri, T.; Della Penna, F.; Baschieri, A.; Carlone, A. Advancements in the recycling of organocatalysts: From classical to alternative approaches. Curr. Opin. Green Sustain. Chem. 2020, 25, 100387. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maturi, M.; Spanu, C.; Baschieri, A.; Comes Franchini, M.; Locatelli, E.; Sambri, L. Iridium-Functionalized Cellulose Microcrystals as a Novel Luminescent Biomaterial for Biocomposites. Biomolecules 2022, 12, 1165. https://doi.org/10.3390/biom12091165
Maturi M, Spanu C, Baschieri A, Comes Franchini M, Locatelli E, Sambri L. Iridium-Functionalized Cellulose Microcrystals as a Novel Luminescent Biomaterial for Biocomposites. Biomolecules. 2022; 12(9):1165. https://doi.org/10.3390/biom12091165
Chicago/Turabian StyleMaturi, Mirko, Chiara Spanu, Andrea Baschieri, Mauro Comes Franchini, Erica Locatelli, and Letizia Sambri. 2022. "Iridium-Functionalized Cellulose Microcrystals as a Novel Luminescent Biomaterial for Biocomposites" Biomolecules 12, no. 9: 1165. https://doi.org/10.3390/biom12091165
APA StyleMaturi, M., Spanu, C., Baschieri, A., Comes Franchini, M., Locatelli, E., & Sambri, L. (2022). Iridium-Functionalized Cellulose Microcrystals as a Novel Luminescent Biomaterial for Biocomposites. Biomolecules, 12(9), 1165. https://doi.org/10.3390/biom12091165










