Impact of Inhibition of Glutamine and Alanine Transport on Cerebellar Glial and Neuronal Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Brain Cerebellar Tissue Slices
2.3. Inhibition of Glutamine Transport
2.4. Preparation of Samples
2.5. NMR Analysis
2.6. Measurement of Metabolic Pool Sizes by LCMS
2.7. Visualization of Expression Data
2.8. Statistical Analysis
3. Results
3.1. Inhibition of Glutamine Transport with 10 mm Histidine under Depolarizing Conditions
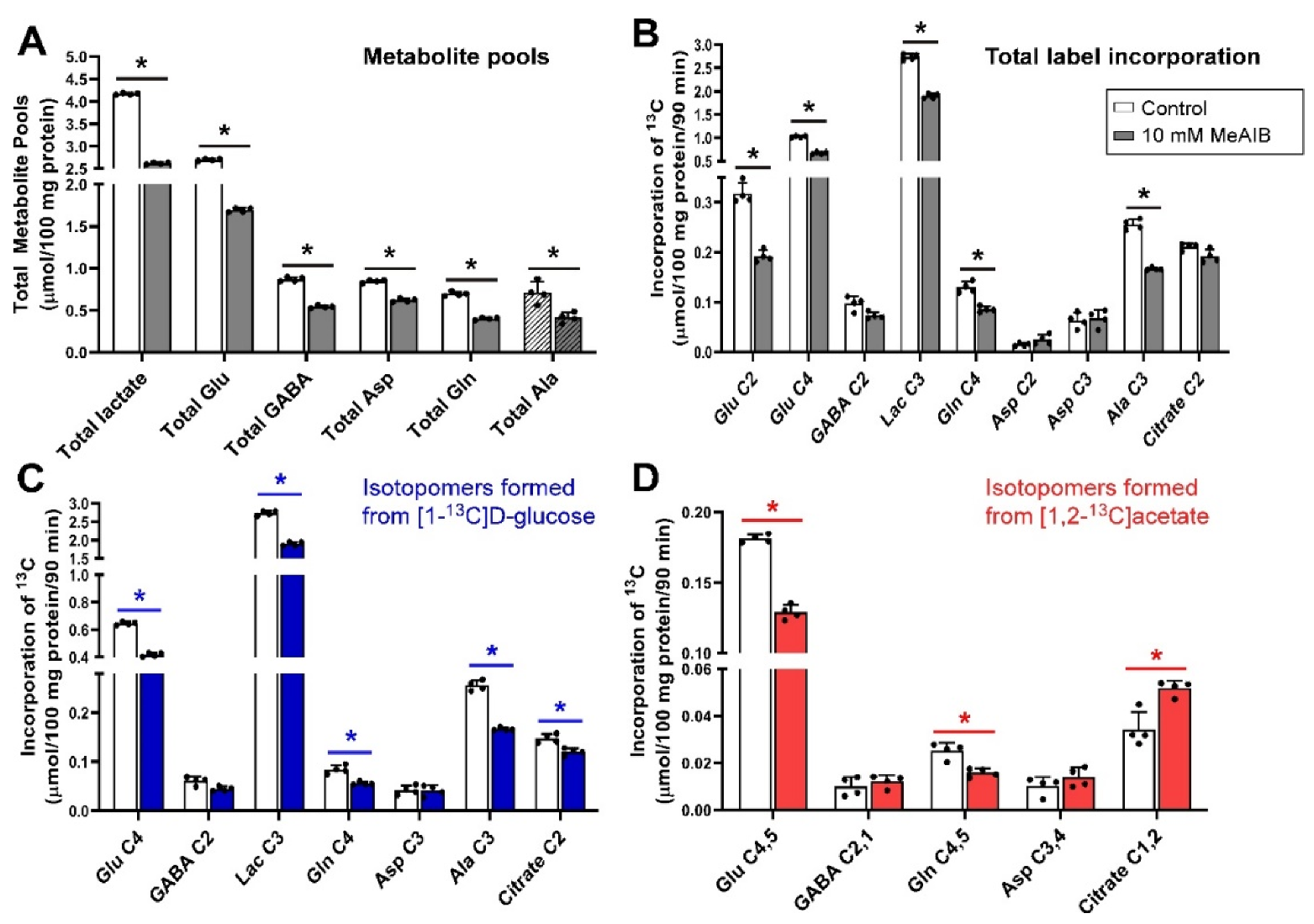
3.2. Inhibition of Glutamine Transport with 10 mm MeAIB under Depolarizing Conditions
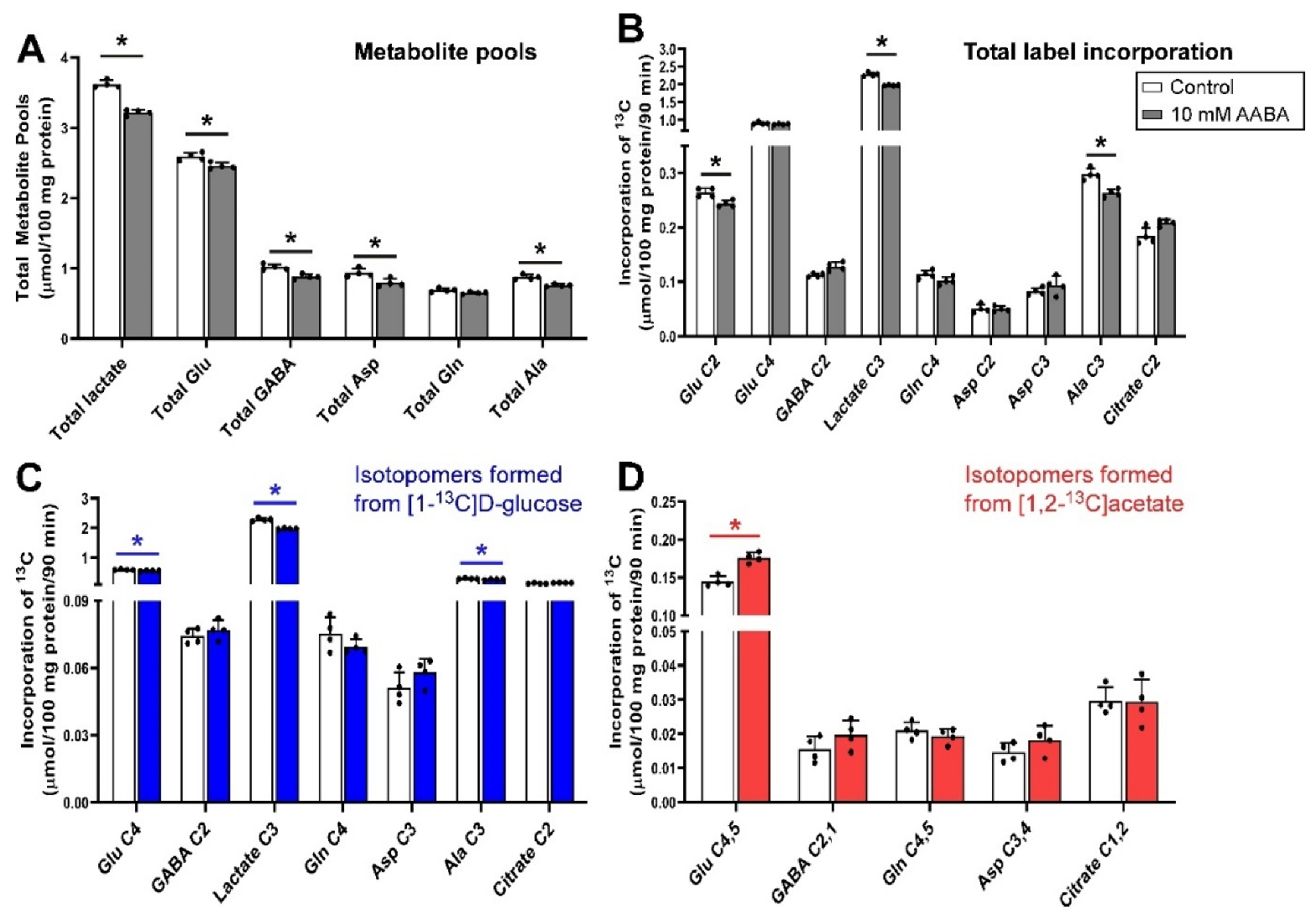
3.3. Inhibition of Glutamine Transport with AABA 100 µm under Depolarizing Conditions
3.4. Inhibition of Glutamine Transport with 10 mm GPNA under Depolarizing Conditions
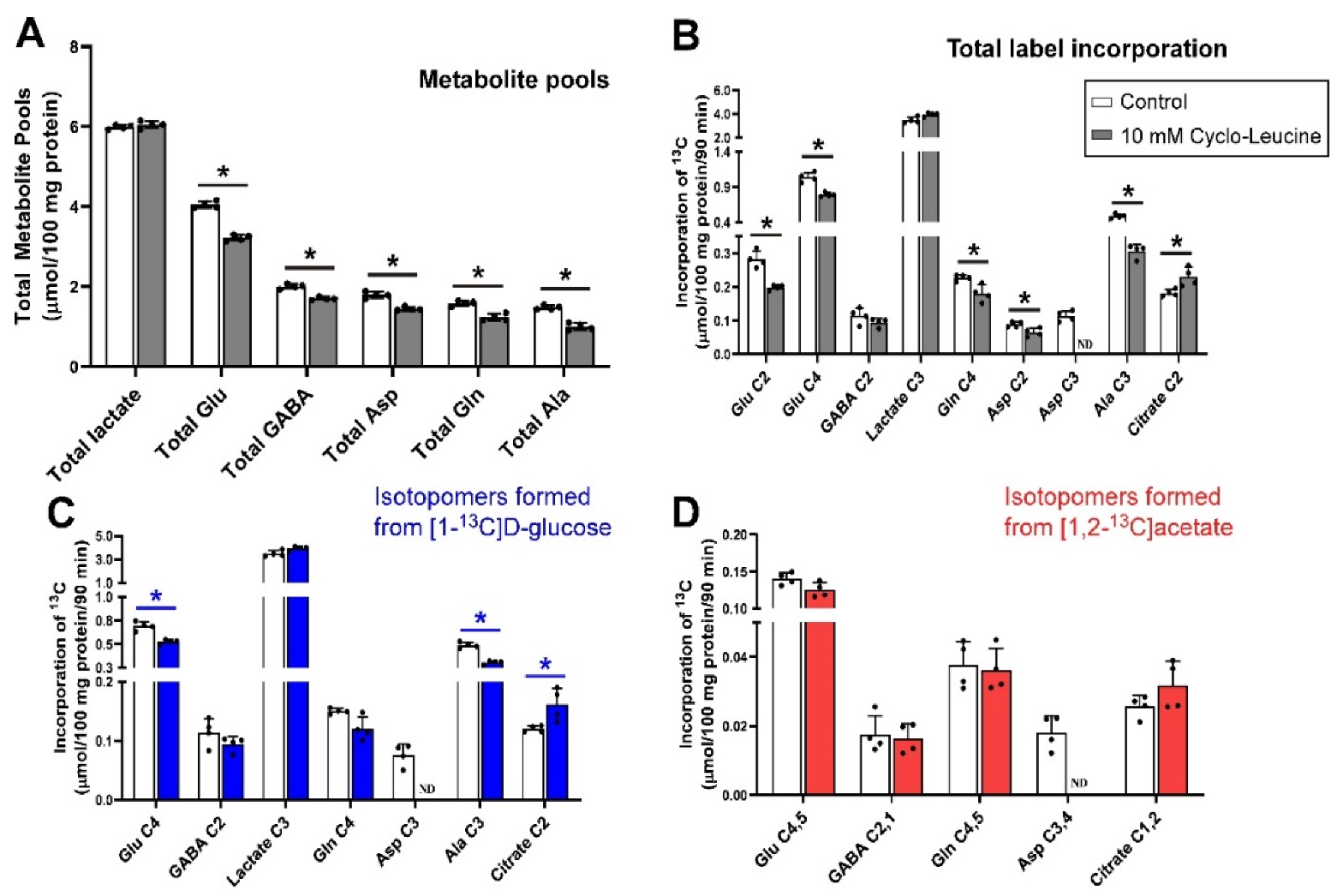
3.5. Inhibition of Glutamine Transport with 10 mm cLeu under Depolarizing Conditions
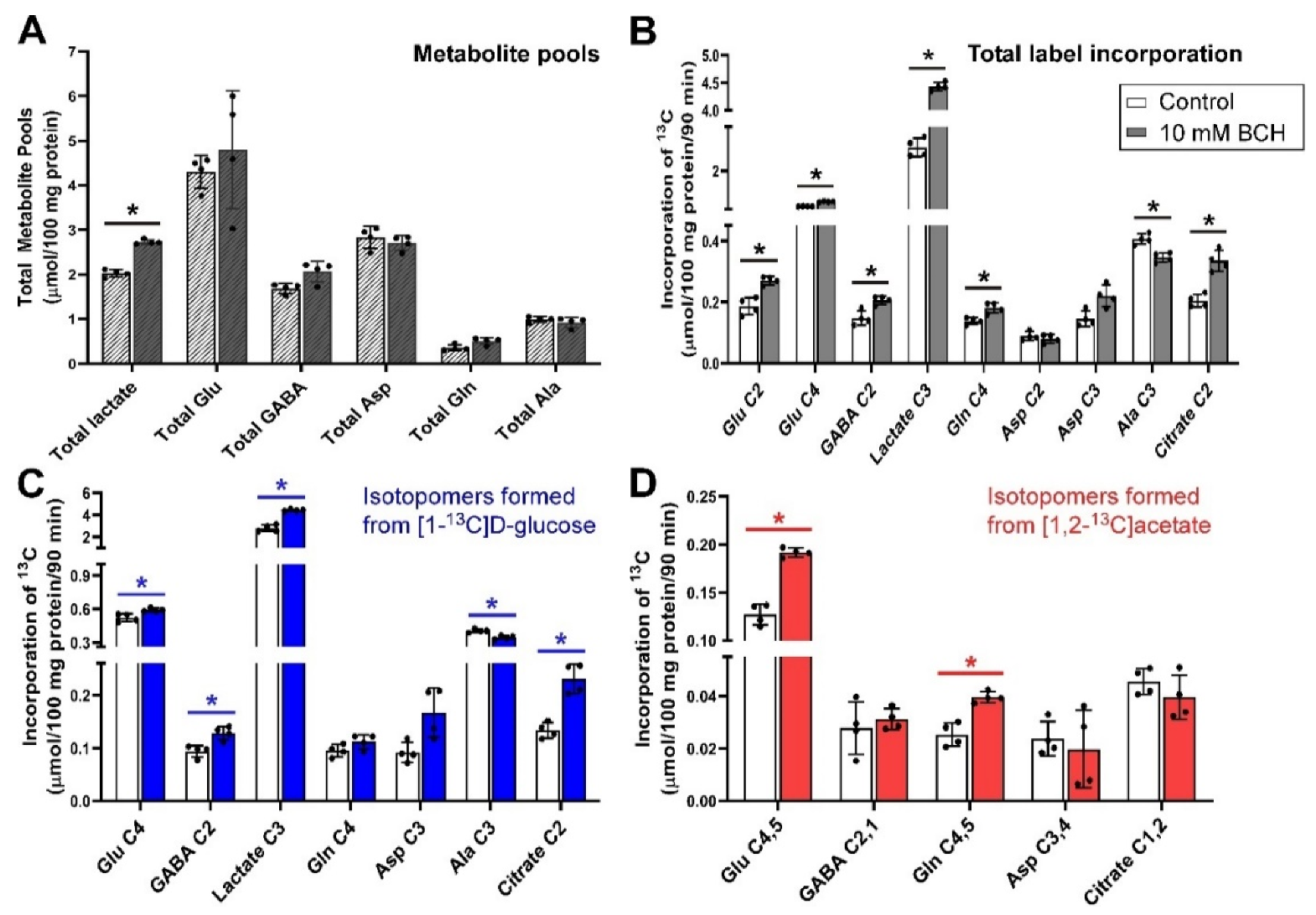
3.6. Inhibition of Glutamine Transport with 10 mm BCH under Depolarizing Conditions
3.7. Visualization of Expression Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassel, B.; Bachelard, H.; Jones, P.; Fonnum, F.; Sonnewald, U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J. Cereb. Blood Flow Metab. 1997, 17, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Brookes, N. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 2001, 77, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U.; Westergaard, N.; Schousboe, A.; Svendsen, J.S.; Unsgård, G.; Petersen, S.B. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int. 1993, 22, 19–29. [Google Scholar] [CrossRef]
- Walls, A.B.; Eyjolfsson, E.M.; Smeland, O.B.; Nilsen, L.H.; Schousboe, I.; Schousboe, A.; Sonnewald, U.; Waagepetersen, H.S. Knockout of GAD65 has Major Impact on Synaptic GABA Synthesized from Astrocyte-Derived Glutamine. J. Cereb. Blood Flow Metab. 2010, 31, 494–503. [Google Scholar] [CrossRef]
- Battaglioli, G.; Martin, D.L. GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochem. Res. 1991, 16, 151–156. [Google Scholar] [CrossRef]
- McKenna, M.C. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef]
- Waagepetersen, H.S.; Sonnewald, U.; Larsson, O.M.; Schousboe, A. A Possible Role of Alanine for Ammonia Transfer between Astrocytes and Glutamatergic Neurons. J. Neurochem. 2000, 75, 471–479. [Google Scholar] [CrossRef]
- Bröer, S.; Bröer, A.; Hansen, J.T.; Bubb, W.A.; Balcar, V.J.; Nasrallah, F.A.; Garner, B.; Rae, C. Alanine metabolism, transport, and cycling in the brain. J. Neurochem. 2007, 102, 1758–1770. [Google Scholar] [CrossRef]
- Griffin, J.L.; Keun, H.; Richter, C.; Moskau, D.; Rae, C.; Nicholson, J.K. Compartmentation of metabolism probed by [2-13C]alanine: Improved 13C NMR sensitivity using a CryoProbe detects evidence of a glial metabolon. Neurochem. Int. 2003, 42, 93–99. [Google Scholar] [CrossRef]
- Schousboe, A.; Sonnewald, U.; Waagepetersen, H.S. Differential roles of alanine in GABAergic and glutamatergic neurons. Neurochem. Int. 2003, 43, 311–315. [Google Scholar] [CrossRef]
- Nagaraja, T.N.; Brookes, N. Glutamine transport in mouse cerebral astrocytes. J. Neurochem. 1996, 66, 1665–1674. [Google Scholar] [CrossRef]
- Su, T.-Z.; Campbell, G.W.; Oxender, D.L. Glutamine transport in cerebellar granule cells in culture. Brain Res. 1997, 757, 69–78. [Google Scholar] [CrossRef]
- Tamarappoo, B.; Raizada, M.; Kilberg, M. Identification of a system N-like Na+-dependent glutamine transport activity in rat brain neurons. J. Neurochem. 1997, 68, 954–960. [Google Scholar] [CrossRef]
- Bröer, A.; Brookes, N.; Ganapathy, V.; Dimmer, K.S.; Wagner, C.; Lang, F.; Bröer, S. The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. J. Neurochem. 1999, 73, 2184–2194. [Google Scholar]
- Chaudhry, F.A.; Reimer, R.J.; Krizaj, D.; Barber, D.; Storm-Mathisen, J.; Copenhagen, D.R.; Edwards, R.H. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 1999, 99, 769–780. [Google Scholar] [CrossRef]
- Dolgodilina, E.; Imobersteg, S.; Laczko, E.; Welt, T.; Verrey, F.; Makrides, V. Brain interstitial fluid glutamine homeostasis is controlled by blood–brain barrier SLC7A5/LAT1 amino acid transporter. J. Cereb. Blood Flow Metab. 2016, 36, 1929–1941. [Google Scholar] [CrossRef]
- Zaia, K.A.; Reimer, R.J. Synaptic vesicle protein NTT4/XT1 (SLC6A17) catalyzes Na+-coupled neutral amino acid transport. J. Biol. Chem. 2009, 284, 8439–8448. [Google Scholar] [CrossRef]
- Varoqui, H.; Zhu, H.; Yao, D.; Ming, H.; Erickson, J.D. Cloning and functional identification of a neuronal glutamine transporter. J. Biol. Chem. 2000, 275, 4049–4054. [Google Scholar] [CrossRef]
- Kanamori, K.; Ross, B.D. Quantitative determination of extracellular glutamine concentration in rat brain, and its elevation in vivo by system A transport inhibitor, α-(methylamino) isobutyrate. J. Neurochem. 2004, 90, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Tietze, N.; Kowalczuk, S.; Chubb, S.; Munzinger, M.; Bak, L.K.; Bröer, S. The orphan transporter v7-3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2). Biochem. J. 2006, 393, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, M.G.; Hellsten, S.V.; Bagchi, S.; Ljungdahl, A.; Nilsson, V.C.; Winnergren, S.; Stephansson, O.; Rumaks, J.; Svirskis, S.; Klusa, V. Characterization of the transporterB 0 AT3 (Slc6a17) in the rodent central nervous system. BMC Neurosci. 2013, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Segawa, H.; Miyamoto, K.-I.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef]
- Broer, S.; Broer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Salganicoff, L.; Robertis, E.D. Subcellular Distribution of the Enzymes of the Glutamic Acid, Glutamine and γ-Aminobutyric Acid Cycles in Rat Brain. J. Neurochem. 1965, 12, 287–309. [Google Scholar] [CrossRef]
- Erecinska, M.; Nelson, D.; Nissim, I.; Daikhin, Y.; Yudkoff, M. Cerebral alanine transport and alanine aminotransferase reaction: Alanine as a source of neuronal glutamate. J. Neurochem. 1994, 62, 1953–1964. [Google Scholar] [CrossRef]
- Fei, Y.-J.; Sugawara, M.; Nakanishi, T.; Huang, W.; Wang, H.; Prasad, P.D.; Leibach, F.H.; Ganapathy, V. Primary structure, genomic organization, and functional and electrogenic characteristics of human system N 1, a Na+-and H+-coupled glutamine transporter. J. Biol. Chem. 2000, 275, 23707–23717. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Li, W.; Mysona, B.A.; Smith, S.B.; Ganapathy, V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3980–3987. [Google Scholar] [CrossRef]
- Boulland, J.L.; Osen, K.K.; Levy, L.M.; Danbolt, N.C.; Edwards, R.H.; Storm-Mathisen, J.; Chaudhry, F.A. Cell-specific expression of the glutamine transporter SN1 suggests differences in dependence on the glutamine cycle. Eur. J. Neurosci. 2002, 15, 1615–1631. [Google Scholar] [CrossRef]
- Rae, C.; Hare, N.; Bubb, W.A.; McEwan, S.R.; Bröer, A.; McQuillan, J.A.; Balcar, V.J.; Conigrave, A.D.; Bröer, S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: Further evidence for metabolic compartmentation. J. Neurochem. 2003, 85, 503–514. [Google Scholar] [CrossRef]
- Christensen, H.N.; Liang, M.; Archer, E.G. A distinct Na+-requiring transport system for alanine, serine, cysteine, and similar amino acids. J. Biol. Chem. 1967, 242, 5237–5246. [Google Scholar] [CrossRef]
- Fairweather, S.J.; Okada, S.; Gauthier-Coles, G.; Javed, K.; Broer, A.; Broer, S. A GC-MS/Single-Cell Method to Evaluate Membrane Transporter Substrate Specificity and Signaling. Front. Mol. Biosci. 2021, 8, 646574. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Sugawara, M.; Devoe, L.D.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem. Biophys. Res. Commun. 2000, 273, 1175–1179. [Google Scholar] [CrossRef]
- Sugawara, M.; Nakanishi, T.; Fei, Y.-J.; Huang, W.; Ganapathy, M.E.; Leibach, F.H.; Ganapathy, V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000, 275, 16473–16477. [Google Scholar] [CrossRef]
- Yao, D.; Mackenzie, B.; Ming, H.; Varoqui, H.; Zhu, H.; Hediger, M.A.; Erickson, J.D. A novel system A isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem. 2000, 275, 22790–22797. [Google Scholar] [CrossRef]
- Armano, S.; Coco, S.; Bacci, A.; Pravettoni, E.; Schenk, U.; Verderio, C.; Varoqui, H.; Erickson, J.D.; Matteoli, M. Localization and functional relevance of system a neutral amino acid transporters in cultured hippocampal neurons. J. Biol. Chem. 2002, 277, 10467–10473. [Google Scholar] [CrossRef]
- Dolińska, M.; Zabłocka, B.; Sonnewald, U.; Albrecht, J. Glutamine uptake and expression of mRNA’s of glutamine transporting proteins in mouse cerebellar and cerebral cortical astrocytes and neurons. Neurochem. Int. 2004, 44, 75–81. [Google Scholar] [CrossRef]
- Maallem, S.; Mutin, M.; González-González, I.M.; Zafra, F.; Tappaz, M.L. Selective tonicity-induced expression of the neutral amino-acid transporter SNAT2 in oligodendrocytes in rat brain following systemic hypertonicity. Neuroscience 2008, 153, 95–107. [Google Scholar] [CrossRef]
- Hägglund, M.G.; Roshanbin, S.; Löfqvist, E.; Hellsten, S.V.; Nilsson, V.C.; Todkar, A.; Zhu, Y.; Stephansson, O.; Drgonova, J.; Uhl, G.R. B0AT2 (SLC6A15) is localized to neurons and astrocytes, and is involved in mediating the effect of leucine in the brain. PLoS ONE 2013, 8, e58651. [Google Scholar]
- El Mestikawy, S.; Wehrle, R.; Masson, J.; Lombard, M.; Hamon, M.; Sotelo, C. Distribution pattern and ultrastructural localization of Rxt1, an orphan Na+/Cl−-dependent transporter, in the central nervous system of rats and mice. Neuroscience 1997, 77, 319–333. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Bröer, A.; Bröer, S. Glutamine efflux from astrocytes is mediated by multiple pathways. J. Neurochem. 2003, 87, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Heckel, T.; Bröer, A.; Wiesinger, H.; Lang, F.; Bröer, S. Asymmetry of glutamine transporters in cultured neural cells. Neurochem. Int. 2003, 43, 289–298. [Google Scholar] [CrossRef]
- Huttunen, J.; Peltokangas, S.; Gynther, M.; Natunen, T.; Hiltunen, M.; Auriola, S.; Ruponen, M.; Vellonen, K.-S.; Huttunen, K.M. L-type amino acid transporter 1 (LAT1/Lat1)-utilizing prodrugs can improve the delivery of Drugs into neurons, astrocytes and microglia. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Albrecht, J.; Zielińska, M. Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology 2019, 161, 107560. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Wagner, C.A.; Lang, F.; Bröer, S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem. J. 2000, 349 Pt 3, 787–795. [Google Scholar] [CrossRef]
- Bodoy, S.; Martín, L.; Zorzano, A.; Palacín, M.; Estévez, R.; Bertran, J. Identification of LAT4, a Novel Amino Acid Transporter with System L Activity. J. Biol. Chem. 2005, 280, 12002–12011. [Google Scholar] [CrossRef]
- Farmer, M.K.; Robbins, M.J.; Medhurst, A.D.; Campbell, D.A.; Ellington, K.; Duckworth, M.; Brown, A.M.; Middlemiss, D.N.; Price, G.W.; Pangalos, M.N. Cloning and Characterization of Human NTT5 and v7-3: Two Orphan Transporters of the Na+/Cl−-Dependent Neurotransmitter Transporter Gene Family. Genomics 2000, 70, 241–252. [Google Scholar] [CrossRef]
- Masson, J.; Pohl, M.; Aidouni, Z.; Giros, B.; Hamon, M.; el Mestikawy, S. The two orphan Na+/Cl(−)-dependent transporters Rxt1 and V-7-3-2 have an overlapping expression pattern in the rat central nervous system. Recept. Channels 1996, 4, 227–242. [Google Scholar]
- Broer, S.; Gether, U. The solute carrier 6 family of transporters. Br. J. Pharm. 2012, 167, 256–278. [Google Scholar] [CrossRef]
- Cuboni, S.; Devigny, C.; Hoogeland, B.; Strasser, A.; Pomplun, S.; Hauger, B.; Höfner, G.; Wanner, K.T.; Eder, M.; Buschauer, A.; et al. Loratadine and Analogues: Discovery and Preliminary Structure—Activity Relationship of Inhibitors of the Amino Acid Transporter B0AT2. J. Med. Chem. 2014, 57, 9473–9479. [Google Scholar] [CrossRef]
- Parra, L.A.; Baust, T.; El Mestikawy, S.; Quiroz, M.; Hoffman, B.; Haflett, J.M.; Yao, J.K.; Torres, G.E. The Orphan Transporter Rxt1/NTT4 (SLC6A17) Functions as a Synaptic Vesicle Amino Acid Transporter Selective for Proline, Glycine, Leucine, and Alanine. Mol. Pharmacol. 2008, 74, 1521–1532. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef]
- Singh, N.; Scalise, M.; Galluccio, M.; Wieder, M.; Seidel, T.; Langer, T.; Indiveri, C.; Ecker, G.F. Discovery of Potent Inhibitors for the Large Neutral Amino Acid Transporter 1 (LAT1) by Structure-Based Methods. Int. J. Mol. Sci. 2018, 20, 27. [Google Scholar] [CrossRef]
- Chiu, M.; Sabino, C.; Taurino, G.; Bianchi, M.G.; Andreoli, R.; Giuliani, N.; Bussolati, O. GPNA inhibits the sodium-independent transport system L for neutral amino acids. Amino Acids 2017, 49, 1365–1372. [Google Scholar] [CrossRef]
- Bröer, A.; Fairweather, S.; Bröer, S. Disruption of Amino Acid Homeostasis by Novel ASCT2 Inhibitors Involves Multiple Targets. Front. Pharmacol. 2018, 9, 785. [Google Scholar] [CrossRef]
- Pineda, M.; Fernández, E.; Torrents, D.; Estévez, R.; López, C.; Camps, M.; Lloberas, J.; Zorzano, A.; Palacín, M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J. Biol. Chem. 1999, 274, 19738–19744. [Google Scholar] [CrossRef]
- Bröer, S. SLC38 Family of Transporters for Neutral Amino Acids. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Membranes and Transport; Lajtha, A., Reith, M.E.A., Eds.; Springer: Boston, MA, USA, 2007; pp. 327–338. [Google Scholar]
- Mackenzie, B.; Schäfer, M.K.H.; Erickson, J.D.; Hediger, M.A.; Weihe, E.; Varoqui, H. Functional Properties and Cellular Distribution of the System a Glutamine Transporter SNAT1 Support Specialized Roles in Central Neurons. J. Biol. Chem. 2003, 278, 23720–23730. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Schmitz, D.; Reimer, R.J.; Larsson, P.; Gray, A.T.; Nicoll, R.; Kavanaugh, M.; Edwards, R.H. Glutamine uptake by neurons: Interaction of protons with system a transporters. J. Neurosci. 2002, 22, 62–72. [Google Scholar] [CrossRef]
- Weiss, M.D.; Derazi, S.; Rossignol, C.; Varoqui, H.; Erickson, J.D.; Kilberg, M.S.; Anderson, K.J. Ontogeny of the neutral amino acid transporter SAT1/ATA1 in rat brain. Dev. Brain Res. 2003, 143, 151–159. [Google Scholar] [CrossRef]
- Bröer, A.; Albers, A.; Setiawan, I.; Edwards, R.H.; Chaudhry, F.A.; Lang, F.; Wagner, C.A.; Bröer, S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol. 2002, 539, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. The SLC38 family of sodium-amino acid co-transporters. Pflügers Arch.-Eur. J. Physiol. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Dominici, S.; Piaggi, S.; Belcastro, E.; Chiu, M.; Taurino, G.; Pacini, S.; Bussolati, O.; Pompella, A. γ-Glutamyltransferase enzyme activity of cancer cells modulates L-γ-glutamyl-p-nitroanilide (GPNA) cytotoxicity. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Rahimi, F.; Bröer, S. Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J. Biol. Chem. 2016, 291, 13194–13205. [Google Scholar] [CrossRef]
- Reimer, R.J.; Chaudhry, F.A.; Gray, A.T.; Edwards, R.H. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 7715–7720. [Google Scholar] [CrossRef]
- Boulland, J.L.; Rafiki, A.; Levy, L.M.; Storm-Mathisen, J.; Chaudhry, F.A. Highly differential expression of SN1, a bidirectional glutamine transporter, in astroglia and endothelium in the developing rat brain. Glia 2003, 41, 260–275. [Google Scholar] [CrossRef]
- Bodoy, S.; Fotiadis, D.; Stoeger, C.; Kanai, Y.; Palacin, M. The small SLC43 family: Facilitator system l amino acid transporters and the orphan EEG1. Mol. Asp. Med. 2013, 34, 638–645. [Google Scholar] [CrossRef]
- Rajendran, A.; Poncet, N.; Oparija-Rogenmozere, L.; Herzog, B.; Verrey, F. Tissue-specific deletion of mouse basolateral uniporter LAT4 (Slc43a2) reveals its crucial role in small intestine and kidney amino acid transport. J. Physiol. 2020, 598, 5109–5132. [Google Scholar] [CrossRef]
- Guetg, A.; Mariotta, L.; Bock, L.; Herzog, B.; Fingerhut, R.; Camargo, S.M.; Verrey, F. Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J. Physiol. 2015, 593, 1273–1289. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030.e16. [Google Scholar] [CrossRef]
- Bröer, S. Amino Acid Transporters as Targets for Cancer Therapy: Why, Where, When, and How. Int. J. Mol. Sci. 2020, 21, 6156. [Google Scholar] [CrossRef]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Martínez-Lozada, Z.; Guillem, A.M.; Flores-Méndez, M.; Hernández-Kelly, L.C.; Vela, C.; Meza, E.; Zepeda, R.C.; Caba, M.; Rodríguez, A.; Ortega, A. GLAST/EAAT1-induced Glutamine release via SNAT3 in Bergmann glial cells: Evidence of a functional and physical coupling. J. Neurochem. 2013, 125, 545–554. [Google Scholar] [CrossRef]
- Balmer, T.S.; Borges-Merjane, C.; Trussell, L.O. Incomplete removal of extracellular glutamate controls synaptic transmission and integration at a cerebellar synapse. eLife 2021, 10, e63819. [Google Scholar] [CrossRef]
- Ferreira, G.C.; Karimi, A.J.; Waddell, J.; McKenna, M.C. Metabolism of [1,6-13C]glucose in the cerebellum of 18-day-old rats: Comparison with cerebral metabolism. J. Neurochem. 2021, 157, 1946–1962. [Google Scholar] [CrossRef]
- Griffin, J.L.; Rae, C.; Radda, G.K.; Matthews, P.M. Delayed labelling of brain glutamate after an intra-arterial [13C]glucose bolus: Evidence for aerobic metabolism of guinea pig brain glycogen store. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 1999, 1450, 297–307. [Google Scholar] [CrossRef]
- Rae, C.; Balcar, V.J. A chip off the old block: The brain slice as a model for metabolic studies of brain compartmentation and neuropharmacology. In Brain Energy Metabolism; Springer: Berlin/Heidelberg, Germany, 2014; pp. 217–241. [Google Scholar]
- Amaral, A.I.; Hadera, M.G.; Tavares, J.M.; Kotter, M.R.; Sonnewald, U. Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells. Glia 2016, 64, 21–34. [Google Scholar] [CrossRef]
- Rowlands, B.D.; Klugmann, M.; Rae, C.D. Acetate metabolism does not reflect astrocytic activity, contributes directly to GABA synthesis, and is increased by silent information regulator 1 activation. J. Neurochem. 2017, 140, 903–918. [Google Scholar] [CrossRef]
- Andersen, J.V.; McNair, L.F.; Schousboe, A.; Waagepetersen, H.S. Specificity of exogenous acetate and glutamate as astrocyte substrates examined in acute brain slices from female mice using methionine sulfoximine (MSO) to inhibit glutamine synthesis. J. Neurosci. Res. 2017, 95, 2207–2216. [Google Scholar] [CrossRef]
- Badar-Goffer, R.S.; Bachelard, H.S.; Morris, P.G. Cerebral metabolism of acetate and glucose studied by 13C-nmr spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem. J. 1990, 266, 133–139. [Google Scholar] [CrossRef]
- McIlwain, H. Biochemistry and the central nervous system. J. Nerv. Ment. Dis. 1955, 122, 499. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishimura, T.; Higuchi, K.; Noguchi, S.; Tega, Y.; Kurosawa, T.; Deguchi, Y.; Tomi, M. Transport of pregabalin via L-type amino acid transporter 1 (SLC7A5) in human brain capillary endothelial cell line. Pharm. Res. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Belle, J.L.; Harris, N.; Williams, S.; Bhakoo, K. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2002, 15, 37–44. [Google Scholar] [CrossRef]
- Rowlands, B.D.; Lau, C.L.; Ryall, J.G.; Thomas, D.S.; Klugmann, M.; Beart, P.M.; Rae, C.D. Silent information regulator 1 modulator resveratrol increases brain lactate production and inhibits mitochondrial metabolism, whereas SRT1720 increases oxidative metabolism. J. Neurosci. Res. 2015, 93, 1147–1156. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Rae, C.; Lawrance, M.L.; Dias, L.S.; Provis, T.; Bubb, W.A.; Balcar, V.J. Strategies for studies of neurotoxic mechanisms involving deficient transport of L-glutamate: Antisense knockout in rat brain in vivo and changes in the neurotransmitter metabolism following inhibition of glutamate transport in guinea pig brain slices. Brain Res. Bull. 2000, 53, 373–381. [Google Scholar] [CrossRef]
- Tapia, R.; Gonzalez, R.M. Glutamine and glutamate as precursors of the releasable pool of gaba in brain cortex slices. Neurosci. Lett. 1978, 10, 165–169. [Google Scholar] [CrossRef]
- Lebon, V.; Petersen, K.F.; Cline, G.W.; Shen, J.; Mason, G.F.; Dufour, S.; Behar, K.L.; Shulman, G.I.; Rothman, D.L. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: Elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci. 2002, 22, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Achanta, L.B.; Rowlands, B.D.; Thomas, D.S.; Housley, G.D.; Rae, C.D. β-Hydroxybutyrate Boosts Mitochondrial and Neuronal Metabolism but is not Preferred Over Glucose under Activated Conditions. Neurochem. Res. 2017, 42, 1710–1723. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Sonnewald, U.; Huang, X.; Stevenson, J.; Zielke, H.R. Exogenous Glutamate Concentration Regulates the Metabolic Fate of Glutamate in Astrocytes. J. Neurochem. 2002, 66, 386–393. [Google Scholar] [CrossRef]
- Erecinska, M.; Pleasure, D.; Nelson, D.; Nissim, I.; Yudkoff, M. Cerebral aspartate utilization—Near-equilibrium relationships in aspartate-aminotransferase reaction. J. Neurochem. 1993, 60, 1696–1706. [Google Scholar] [CrossRef]
- Griffin, J.L.; Rae, C.; Dixon, R.M.; Radda, G.K.; Matthews, P.M. Excitatory amino acid synthesis in hypoxic brain slices: Does alanine act as a substrate for glutamate production in hypoxia? J. Neurochem. 1998, 71, 2477–2486. [Google Scholar] [CrossRef]
- Gauthier-Coles, G.; Vennitti, J.; Zhang, Z.; Comb, W.C.; Xing, S.; Javed, K.; Bröer, A.; Bröer, S. Quantitative modelling of amino acid transport and homeostasis in mammalian cells. Nat. Commun. 2021, 12, 1–18. [Google Scholar]
- Schulte, M.L.; Khodadadi, A.B.; Cuthbertson, M.L.; Smith, J.A.; Manning, H.C. 2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids: A novel scaffold for inhibition of ASCT2-mediated glutamine transport. Bioorganic Med. Chem. Lett. 2016, 26, 1044–1047. [Google Scholar] [CrossRef]
- Esslinger, C.S.; Cybulski, K.A.; Rhoderick, J.F. Nγ-Aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 2005, 13, 1111–1118. [Google Scholar] [CrossRef]
- Pajari, M. Properties of γ-glutamyltransferase in developing rat brain. Int. J. Dev. Neurosci. 1984, 2, 197–202. [Google Scholar] [CrossRef]
- Rae, C.D.; Williams, S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 127–143. [Google Scholar] [CrossRef]
- Furlong, T.M.; Duncan, J.R.; Corbit, L.H.; Rae, C.D.; Rowlands, B.D.; Maher, A.D.; Nasrallah, F.A.; Milligan, C.J.; Petrou, S.; Lawrence, A.J.; et al. Toluene inhalation in adolescent rats reduces flexible behaviour in adulthood and alters glutamatergic and GABAergic signalling. J. Neurochem. 2016, 139, 806–822. [Google Scholar] [CrossRef]
- Snell, L.D.; Johnson, K.M. Cycloleucine competitively antagonizes the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 151, 165–166. [Google Scholar] [CrossRef]
- Blot, A.; Billups, D.; Bjørkmo, M.; Quazi, A.Z.; Uwechue, N.M.; Chaudhry, F.A.; Billups, B. Functional expression of two system a glutamine transporter isoforms in rat auditory brainstem neurons. Neuroscience 2009, 164, 998–1008. [Google Scholar] [CrossRef][Green Version]
- Gylfe, E. Comparison of the effects of leucines, non-metabolizable leucine analogues and other insulin secretagogues on the activity of glutamate dehydrogenase. Acta Diabetol. Lat. 1976, 13, 20–24. [Google Scholar] [CrossRef]
- McKenna, M.C. Glutamate dehydrogenase in brain mitochondria: Do lipid modifications and transient metabolon formation influence enzyme activity? Neurochem. Int. 2011, 59, 525–533. [Google Scholar] [CrossRef]
- Spanaki, C.; Zaganas, I.; Kleopa, K.A.; Plaitakis, A. Human GLUD2 Glutamate Dehydrogenase Is Expressed in Neural and Testicular Supporting Cells. J. Biol. Chem. 2010, 285, 16748–16756. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Stevenson, J.H.; Huang, X.; Hopkins, I.B. Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem. Int. 2000, 37, 229–241. [Google Scholar] [CrossRef]
- Tveit, B.; Svenneby, G.; Kvamme, E. Kinetic properties of glutaminase from pig renal cortex. Eur. J. Biochem. 1970, 14, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Marafi, D.; Fatih, J.M.; Kaiyrzhanov, R.; Ferla, M.P.; Gijavanekar, C.; Al-Maraghi, A.; Liu, N.; Sites, E.; Alsaif, H.S.; Al-Owain, M.; et al. Biallelic variants in SLC38A3 encoding a glutamine transporter cause epileptic encephalopathy. Brain 2021, 145, 909–924. [Google Scholar] [CrossRef]
- Farina, M.G.; Sandhu, M.R.S.; Parent, M.; Sanganahalli, B.G.; Derbin, M.; Dhaher, R.; Wang, H.; Zaveri, H.P.; Zhou, Y.; Danbolt, N.C.; et al. Small loci of astroglial glutamine synthetase deficiency in the postnatal brain cause epileptic seizures and impaired functional connectivity. Epilepsia 2021, 62, 2858–2870. [Google Scholar] [CrossRef]
- Tani, H.; Dulla, C.G.; Farzampour, Z.; Taylor-Weiner, A.; Huguenard, J.R.; Reimer, R.J. A Local Glutamate-Glutamine Cycle Sustains Synaptic Excitatory Transmitter Release. Neuron 2014, 81, 888–900. [Google Scholar] [CrossRef]
- Kam, K.; Nicoll, R. Excitatory Synaptic Transmission Persists Independently of the Glutamate Glutamine Cycle. J. Neurosci. 2007, 27, 9192–9200. [Google Scholar] [CrossRef]
- Pawlik, M.J.; Aldana, B.I.; Belfiori-Carrasco, L.F.; Obara-Michlewska, M.; Popek, M.P.; Czarnecka, A.M.; Albrecht, J. Inhibition of Glutamate Release, but Not of Glutamine Recycling to Glutamate, Is Involved in Delaying the Onset of Initial Lithium-Pilocarpine-Induced Seizures in Young Rats by a Non-Convulsive MSO Dose. Int. J. Mol. Sci. 2021, 22, 11127. [Google Scholar] [CrossRef]



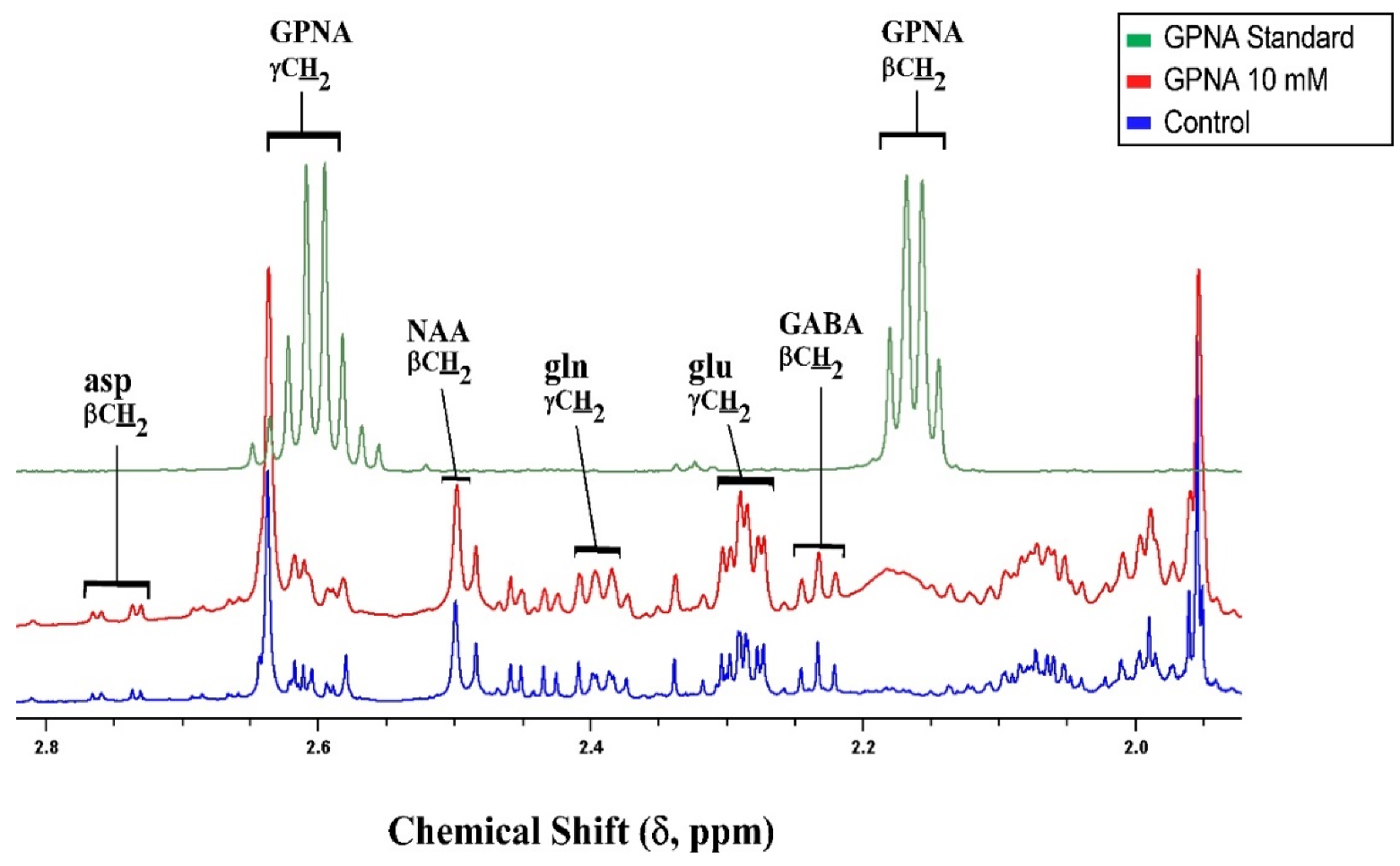
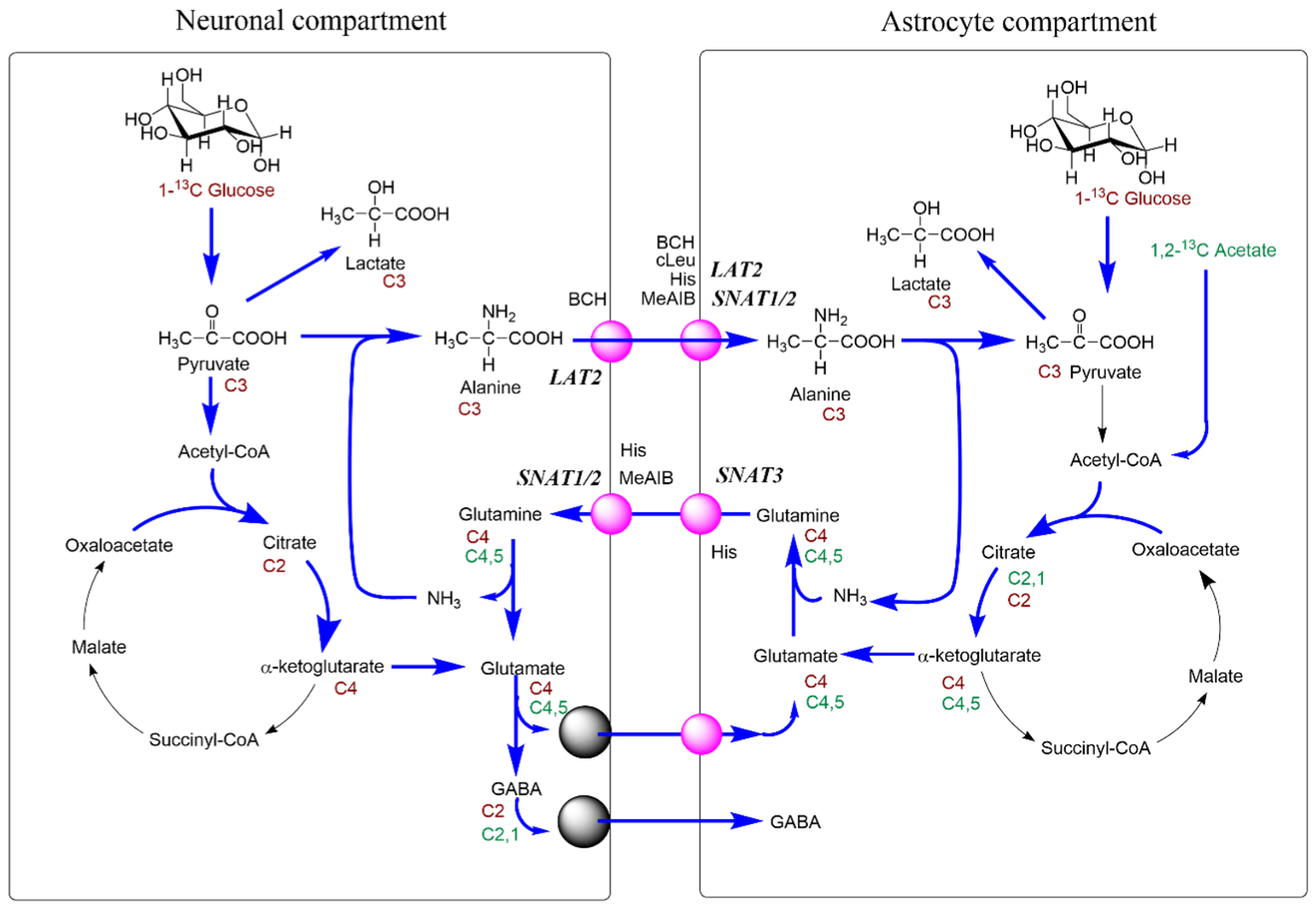
| SLC | Transporter Molecule | Transport System | Cerebellar Expression | Properties | Transport Mechanism | Affinity for Gln (KM) | Substrates Other than Gln | Inhibitors | References |
|---|---|---|---|---|---|---|---|---|---|
| SLC6A15 | B0AT2 | B0 | Glutamatergic (pyramidal cells) and GABAergic neurons (Purkinje cells) | Electrogenic in nature; Na+-dependent and Cl−-independent; pH dependent | 1:1 Na+/amino acid co-transport | 5.3 ± 2.7 mm | Leu, Ile, Val, Prol, Met, Ala (KM 670 ± 92 μM), and Phe. Gln is a poor substrate. | Aminoisobutyric acid, BCH, His, hydroxyproline, nipecotic acid, pipecolic acid, loratadine. | [21,39,47,48,49,50] |
| SLC6A17 | NTT4 | B0 | Glutamatergic (synaptic vesicles) and GABAergic neurons (Purkinje cells) | Electrogenic in nature; Na+-dependent and Cl−-independent; pH dependent | 1:1 Na+/amino acid co-transport | 5.2 ± 1.5 mm | Leu, Ile, Val, Pro, Met, Gly, and Ala | Unknown | [18,22,49,51] |
| SLC7A5 | LAT1 | L | Astrocyte, neurons, and BBB | Na+- and pH independent | Obligatory exchanger (1:1 stoichiometry) | 2.2 mm | Large neutral amino acids (LNAA), branched or aromatic AA | BCH, KYT-0353 (JPH203), and (Z)-4-chloro-N-(4-(trifluoromethoxy)phenyl)-5H-1,2,3-dithiazol-5-imine, GPNA, AABA | [2,44,52,53,54,55,56] |
| SLC7A8 | LAT2 | L | Astrocyte, neurons, and BBB | Na+- and pH independent | Obligatory exchanger (1:1 stoichiometry) | 151–275 µM | Broad specificity small and large zwitterionic amino acidsAla KM = 978 ± 143 μm | BCH, GPNA, cycloleucine | [2,9,44,52,55,57] |
| SLC38A1 | SNAT1 | A | Glutamatergic and GABAergic neurons | Electrogenic, Na+- and pH dependent | 1:1 Na+/amino acid co-transport | 230 µM | Gly, Ala (KM = 0.3 ± 0.02 mm), Ser, Cys, Asn, His, Met | His, MeAIB, GPNA | [19,58,59,60,61,62,63,64,65] |
| SLC38A2 | SNAT2 | A | Glutamatergic and GABAergic neurons | Electrogenic, Na+- and pH dependent | 1:1 Na+/amino acid co-transport | 1.65 ± 0.27 mm | Gly, Pro, Ala (KM = 529 ± 50 μM), Ser, Cys, Asn, His, Met | His, MeAIB, GPNA, AABA | [35,42,56,58,60,62,63,64,65,66] |
| SLC38A3 | SNAT3 | N | Astrocyte (Bergmann glia; granular cells; adjacent to glutamatergic, GABAergic, and glycinergic synapses) | Na+- and pH dependent; Bidirectional/reversible | 1:1 Na+-AA cotransport; H+-antiport | 2.4 mm | Asn, His | His, Ala Asn, Ser, Thr, Met, Leu, Trp, Val, Phe, Ile, Gly, Tyr, and Cys | [16,28,29,30,58,67] |
| SLC43A2 | LAT4 | L | Astrocyte and neurons | Na+ and Cl− independent; pH-independent; shows two kinetic components | Uniport of AA | - | Neutral and branched chain AA (leucine, isoleucine, valine), methionine and phenylalanine | Leu, BCH, JPH203, Acivicin, 3-iodo-L-tyrosine, ESK242, ESK246 | [46,68,69,70,71] |
| Transporter | Name | Granule | Purkinje | Bergmann | Comment |
|---|---|---|---|---|---|
| Slc1a1 | EAAT3 | 1.1 | 0 | 0 | Neuronal Glu transporter |
| Slc1a2 | GLT-1 | 1.79 | 1.61 | 5.56 | |
| Slc1a3 | GLAST | 1.95 | 1.61 | 7.28 | Glial Glu transporter |
| Slc1a4 | ASCT1 | 0 | 0 | 2.3 | |
| Slc1a5 | ASCT2 | 0 | 0 | 0 | |
| Slc1a6 | EAAT4 | 0 | 4.84 | 0 | |
| Slc1a7 | EAAT5 | nd | nd | nd | |
| Slc6a1 | GAT1 | 0.69 | 1.1 | 4.17 | GABA transporter |
| Slc6a5 | GLYT2 | nd | nd | nd | |
| Slc6a7 | PROT | 0 | 0.7 | 0 | |
| Slc6a9 | GLYT1 | 0.69 | 0.69 | 3.3 | |
| Slc6a11 | GAT3 | 0 | 0 | 3.2 | |
| Slc6a12 | BGT1 | 0 | 0 | 0 | |
| Slc6a13 | GAT2 | 0 | 0 | 0 | |
| Slc6a14 | ATB0+ | nd | nd | nd | |
| Slc6a15 | B0AT2 | 1.95 | 1.79 | 0 | Neuronal BCAA transporter |
| Slc6a16 | NTT5 | nd | nd | nd | |
| Slc6a17 | NTT4 | 1.61 | 2.77 | 0 | Neuronal BCAA transporter |
| Slc6a18 | B0AT3 | nd | nd | nd | |
| Slc6a19 | B0AT1 | nd | nd | nd | |
| Slc6a20a | SIT | 0 | 0 | 0 | |
| Slc6a20b | SIT | nd | nd | nd | |
| Slc7a1 | CAT1 | 0.69 | 1.1 | 0.69 | Cationic AA transporter |
| Slc7a2 | CAT2 | 0 | 0 | 1.79 | Cationic AA transporter |
| Slc7a3 | CAT3 | 0 | 0 | 0 | |
| Slc7a4 | CAT4 | 1.39 | 0.69 | 0 | |
| Slc7a5 | LAT1 | 0.69 | 0 | 1.39 | Large neutral AA transporter |
| Slc7a6 | yLAT2 | 0.69 | 0 | 0 | |
| Slc7a7 | yLAT1 | 0 | 0 | 0 | |
| Slc7a8 | LAT2 | 1.1 | 1.1 | 1.1 | |
| Slc7a9 | b0+AT | nd | nd | nd | |
| Slc7a10 | asc1 | 0 | 0.69 | 3.9 | Glial D-serine transporter |
| Slc7a11 | xCT | 0 | 0 | 0 | Cystine/Glu exchanger |
| Slc7a12 | asc2 | nd | nd | nd | |
| Slc7a13 | AGT1 | nd | nd | nd | |
| Slc7a14 | 2.3 | 1.39 | 0 | ||
| Slc7a15 | nd | nd | nd | ||
| Slc16a10 | TAT1 | 0 | 0 | 0 | |
| Slc17a6 | VGLUT2 | 0 | 0 | 0 | Vesicular Glu transporter |
| Slc17a7 | VGLUT1 | 3.2 | 1.1 | 0.69 | Vesicular Glu transporter |
| Slc17a8 | VGLUT3 | nd | nd | nd | |
| Slc25a12 | AGC1 | 2.56 | 2.64 | 1.1 | Mito Asp/Glu carrier |
| Slc25a13 | AGC2 | 0 | 0.69 | 0 | Mito Asp/Glu carrier |
| Slc25a18 | GC2 | 0 | 0 | 3.1 | Mito Glu carrier |
| Slc25a22 | GC1 | 2.56 | 2.2 | 0.69 | Mito Glu carrier |
| Slc32a1 | VIAAT | 0 | 4.14 | 0 | Vesicular GABA transporter |
| Slc36a1 | PAT1 | 0.69 | 2.1 | 0.69 | |
| Slc36a2 | PAT2 | 0 | 0 | 0 | |
| Slc36a3 | PAT3 | nd | nd | nd | |
| Slc36a4 | PAT4 | 1.6 | 1.79 | 0.69 | |
| Slc38a1 | SNAT1 | 2.4 | 3.3 | 4.65 | Small neutral AA transporter |
| Slc38a2 | SNAT2 | 2.56 | 1.79 | 1.79 | Small neutral AA transporter |
| Slc38a3 | SNAT3 | 0 | 0.69 | 2.77 | Gln transporter |
| Slc38a4 | SNAT4 | nd | nd | nd | |
| Slc38a5 | SNAT5 | 0 | 0 | 0 | Gln transporter |
| Slc38a6 | SNAT6 | 0 | 0.69 | 0 | |
| Slc38a7 | SNAT7 | 0.69 | 0.69 | 1.1 | Lysosomal AA transporter |
| Slc38a8 | SNAT8 | nd | nd | nd | |
| Slc38a9 | SNAT9 | 1.1 | 1.4 | 0.69 | Lysosomal AA transporter |
| Slc38a10 | SNAT10 | 1.39 | 1.79 | 1.39 | |
| Slc38a11 | SNAT11 | 0 | 0 | 0 | |
| Slc43a1 | LAT3 | nd | nd | nd | |
| Slc43a2 | LAT4 | 2.2 | 0.69 | 1.95 | Large neutral AA transporter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Gauthier-Coles, G.; Bröer, S.; Rae, C.D. Impact of Inhibition of Glutamine and Alanine Transport on Cerebellar Glial and Neuronal Metabolism. Biomolecules 2022, 12, 1189. https://doi.org/10.3390/biom12091189
Das A, Gauthier-Coles G, Bröer S, Rae CD. Impact of Inhibition of Glutamine and Alanine Transport on Cerebellar Glial and Neuronal Metabolism. Biomolecules. 2022; 12(9):1189. https://doi.org/10.3390/biom12091189
Chicago/Turabian StyleDas, Abhijit, Gregory Gauthier-Coles, Stefan Bröer, and Caroline D. Rae. 2022. "Impact of Inhibition of Glutamine and Alanine Transport on Cerebellar Glial and Neuronal Metabolism" Biomolecules 12, no. 9: 1189. https://doi.org/10.3390/biom12091189
APA StyleDas, A., Gauthier-Coles, G., Bröer, S., & Rae, C. D. (2022). Impact of Inhibition of Glutamine and Alanine Transport on Cerebellar Glial and Neuronal Metabolism. Biomolecules, 12(9), 1189. https://doi.org/10.3390/biom12091189







