Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Chickens and Treatment
2.3. Sample Collection and Viscera Index
2.4. Histological Observation
2.5. Counting and Karyoplasmic Area Ratio of Plasma Cells
2.6. RNA Extraction and cDNA Synthesis
2.7. Quantitative Real-Time PCR (qPCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Observation of Arbor Acres Broilers’ Livers
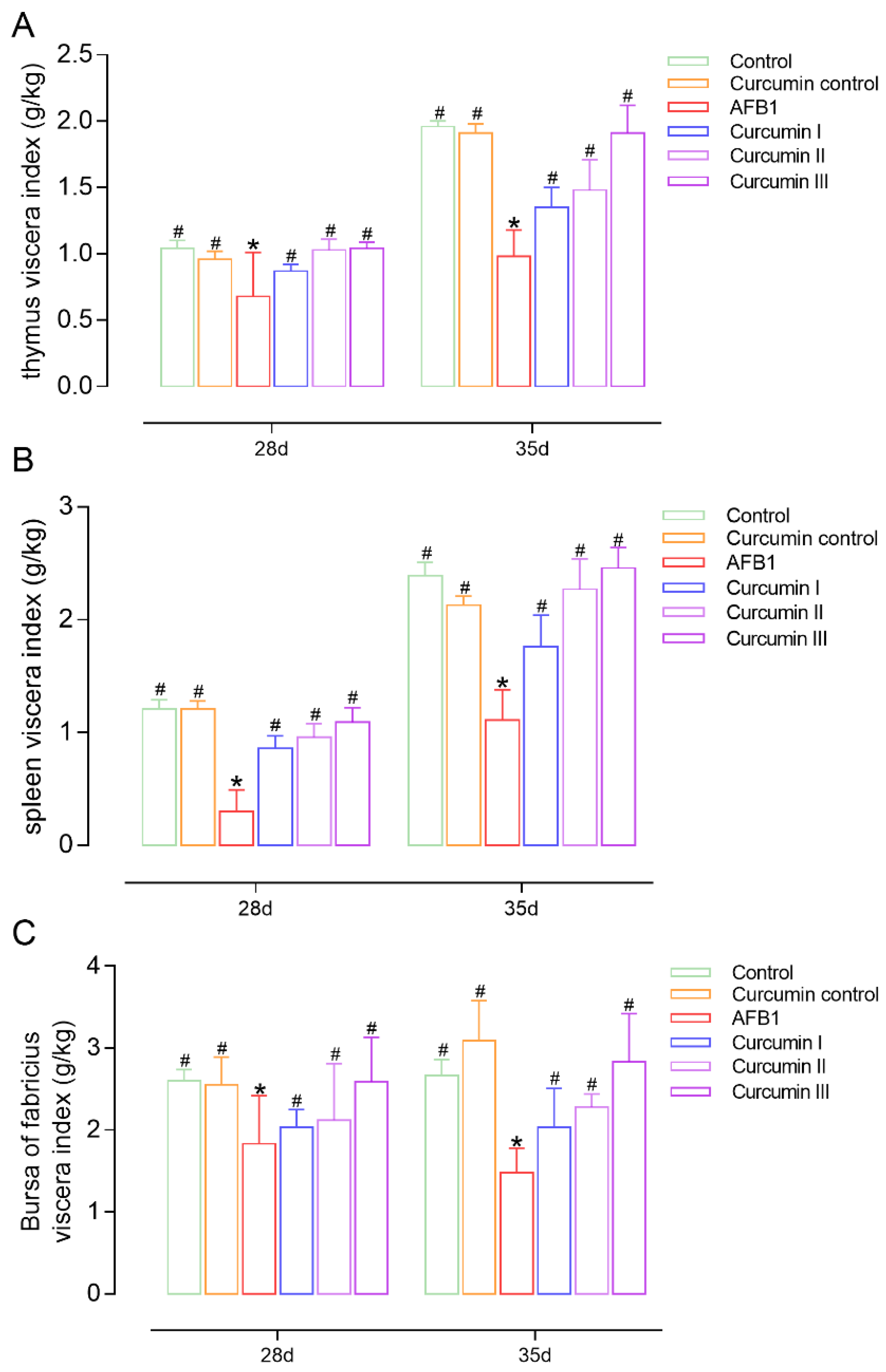
3.2. Effects on Viscera Index of Thymus, Spleen and Bursa of Fabricius
3.3. Histopathological Changes in Immune Organs
3.3.1. Histopathological Changes in Thymus
3.3.2. Histopathological Changes in Spleen
3.3.3. Histopathological Changes in Bursa of Fabricius
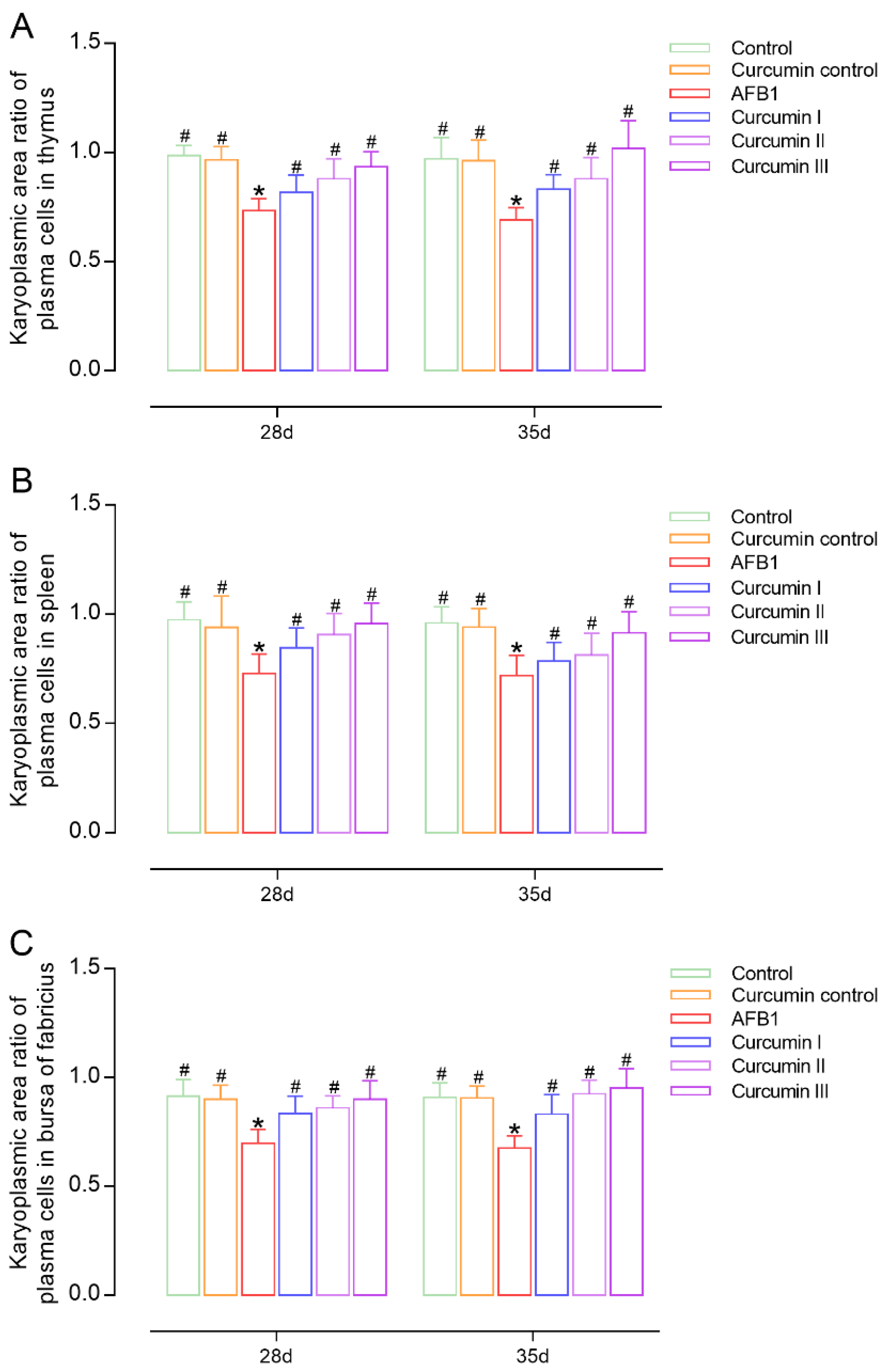
3.4. Effects on Number and Karyoplasmic Area Ratio of Plasma Cells in Immune Organs
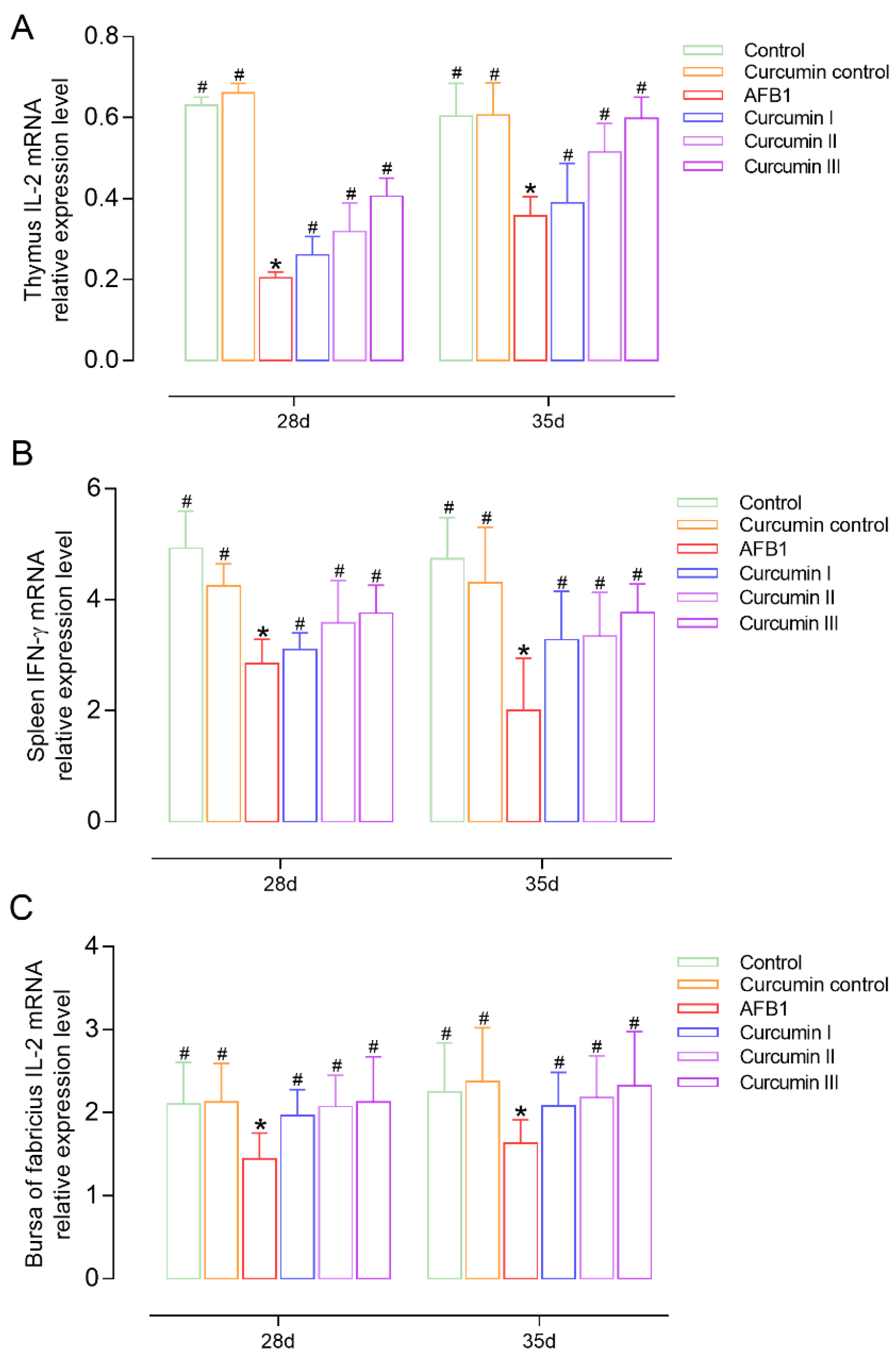
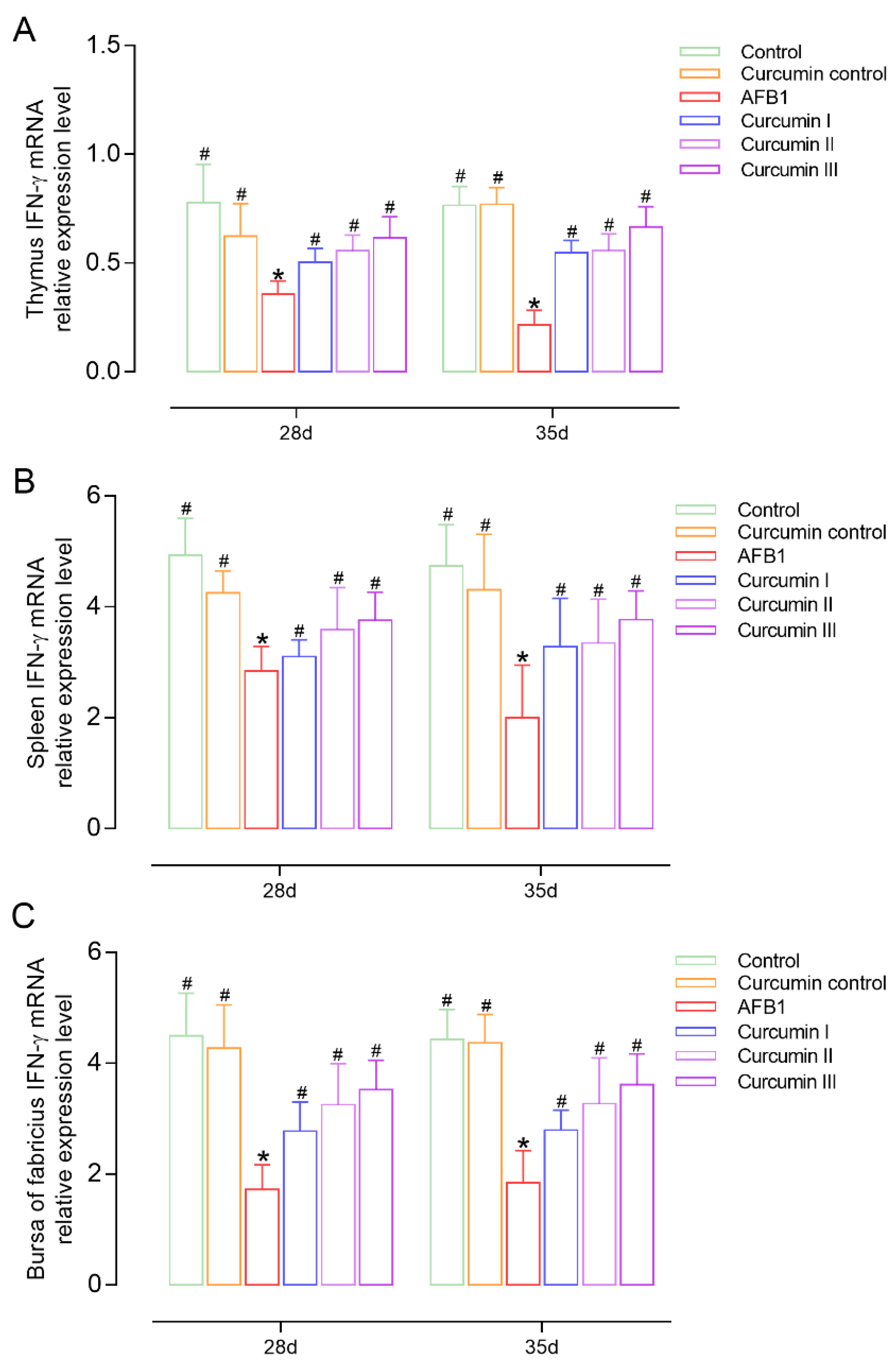
3.5. Effect of AFB1 and Curcumin on Cytokines’ Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B(1) metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B(1), deoxynivalenol and zearalenone in feeds in China during 2018-2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.T.; Xie, W.M.; Zhang, N.Y.; Dai, J.F.; Wang, Y.; Rajput, S.A.; Qi, D.S.; et al. Individual and Combined Occurrence of Mycotoxins in Feed Ingredients and Complete Feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Ferenčík, M.; Ebringer, L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol. 2003, 48, 417. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.; Yu, R.-C.; Chou, C.-C.; Liu, J.-R.; Cheng, K.-C. Protective and Detoxifying Effects Conferred by Dietary Selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Randhawa, M.A.; Saeed, F.; Waqas, K. Aflatoxins: Biosynthesis, Occurrence, Toxicity, and Remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W. Biotransformation of Aflatoxin B1 and Its Relationship with the Differential Toxicological Response to Aflatoxin in Commercial Poultry Species; Guevara-González, R.G., Ed.; InTech: Vienna, Austria, 2011. [Google Scholar]
- Zhou, Y.; Mao, S.; Zhou, M. Effect of the flavonoid baicalein as a feed additive on the growth performance, immunity, and antioxidant capacity of broiler chickens. Poult. Sci. 2019, 98, 2790–2799. [Google Scholar] [CrossRef]
- Noble, B.T.; Brennan, F.H.; Popovich, P.G. The spleen as a neuroimmune interface after spinal cord injury. J. Neuroimmunol. 2018, 321, 1–11. [Google Scholar] [CrossRef]
- Fleischer, B. The avian immune system. Immunol. Today 1981, 2, 195–200. [Google Scholar] [CrossRef]
- Guo, S.; Shi, D.; Liao, S.; Su, R.; Lin, Y.; Pan, J.; Tang, Z. Influence of selenium on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B₁. Biol. Trace Elem. Res. 2012, 146, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhang, Y.; Li, P.; Yang, S.-H.; Zhang, W.-K.; Han, J.-X.; Wang, Y.; He, J.-B. Intervention of Grape Seed Proanthocyanidin Extract on the Subchronic Immune Injury in Mice Induced by Aflatoxin B1. Int. J. Mol. Sci. 2016, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shu, G.; Peng, X.; Fang, J.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; et al. Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem. Toxicol. 2013, 59, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Bai, S.; Ding, X.; Zeng, Q.; Zhang, K.; Fang, J. Pathological changes in the immune organs of broiler chickens fed on corn naturally contaminated with aflatoxins B1 and B2. Avian Pathol. 2015, 44, 192–199. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, S.; Chen, J.; Peng, X.; Wang, F.; Cui, H.; Fang, J. Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-γ induced by aflatoxin B₁ in broilers. Res. Vet. Sci. 2013, 95, 143–145. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Gowda, N.; Ledoux, D.; Rottinghaus, G.; Bermudez, A.; Chen, Y.J.B.J.N. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009, 102, 1629–1634. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Qi, M.; Zhao, L.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; et al. Curcumin Prevents Aflatoxin B(1) Hepatoxicity by Inhibition of Cytochrome P450 Isozymes in Chick Liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Muhammad, I.; Li, W.; Sun, X.; Cheng, P.; Zhang, X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-Ⅱ enzymes and Nrf2. Environ. Toxicol Pharm. 2018, 59, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Tang, L.; Rao, G.; Zhong, G.; Jiang, X.; Wu, S.; Huang, R.; Tang, Z.; Ruan, Z.; Chen, Z.; et al. Curcumin activates the Nrf2 Pathway to alleviate AFB1-induced immunosuppression in the spleen of ducklings. Toxicon 2022, 209, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.; Naeem, M.; Ali, S.; Zhang, J.F.; Zhang, L.; Wang, T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on broiler pigmentation and immunity. Poult. Sci. 2013, 92, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin Successfully Inhibited the Computationally Identified CYP2A6 Enzyme-Mediated Bioactivation of Aflatoxin B1 in Arbor Acres broiler. Front. Pharmacol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Wang, J.; Yi, M.; Chen, X.; Muhammad, I.; Liu, F.; Li, R.; Li, J.; Li, J. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain. Neurotoxicol. Teratol. 2016, 55, 32–37. [Google Scholar] [CrossRef]
- Wang, X.; Muhammad, I.; Sun, X.; Han, M.; Hamid, S.; Zhang, X. Protective role of curcumin in ameliorating AFB1-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018, 45, 881–891. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef]
- Freire, F.D.C.O.; da Rocha, M.E.B. Impact of Mycotoxins on Human Health. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 239–261. [Google Scholar]
- Mohajeri, M.; Behnam, B.; Cicero, A.F.G.; Sahebkar, A. Protective effects of curcumin against aflatoxicosis: A comprehensive review. J. Cell. Physiol. 2018, 233, 3552–3577. [Google Scholar] [CrossRef]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Kaspers, B.; Göbel, T.W.F. The Avian Immune System. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 498–503. [Google Scholar]
- Quist, C.F.; Bounous, D.I.; Kilburn, J.V.; Nettles, V.F.; Wyatt, R.D. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 2000, 36, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M.; Umakantha, M.; Ali, M.N.; Swamy, H.N. Study of the Combination Effects of Aflatoxin and T-2 Toxin on Performance Parameters and Internal Organs of Commercial Broilers. Glob. Vet. 2012, 8, 393–396. [Google Scholar]
- Santin, E.; Paulillo, A.C.; Nakagui, L.S.; Alessi, A.C.; Maiorka, A. Evaluation of yeast cell wall on the performance of broiles fed diets with or without mycotoxins. Rev. Bras. Ciência Avícola 2006, 8, 221–225. [Google Scholar] [CrossRef]
- Sawarkar, A.R.; Sonkusale, P.M.; Kurkure, N.V.; Jangade, C.R. Experimental Afla and Ochratoxin Induced Mixed Mycotoxicosis in Broilers and its Amelioration with Herbomineral Toxin Binder Toxiroak Gold. Int. J. Poult. Sci. 2011, 10, 560–566. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 1985, 64, 1678–1684. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of purified aflatoxin on broiler chickens. Poult. Sci. 1985, 64, 852–858. [Google Scholar] [CrossRef]
- Fernandez, A.; Verde, M.T.; Gascon, M.; Ramos, J.; Gomez, J.; Luco, D.F.; Chavez, G. Variations of clinical biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed. Avian Pathol. 1994, 23, 37–47. [Google Scholar] [CrossRef]
- Umaya, S.R.; Vijayalakshmi, Y.C.; Sejian, V. Exploration of plant products and phytochemicals against aflatoxin toxicity in broiler chicken production: Present status. Toxicon 2021, 200, 55–68. [Google Scholar] [CrossRef]
- Gómez-Espinosa, D.; Cervantes-Aguilar, F.J.; Del Río-García, J.C.; Villarreal-Barajas, T.; Vázquez-Durán, A.; Méndez-Albores, A. Ameliorative Effects of Neutral Electrolyzed Water on Growth Performance, Biochemical Constituents, and Histopathological Changes in Turkey Poults during Aflatoxicosis. Toxins 2017, 9, 104. [Google Scholar] [CrossRef]
- Chen, K.; Fang, J.; Peng, X.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; Lai, W.; et al. Effect of selenium supplementation on aflatoxin B1-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014, 74, 91–97. [Google Scholar] [CrossRef]
- Ortatatli, M.; Oğuz, H.; Hatipoğlu, F.; Karaman, M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005, 78, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, C.R.; Gómez, G.V.; Del Río, J.C.G.; Cuevas, A.C.; Arce, J.M.; Ávila, E.G. Effect of the Addition of Saccharomyces Cerevisiae Yeast Cell Walls to Diets with Mycotoxins on the Performance and Immune Responses of Broilers. J. Poult. Sci. 2018, 55, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bigorra, L.; Merino, A.; Alférez, S.; Rodellar, J. Feature Analysis and Automatic Identification of Leukemic Lineage Blast Cells and Reactive Lymphoid Cells from Peripheral Blood Cell Images. J. Clin. Lab. Anal. 2017, 31, e22024. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, W.; Zhang, D.; Pang, Y.; Shi, J.; Wan, S.; Cheng, K.; Wang, J.; Cheng, S. Circulating tumor cell detection in hepatocellular carcinoma based on karyoplasmic ratios using imaging flow cytometry. Sci. Rep. 2016, 6, 39808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Y.; Deng, J.; Zhang, N.-Y.; Zhang, W.-P.; Liu, X.-L.; Rajput, S.A.; Qi, D.-S.; Sun, L.-H. Selenium Deficiency Aggravates Aflatoxin B1–Induced Immunotoxicity in Chick Spleen by Regulating 6 Selenoprotein Genes and Redox/Inflammation/Apoptotic Signaling. J. Nutr. 2019, 149, 894–901. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mashayekhi, K.; Navashenaq, J.G.; Haftcheshmeh, S.M. Curcumin as a Natural Modulator of B Lymphocytes: Evidence from In Vitro and In Vivo Studies. Mini Rev. Med. Chem. 2022, 22, 2361–2370. [Google Scholar] [CrossRef]
- Fairweather, D.L.; Afanasyeva, M.; Rose, N.R. Chapter 1—Cellular Immunity: A Role for Cytokines. In Handbook of Systemic Autoimmune Diseases; Atzeni, F., Doria, A., Nurmohamed, M., Pauletto, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 14, pp. 1–29. [Google Scholar]
- Zhu, J.F.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Erf, G.F.; Bottje, W.G.; Bersi, T.K. CD4, CD8 and TCR defined T-cell subsets in thymus and spleen of 2- and 7-week old commercial broiler chickens. Vet. Immunol. Immunopathol. 1998, 62, 339–348. [Google Scholar] [CrossRef]
- Borthwick, N.J.; Lowdell, M.; Salmon, M.; Akbar, A.N. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int. Immunol. 2000, 12, 1005–1013. [Google Scholar] [CrossRef]
- Seder, R.A.; Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003, 4, 835–842. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugyala, R.R.; Sharma, R.P. The effect of aflatoxin B1 on cytokine mrna and corresponding protein levels in peritoneal macrophages and splenic lymphocytes. Int. J. Immunopharmacol. 1996, 18, 599–608. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Attia, S.M.; Alwetaid, M.Y.; Ansari, M.A.; Zoheir, K.M.A.; Nadeem, A.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Ahmad, S.F. β-1,3-Glucan reverses aflatoxin B1-mediated suppression of immune responses in mice. Life Sci. 2016, 152, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Bunaciu, R.P.; Pascale, F.; Tudor, D.S.; Avram, N.; Sarca, M.; Cureu, I.; Criste, R.D.; Suta, V.; et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 2002, 80, 1250–1257. [Google Scholar] [CrossRef]

| Gene | Primers (from 5′ to 3′) | Length (bp) |
|---|---|---|
| GAPDH | Forward GCACGCCATCACTATCTT | 82 |
| Reverse GGACTCCACAACATACTCAG | ||
| IL-2 | Forward GGATCCATGATGTGCAAAGTACTG | 80 |
| Reverse CGGTCGACTTATTTTTGCAGATATCT | ||
| IFN-γ | Forward TCCTTCTGAAAGCTCTCGCC | 175 |
| Reverse CTGGTGTCCAGGATGGTGTC |
| Group | Body Size | Eyes | Feathers | Behavior |
|---|---|---|---|---|
| Control | normal | bright eyes | glossy feathers | normal appetite, growth and behavior |
| Curcumin Control | normal or bigger | bright eyes | glossy feathers | normal appetite, growth and behavior |
| AFB1 | smaller | dull eyes | dull feathers | decreased appetite, delayed growth and depressed behavior |
| Curcumin I | normal | bright eyes | glossy feathers | normal appetite, growth and behavior |
| Curcumin II | normal | bright eyes | glossy feathers | normal appetite, growth and behavior |
| Curcumin III | normal | bright eyes | glossy feathers | normal appetite, growth and behavior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Han, M.; Zhang, Y.; Ishfaq, M.; Liu, R.; Wei, G.; Zhang, X.; Zhang, X. Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules 2022, 12, 1188. https://doi.org/10.3390/biom12091188
Li S, Han M, Zhang Y, Ishfaq M, Liu R, Wei G, Zhang X, Zhang X. Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules. 2022; 12(9):1188. https://doi.org/10.3390/biom12091188
Chicago/Turabian StyleLi, Sihong, Meiyu Han, Yixin Zhang, Muhammad Ishfaq, Ruimeng Liu, Gaoqiang Wei, Xiuying Zhang, and Xiuying Zhang. 2022. "Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1" Biomolecules 12, no. 9: 1188. https://doi.org/10.3390/biom12091188
APA StyleLi, S., Han, M., Zhang, Y., Ishfaq, M., Liu, R., Wei, G., Zhang, X., & Zhang, X. (2022). Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules, 12(9), 1188. https://doi.org/10.3390/biom12091188







