Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications
Abstract
:1. Introduction
2. Applications in Diabetes
2.1. Urine-Glucose Testing
2.2. Blood Glucose Testing
2.3. Non-Invasive Glucose Testing
3. Applications in Cancer Treatment
3.1. Stimuli-Responsive Polymers
3.1.1. Gox-Based Systems
3.1.2. Redox Responsive Systems
3.1.3. DNA-Based Systems
3.1.4. Other Polymer-Based Microdevices
3.2. Implantable BioMEMS and Electrochemical Systems
4. Applications in Cardiovascular Diseases
4.1. PH-Dependent Drug Delivery
4.2. Hypoxia-Sensing Drug Delivery Systems
4.3. Reactive Oxygen Species-Responsive Drug Delivery
4.4. Enzyme-Responsive Drug Delivery
4.5. Shear-Responsive Systems
4.6. Advanced BioMEMs
5. Applications in Regenerative Medicine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera, D.; Monaghan, M.G. Electroactive material-based biosensors for detection and drug delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Santana, W.; Dolabella, S.S.; Santos, A.L.S.; Souto, E.B.; Severino, P. Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania? Pharmaceutics 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From oral formulations to drug-eluting implants: Using 3D and 4D printing to develop drug delivery systems and personalized medicine. BIO-DES MANUF 2022, 5, 85–106. [Google Scholar] [CrossRef]
- Ngoepe, M.; Choonara, Y.E.; Tyagi, C.; Tomar, L.K.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.; Pillay, V. Integration of biosensors and drug delivery technologies for early detection and chronic management of illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef]
- Akolpoglu, M.B.; Bozuyuk, U.; Erkoc, P.; Kizilel, S. Biosensing–Drug Delivery Systems for In Vivo Applications. In Advanced Biosensors for Health Care Applications, Inamuddin; Raju, K., Ali, M., Abdullah, M.A., Eds.; Elsevier: New York, NY, USA, 2019; pp. 249–262. [Google Scholar]
- Yazdi, M.K.; Zarrintaj, P.; Bagheri, B.; Kim, Y.C.; Ganjali, M.R.; Saeb, M.R. Nanotechnology-based biosensors in drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Masoud, M., Ed.; Elsevier: New York, NY, USA, 2020; pp. 767–779. [Google Scholar]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine–A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Jayanthi, V.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab. Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Sofi, H.S.; Ashraf, R.; Khan, A.H.; Beigh, M.A.; Majeed, S.; Sheikh, F.A. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater. Sci. Eng. C. 2019, 94, 1102–1124. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Yan, J.; Kahkoska, A.R.; Gu, Z. Advances in bioresponsive closed-loop drug delivery systems. Int. J. Pharm. 2018, 544, 350–357. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2020, 32, 1902004. [Google Scholar] [CrossRef]
- Coffel, J.; Nuxoll, E. BioMEMS for biosensors and closed-loop drug delivery. Int. J. Pharm. 2018, 544, 335–349. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; Argyle, D. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Biosensors and Bioelectronics Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-responsive polymers for sensing and actuation. Mater. Horiz. 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Alsuraifi, A.; Curtis, A.; Lamprou, D.A.; Hoskins, C. Stimuli responsive polymeric systems for cancer therapy. Pharmaceutics 2018, 10, 136. [Google Scholar] [CrossRef]
- Traitel, T.; Cohen, Y.; Kost, J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials 2000, 21, 1679–1687. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- American Diabetes Association. Insulin Basics. Available online: http://www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-basics.html (accessed on 3 May 2022).
- Center for Disease Control and Prevention “Gestational Diabetes”. Available online: https://www.cdc.gov/diabetes/basics/gestational.html (accessed on 3 May 2022).
- Swidorski, D. Diabetes History. 2014. Available online: https://www.defeatdiabetes.org/diabetes-history/ (accessed on 3 May 2022).
- American Diabetes Association. Severe Hypoglycemia Predicts Mortality in Diabetes. Available online: https://care.diabetesjournals.org/content/35/9/1814 (accessed on 3 May 2022).
- Storey, H.L.; van Pelt, M.H.; Bun, S.; Daily, F.; Neogi, T.; Thompson, M.; McGuire, H.; Weigl, B.H. Diagnostic accuracy of self-administered urine glucose test strips as a diabetes screening tool in a low-resource setting in Cambodia. BMJ Open 2018, 8, e019924. [Google Scholar] [CrossRef]
- Patel, B.; Priefer, R. Infections associated with diabetic-care devices. Diabetes Metab. Syndr. 2021, 15, 519–524. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: Current problems and future promises. JDST 2010, 4, 1540–1562. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Burgess, D.; Jain, F.; Papadimitrakopoulos, F. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosens. Bioelectron. 2009, 24, 1557–1562. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Singh, H.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Enhanced glucose sensor linearity using poly (vinyl alcohol) hydrogels. JDST 2009, 3, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal electrodes. I. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- Pishko, M.V.; Katakis, I.; Lindquist, S.E.; Ye, L.; Gregg, B.A.; Heller, A. Direct electrical communication between graphite electrodes and surface adsorbed glucose oxidase/redox polymer complexes. Angew. Chem. Int. Ed. Engl. 1990, 29, 82–84. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Liu, B. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosens. Bioelectron. 1992, 7, 215–222. [Google Scholar] [CrossRef]
- Joshi, P.P.; Merchant, S.A.; Wang, Y.; Schmidtke, D.W. Amperometric biosensors based on redox polymer− carbon nanotube− enzyme composites. Anal. Chem. 2005, 77, 3183–3188. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Tomazos, I.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosens. Bioelectron. 2010, 25, 1553–1565. [Google Scholar] [CrossRef]
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Gorton, L.; Bremle, G.; Csöregi, E.; Jönsson-Pettersson, G.; Persson, B. Amperometric glucose sensors based on immobilized glucose-oxidizing enzymes and chemically modified electrodes. Anal. Chim. Acta 1991, 249, 43–54. [Google Scholar] [CrossRef]
- Mizutani, F.; Yabuki, S.; Katsura, T. Amperometric enzyme electrode with the use of dehydrogenase and NAD (P) H oxidase. Sens. Actuators B Chem. 1993, 14, 574–575. [Google Scholar] [CrossRef]
- Skoog, M.; Johansson, G. Internal supply of coenzyme to an amperometric glucose biosensor based on a chemically modified electrode. Biosens. Bioelectron. 1991, 6, 407–412. [Google Scholar] [CrossRef]
- Laurinavicius, V.; Kurtinaitiene, B.; Liauksminas, V.; Ramanavicius, A.; Meskys, R.; Rudomanskis, R.; Skotheim, T.; Boguslavsky, L. Oxygen insensitive glucose biosensor based on PQQ-dependent glucose dehydrogenase. Anal. Lett. 1999, 32, 299–316. [Google Scholar] [CrossRef]
- Loew, N.; Scheller, F.W.; Wollenberger, U. Characterization of Self-Assembling of Glucose Dehydrogenase in Mono-and Multilayers on Gold Electrodes. Electroanalysis 2004, 16, 1149–1154. [Google Scholar] [CrossRef]
- HabermuÈller, K.; Ramanavicius, A.; Laurinavicius, V.; Schuhmann, W. An oxygen-insensitive reagentless glucose biosensor based on osmium-complex modified polypyrrole. Electroanalysis 2000, 12, 1383–1389. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef]
- Zhou, Y.-G.; Yang, S.; Qian, Q.-Y.; Xia, X.-H. Gold nanoparticles integrated in a nanotube array for electrochemical detection of glucose. Electrochem. Commun. 2009, 11, 216–219. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, K.; Xia, X. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Funct. Mater. 2005, 15, 803–809. [Google Scholar] [CrossRef]
- Myung, Y.; Jang, D.M.; Cho, Y.J.; Kim, H.S.; Park, J.; Kim, J.-U.; Choi, Y.; Lee, C.J. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. J. Phys. Chem. C. 2009, 113, 1251–1259. [Google Scholar] [CrossRef]
- Clarke, S.; Foster, J. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Olczuk, D.; Priefer, R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab. Syndr. 2018, 12, 181–187. [Google Scholar] [CrossRef]
- Bolla, A.S.; Priefer, R. Blood glucose monitoring-an overview of current and future non-invasive devices. Diabetes Metab. Syndr. 2020, 14, 739–751. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151. [Google Scholar] [CrossRef]
- Boatemaa, M.A.; Doss, S. Non-invasive glucose estimation based on near infrared laser diode spectroscopy. Asian J. Biomed. Pharmaceut. Sci. 2017, 1, 22–28. [Google Scholar]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In vivo noninvasive monitoring of glucose concentration in human epidermis by mid-infrared pulsed photoacoustic spectroscopy. Anal. Chem. 2013, 85, 1013–1020. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Yang, S.; Zhao, F.; Schmidt, D.J. Noninvasive Glucose Monitoring by Mid-infrared Self-emission Method. In Proceedings of the 7th International Conference on Biomedical Electronics and Devices (BIODEVICES 2014), Eeseo, France, 3–6 March 2014; pp. 107–111. [Google Scholar]
- Abd Salam, N.A.B.; bin Mohd Saad, W.H.; Manap, Z.B.; Salehuddin, F. The evolution of non-invasive blood glucose monitoring system for personal application. JTEC 2016, 8, 59–65. [Google Scholar]
- Nawaz, A.; Øhlckers, P.; Sælid, S.; Jacobsen, M.; Nadeem Akram, M. Review: Non-invasive continuous blood glucose measurement techniques. J. Bioinform. Diabetes 2016, 1, 1–27. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Naidis, E.; Mayzel, Y. Noninvasive glucose monitoring: Increasing accuracy by combination of multi-technology and multi-sensors. JDST 2010, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Chowdhury, M.K.; Sharma, S.; Sharma, N. Measurement of glucose by using modulating ultrasound with optical technique in normal and diabetic human blood serum. In Proceedings of the 2014 International Conference on Advances in Engineering & Technology Research (ICAETR-2014), Unnao, India, 1–2 August 2014; pp. 1–5. [Google Scholar]
- Kit, S.Y.H.; Kassim, N.M. Non-invasive blood glucose measurement using temperature-based approach. J. Teknol. 2013, 64. [Google Scholar]
- Cho, O.K.; Kim, Y.O.; Mitsumaki, H.; Kuwa, K. Noninvasive measurement of glucose by metabolic heat conformation method. Clin. Chem. 2004, 50, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, C.-S.; Choi, B.-C.; Ham, K.-Y. The correlation of the complex dielectric constant and blood glucose at low frequency. Biosens. Bioelectron. 2003, 19, 321–324. [Google Scholar] [CrossRef]
- So, C.-F.; Choi, K.-S.; Wong, T.K.; Chung, J.W. Recent advances in noninvasive glucose monitoring. Med. Devices 2012, 5, 45. [Google Scholar]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Murray, K.; Hughson, R. Glucose Levels Detection Using mm-Wave Radar. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Cano-Garcia, H.; Saha, S.; Sotiriou, I.; Kosmas, P.; Gouzouasis, I.; Kallos, E. Millimeter-Wave Sensing of Diabetes-Relevant Glucose Concentration Changes in Pigs. J. Infrared. Millim Terahertz Waves 2018, 39, 761–772. [Google Scholar] [CrossRef]
- Mohd Bahar, A.A.; Zakaria, Z.; Md Arshad, M.K.; Isa, A.A.M.; Dasril, Y.; Alahnomi, R.A. Real Time Microwave Biochemical Sensor Based on Circular SIW Approach for Aqueous Dielectric Detection. Sci. Rep. 2019, 9, 5467. [Google Scholar] [CrossRef]
- Cameron, B.D.; Gorde, H.W.; Satheesan, B.; Cote, G.L. The use of polarized laser light through the eye for noninvasive glucose monitoring. Diabetes Technol. Ther. 1999, 1, 135–143. [Google Scholar] [CrossRef]
- Purvinis, G.; Cameron, B.D.; Altrogge, D.M. Noninvasive polarimetric-based glucose monitoring: An in vivo study. JDST 2011, 5, 380–387. [Google Scholar] [CrossRef]
- Woods, J.; Smith, J.; Rice, M.; Routt, W.; Messerschmidt, R.; Ou, J. Non-Invasive Measurement of Blood Glucose Using Retinal Imaging. U.S. Patent 20050267343A1, 3 May 2022. [Google Scholar]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators B Chem. 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Choi, S.-J.; Lee, I.; Jang, B.-H.; Youn, D.-Y.; Ryu, W.-H.; Park, C.O.; Kim, I.-D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef]
- Ryabtsev, S.; Shaposhnick, A.; Lukin, A.; Domashevskaya, E. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuators B Chem. 1999, 59, 26–29. [Google Scholar] [CrossRef]
- Paldus, B.A.; Kachanov, A.A. An historical overview of cavity-enhanced methods. Can. J. Phys. 2005, 83, 975–999. [Google Scholar] [CrossRef]
- Ronny Priefer; Rust, M. Breath Acetone Monitor and Method of Detecting Breath Acetone. Utility Patent US9921209B2, 3 May 2022.
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. JPhys 2019, 2, 032004. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef]
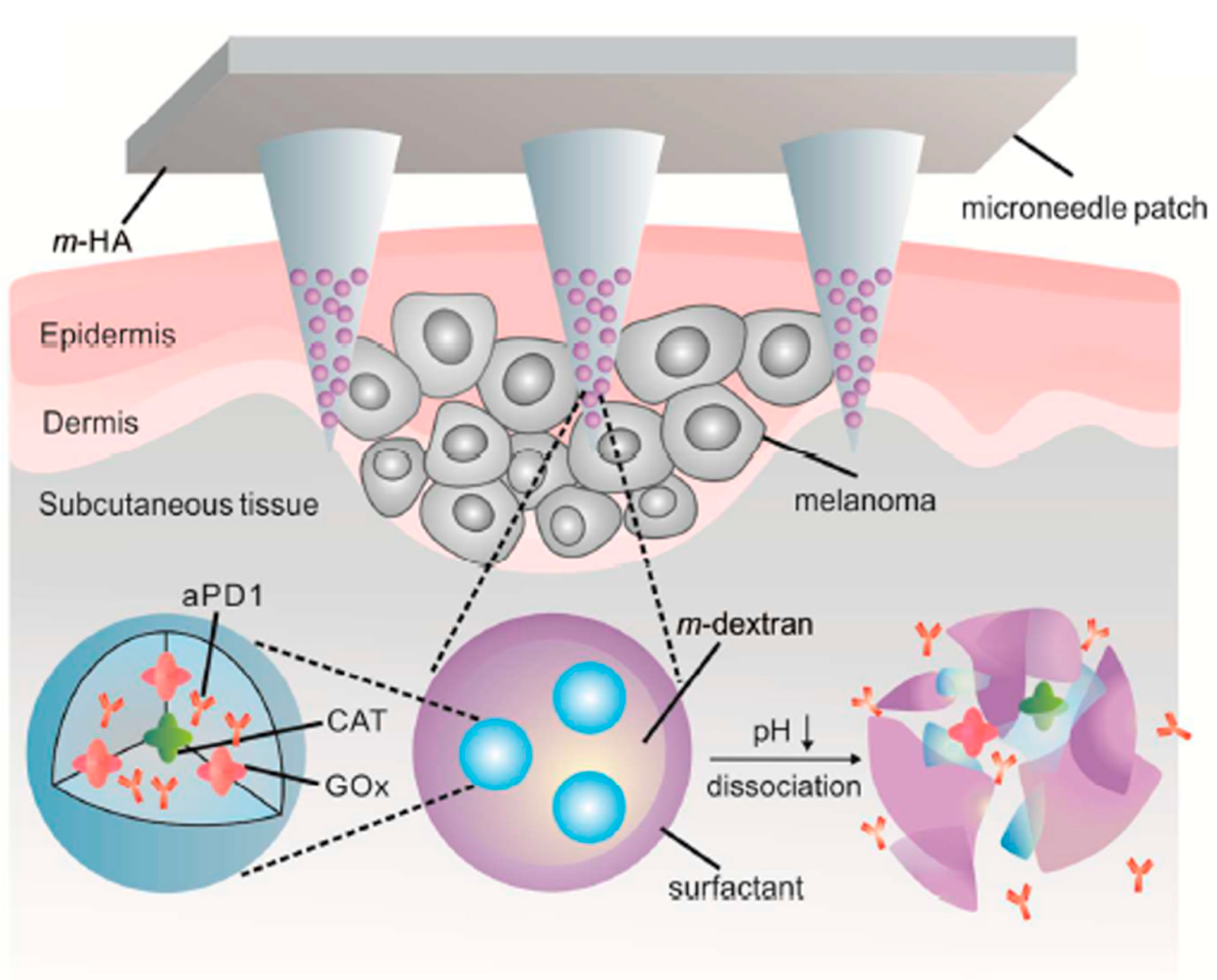
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Su, T.; Tang, Z.; He, H.; Li, W.; Wang, X.; Liao, C.; Sun, Y.; Wang, Q. Glucose oxidase triggers gelation of N-hydroxyimide–heparin conjugates to form enzyme-responsive hydrogels for cell-specific drug delivery. Chem. Sci. 2014, 5, 4204–4209. [Google Scholar] [CrossRef]
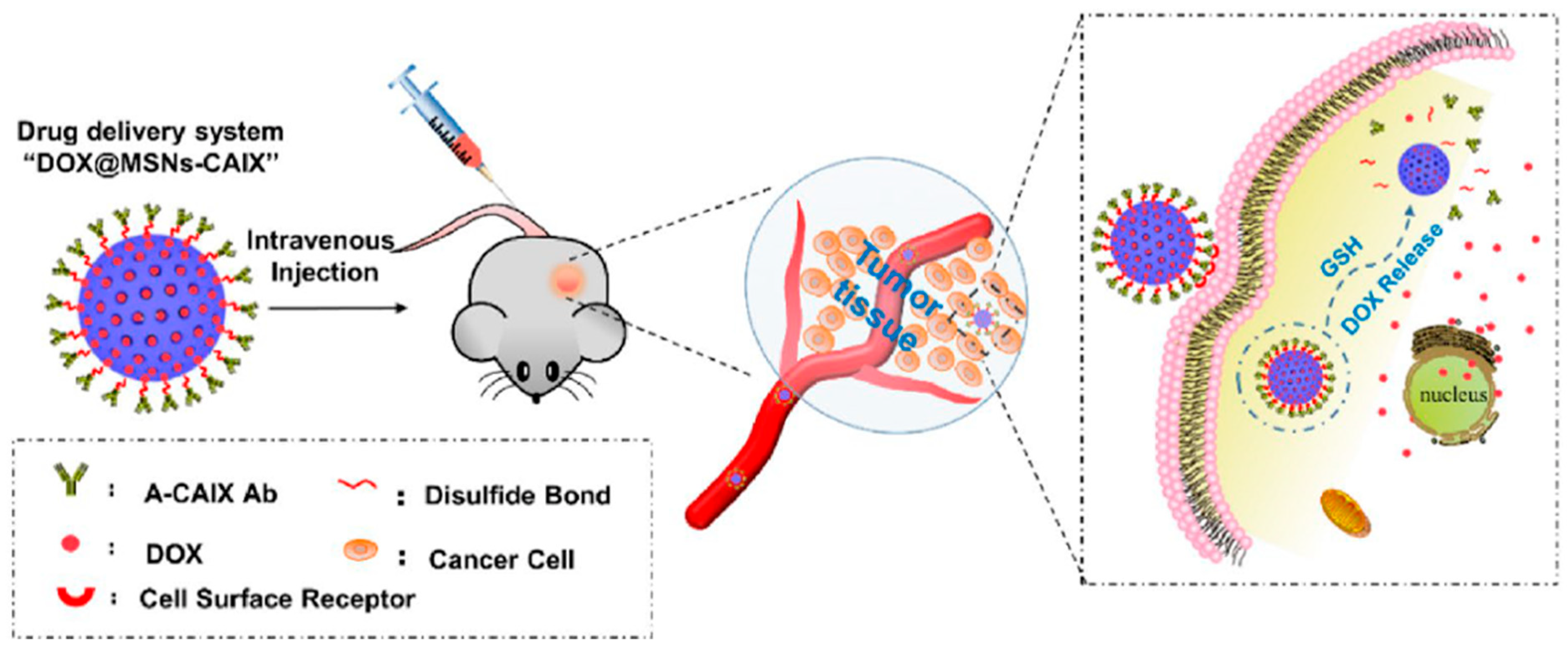
- Chen, M.; Hu, J.; Wang, L.; Li, Y.; Zhu, C.; Chen, C.; Shi, M.; Ju, Z.; Cao, X.; Zhang, Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020, 10, 14447. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Nizak, C.; Surana, S.; Halder, S.; Krishnan, Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. Nat. Nanotechnol. 2013, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Bhatia, D.; Surana, S.; Chakraborty, S.; Koushika, S.P.; Krishnan, Y. A synthetic icosahedral DNA-based host–cargo complex for functional in vivo imaging. Nat. Commun. 2011, 2, 339. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258. [Google Scholar] [CrossRef]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef]
- Zhang, P.; Cheng, F.; Zhou, R.; Cao, J.; Li, J.; Burda, C.; Min, Q.; Zhu, J.J. DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microRNA-responsive controlled drug delivery. Angew. Chem. 2014, 126, 2403–2407. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.-H.; Deng, Z.J.; Cho, N.-J.; Hammond, P.T. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano 2014, 8, 8374–8382. [Google Scholar] [CrossRef]
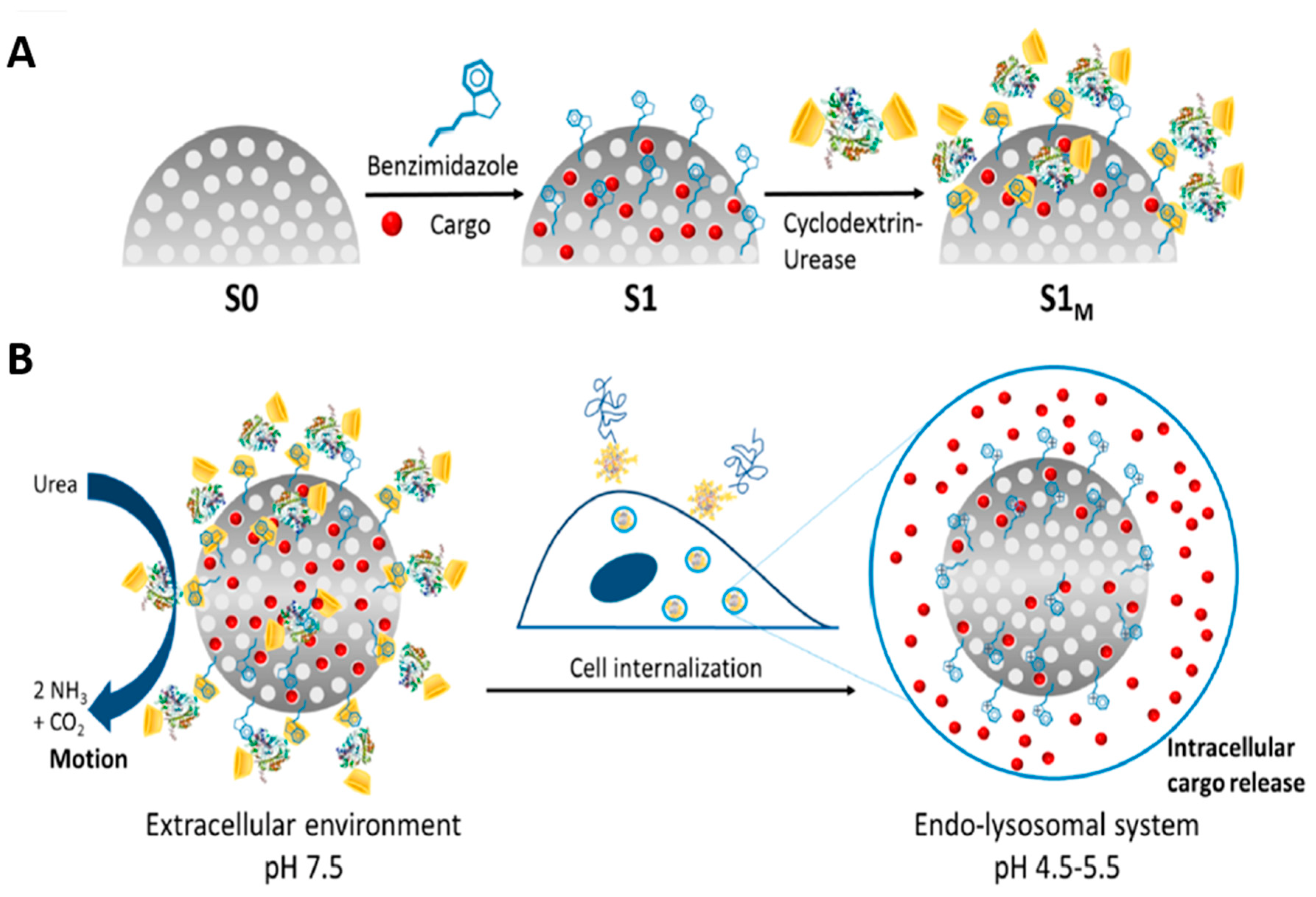
- Llopis-Lorente, A.; Garcia-Fernandez, A.; Murillo-Cremaes, N.; Hortelão, A.C.; Patino, T.; Villalonga, R.; Sancenon, F.; Martinez-Manez, R.; Sanchez, S. Enzyme-powered gated mesoporous silica nanomotors for on-command intracellular payload delivery. ACS Nano 2019, 13, 12171–12183. [Google Scholar] [CrossRef]
- Mage, P.; Ferguson, B.; Maliniak, D.; Ploense, K.; Kippin, T.; Soh, H. Closed-loop control of circulating drug levels in live animals. Nat. Biomed. Eng. 2017, 1, 0070. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Hoggarth, D.A.; Maliniak, D.; Ploense, K.; White, R.J.; Woodward, N.; Hsieh, K.; Bonham, A.J.; Eisenstein, M.; Kippin, T.E. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Science 2013, 5, ra165–ra213. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, M.S.; Brem, H. Targeted therapy for brain tumours. Nat. Rev. Drug Discov. 2004, 3, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncol. 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Masi, B.C.; Tyler, B.M.; Bow, H.; Wicks, R.T.; Xue, Y.; Brem, H.; Langer, R.; Cima, M.J. Intracranial MEMS based temozolomide delivery in a 9L rat gliosarcoma model. Biomaterials 2012, 33, 5768–5775. [Google Scholar] [CrossRef]
- Jonas, O.; Landry, H.M.; Fuller, J.E.; Santini, J.T.; Baselga, J.; Tepper, R.I.; Cima, M.J.; Langer, R. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Science 2015, 7, ra257–ra284. [Google Scholar] [CrossRef]
- Sellner, S.; Kocabey, S.; Zhang, T.; Nekolla, K.; Hutten, S.; Krombach, F.; Liedl, T.; Rehberg, M. Dexamethasone-conjugated DNA nanotubes as anti-inflammatory agents in vivo. Biomaterials 2017, 134, 78–90. [Google Scholar] [CrossRef]
- Lin, Y.X.; Liu, J.F.; Bai, R.; Shi, J.M.; Zhu, X.M.; Liu, J.; Guo, J.; Zhang, W.; Liu, H.L.; Liu, Z.Q. Mitochondria-Inspired Nanoparticles with Microenvironment-Adapting Capacities for On-Demand Drug Delivery after lschemic Injury. ACS Nano 2020, 14, 11846–11859. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Guo, R.; Li, S.; Ni, J.Y.; Gao, S.; Gao, X.M.; Mao, J.Y.; Zhu, Y.; Wu, P.L.; et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. JCR 2020, 317, 259–272. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.P.; Wang, X.Q.; Li, X.R.; Xu, Q.W.; Xin, H.L. Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef]
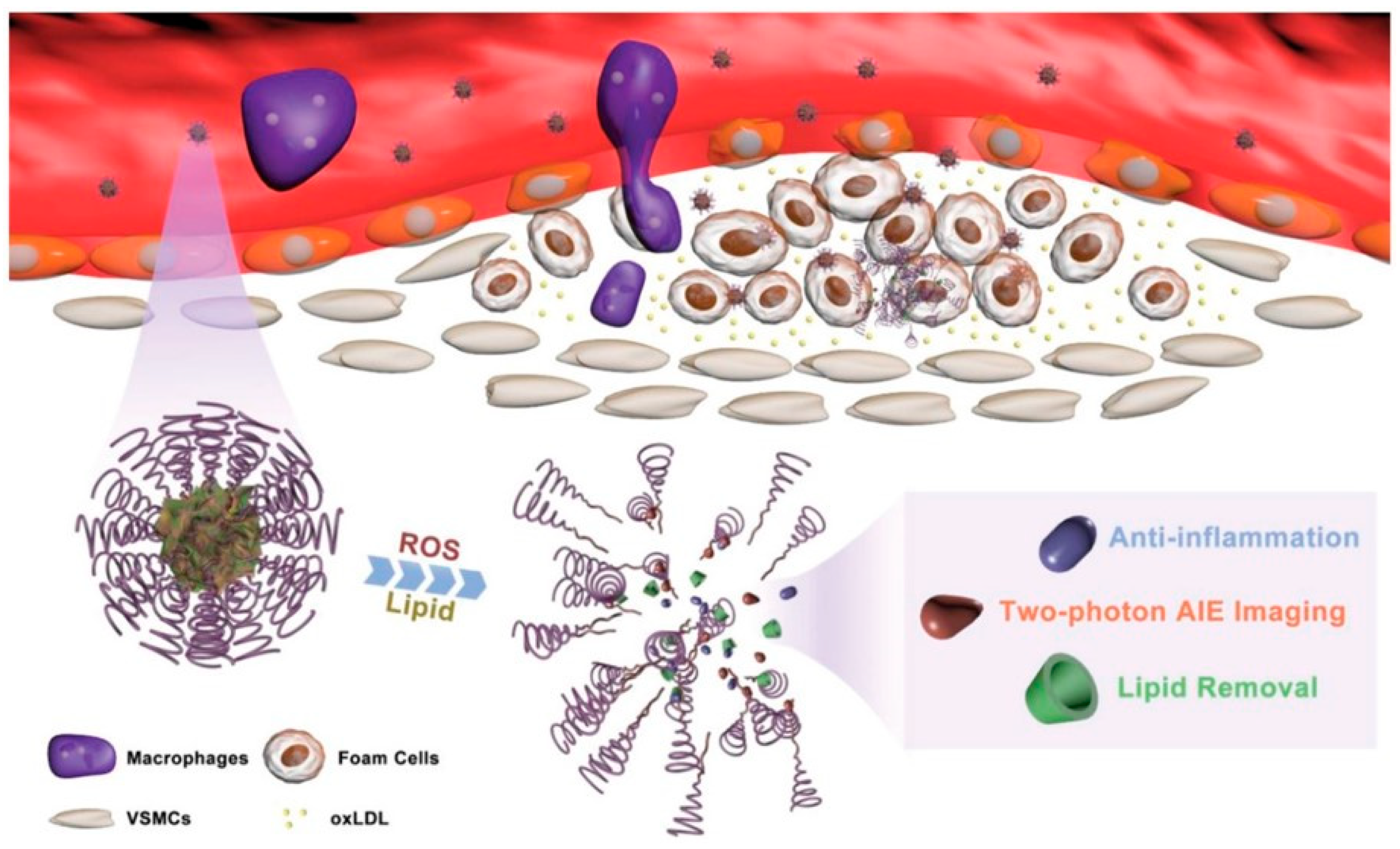
- Ma, B.X.; Xu, H.; Zhuang, W.H.; Wang, Y.N.; Li, G.C.; Wang, Y.B. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and “Two-Pronged” Therapy. Small 2020, 16, e2003253. [Google Scholar] [CrossRef]
- Mu, D.; Li, J.H.; Qi, Y.; Sun, X.; Liu, Y.H.; Shen, S.; Li, Y.Y.; Xu, B.; Zhang, B. Hyaluronic acid-coated polymeric micelles with hydrogen peroxide scavenging to encapsulate statins for alleviating atherosclerosis. J. Nanobiotechnol. 2020, 18, 179. [Google Scholar] [CrossRef]
- Wang, L.L.; Chung, J.J.; Li, E.C.; Uman, S.; Atluri, P.; Burdick, J.A. Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. JCR 2018, 285, 152–161. [Google Scholar] [CrossRef]
- Peters, E.B.; Tsihlis, N.D.; Karver, M.R.; Chin, S.M.; Musetti, B.; Ledford, B.T.; Bahnson, E.M.; Stupp, S.I.; Kibbe, M.R. Atheroma Niche-Responsive Nanocarriers for Immunotherapeutic Delivery. Adv. Healthc. Mater. 2019, 8, e1801545. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, H.; He, J.H.; Jiang, C.P.; Lu, J.; Zhang, W.L.; Yang, H.; Liu, J.P. Co-delivery of LOX-1 siRNA and statin to endothelial cells and macrophages in the atherosclerotic lesions by a dual-targeting core-shell nanoplatform: A dual cell therapy to regress plaques. JCR 2018, 283, 241–260. [Google Scholar] [CrossRef]
- Korin, N. Mechanoresponsive nanotherapeutic for localized drug delivery to flow obstructed blood vessels. Ther. Deliv. 2015, 6, 895–897. [Google Scholar] [CrossRef]
- Korin, N.; Kanapathipillai, M.; Matthews, B.D.; Crescente, M.; Brill, A.; Mammoto, T.; Ghosh, K.; Jurek, S.; Bencherif, S.A.; Bhatta, D.; et al. Shear-Activated Nanotherapeutics for Drug Targeting to Obstructed Blood Vessels. Science 2012, 337, 738–742. [Google Scholar] [CrossRef]
- Yao, S.Y.; Shen, M.L.; Li, S.J.; Wu, X.D.; Zhang, M.M.; Ma, L.N.; Li, Y.P. Application of a mechanically responsive, inflammatory macrophage-targeted dual-sensitive hydrogel drug carrier for atherosclerosis. Colloids Surf. B Biointerfaces 2020, 186, 110718. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Lee, D.J.; Ghaffari, R.; Yun, S.; Kim, S.J.; Lee, J.E.; Cho, H.R.; Yoon, S.; Yang, S.X.; et al. Bioresorbable Electronic Stent Integrated with Therapeutic Nanoparticles for Endovascular Diseases. ACS Nano 2015, 9, 5937–5946. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials-Recent advances and challenges. Appl. Surf. Sci. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Mohanraj, B.; Duan, G.; Peredo, A.; Kim, M.; Tu, F.Q.; Lee, D.; Dodge, G.R.; Mauck, R.L. Mechanically Activated Microcapsules for “On-Demand” Drug Delivery in Dynamically Loaded Musculoskeletal Tissues. Adv. Funct. Mater. 2019, 29, 1807909. [Google Scholar] [CrossRef]
- Lan, Q.M.; Lu, R.B.; Chen, H.M.; Pang, Y.F.; Xiong, F.; Shen, C.; Qin, Z.N.; Zheng, L.; Xu, G.J.; Zhao, J.M. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J. Nanobiotechnol. 2020, 18, 117. [Google Scholar] [CrossRef]
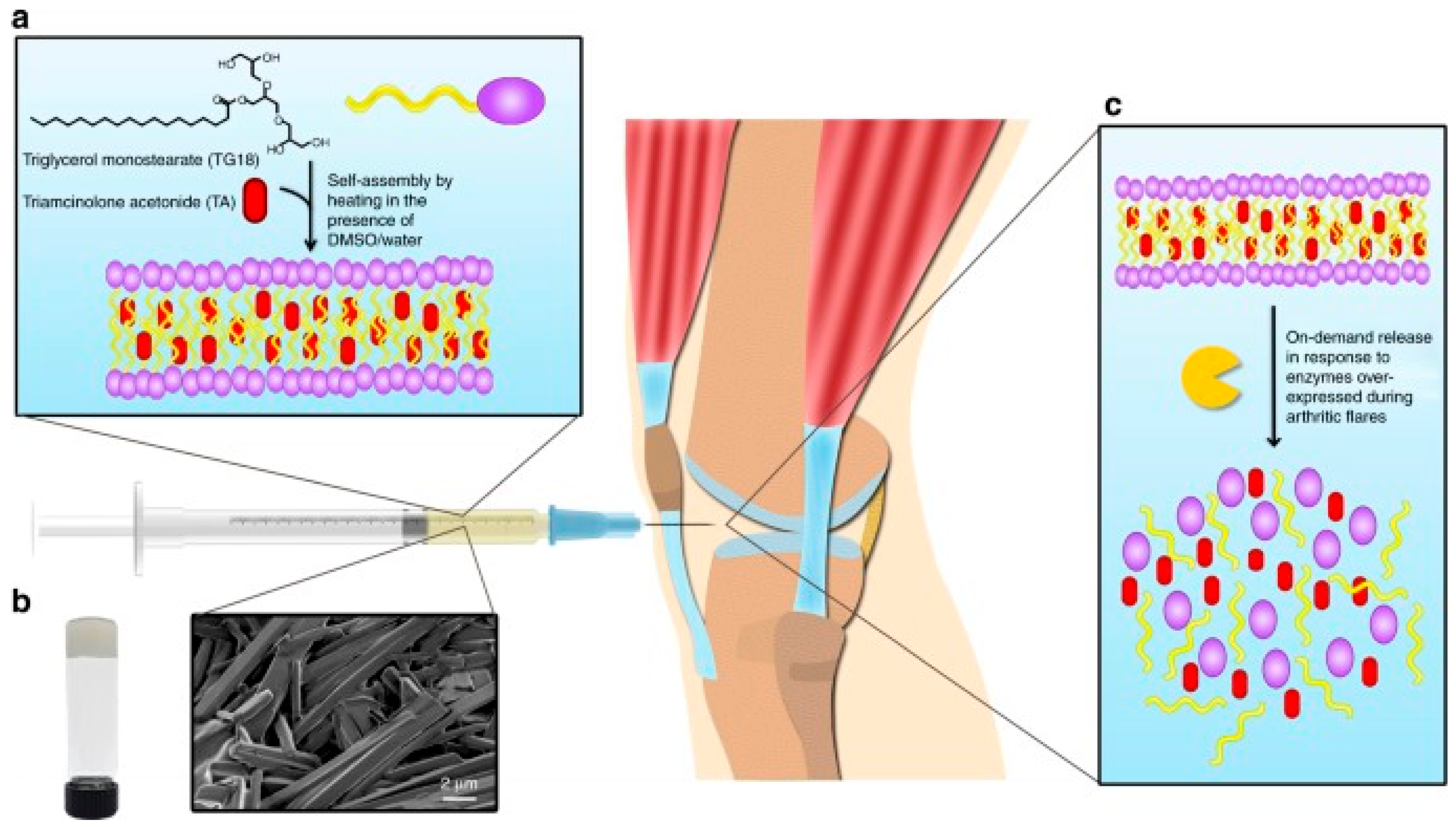
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.F.; Riegel, L.; He, X.Y.; et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Ryma, M.; Blohbaum, J.; Singh, R.; Sancho, A.; Matuszak, J.; Cicha, I.; Groll, J. Easy-to-Prepare Coating of Standard Cell Culture Dishes for Cell-Sheet Engineering Using Aqueous Solutions of Poly (2-n-propyl-oxazoline). Acs Biomater. Sci. Eng. 2019, 5, 1509–1517. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermoresponsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Chen, F.M.; Lu, H.; Wu, L.A.; Gao, L.N.; An, Y.; Zhang, J. Surface-engineering of glycidyl methacrylated dextran/gelatin microcapsules with thermo-responsive poly (N-isopropylacrylamide) gates for controlled delivery of stromal cell-derived factor-1 alpha. Biomaterials 2013, 34, 6515–6527. [Google Scholar] [CrossRef]
- Wang, M.; He, F.; Li, H.; Yang, S.H.; Zhang, J.H.; Ghosh, P.; Wang, H.H.; Nie, Z. Near-Infrared Light-Activated DNA-Agonist Nanodevice for Nongenetically and Remotely Controlled Cellular Signaling and Behaviors in Live Animals. Nano Lett. 2019, 19, 2603–2613. [Google Scholar] [CrossRef]
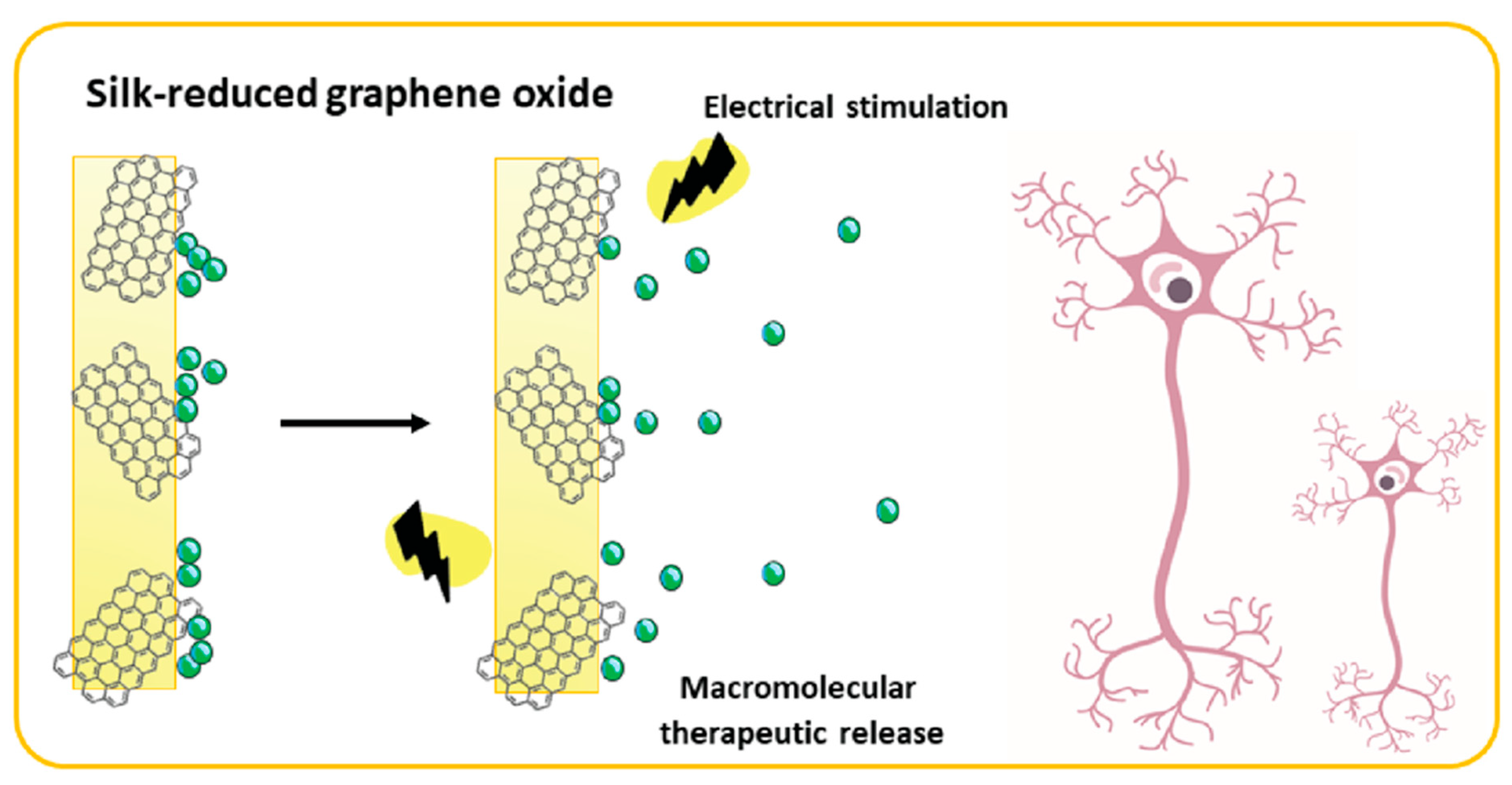
- Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12, 742. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. https://doi.org/10.3390/biom12091198
Cicha I, Priefer R, Severino P, Souto EB, Jain S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules. 2022; 12(9):1198. https://doi.org/10.3390/biom12091198
Chicago/Turabian StyleCicha, Iwona, Ronny Priefer, Patrícia Severino, Eliana B. Souto, and Sona Jain. 2022. "Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications" Biomolecules 12, no. 9: 1198. https://doi.org/10.3390/biom12091198
APA StyleCicha, I., Priefer, R., Severino, P., Souto, E. B., & Jain, S. (2022). Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules, 12(9), 1198. https://doi.org/10.3390/biom12091198











