Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases
Abstract
:1. Introduction
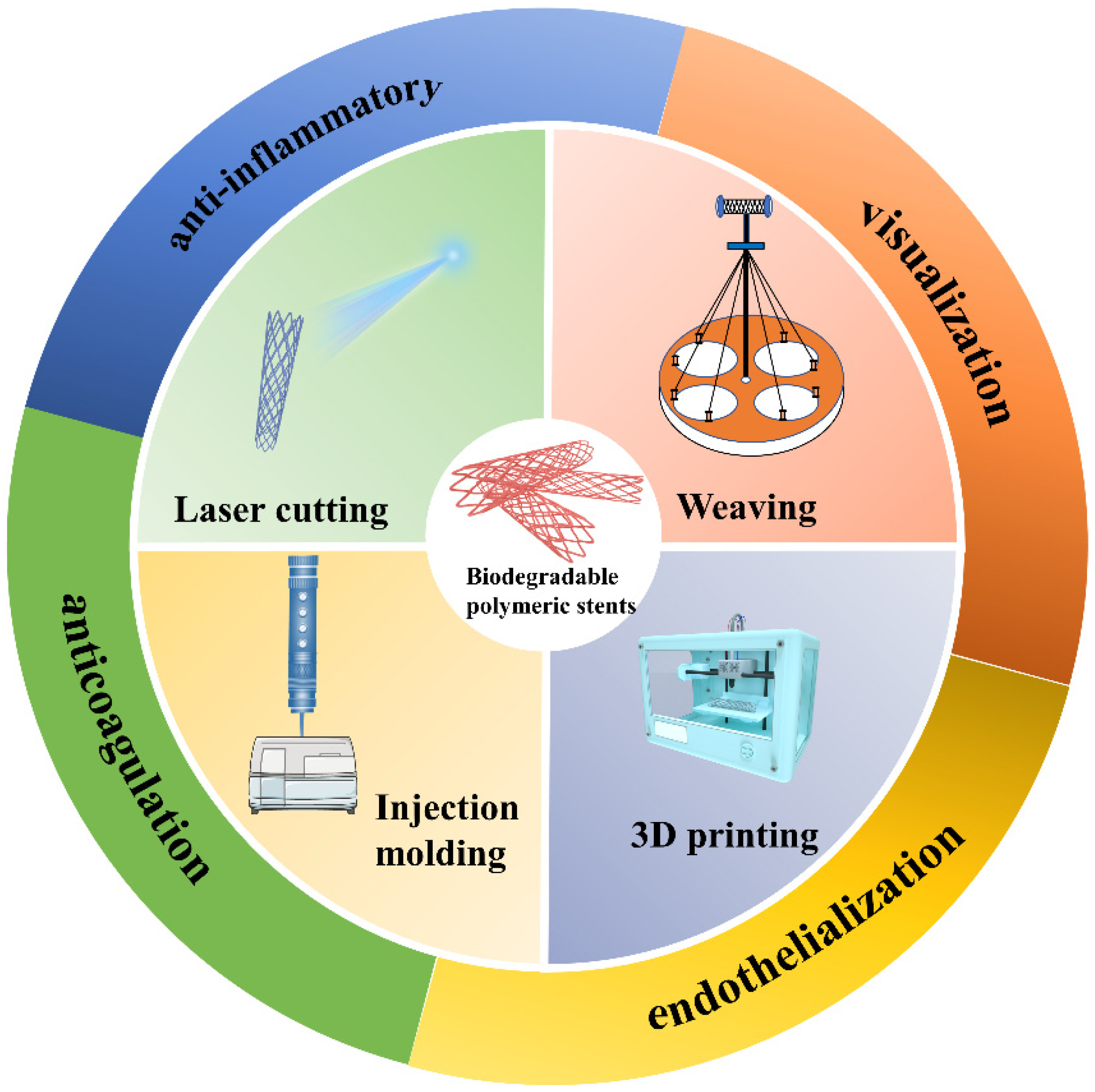
2. Manufacturing Methods of Biodegradable Polymeric Stents
2.1. Injection Molding
2.2. Laser Cutting
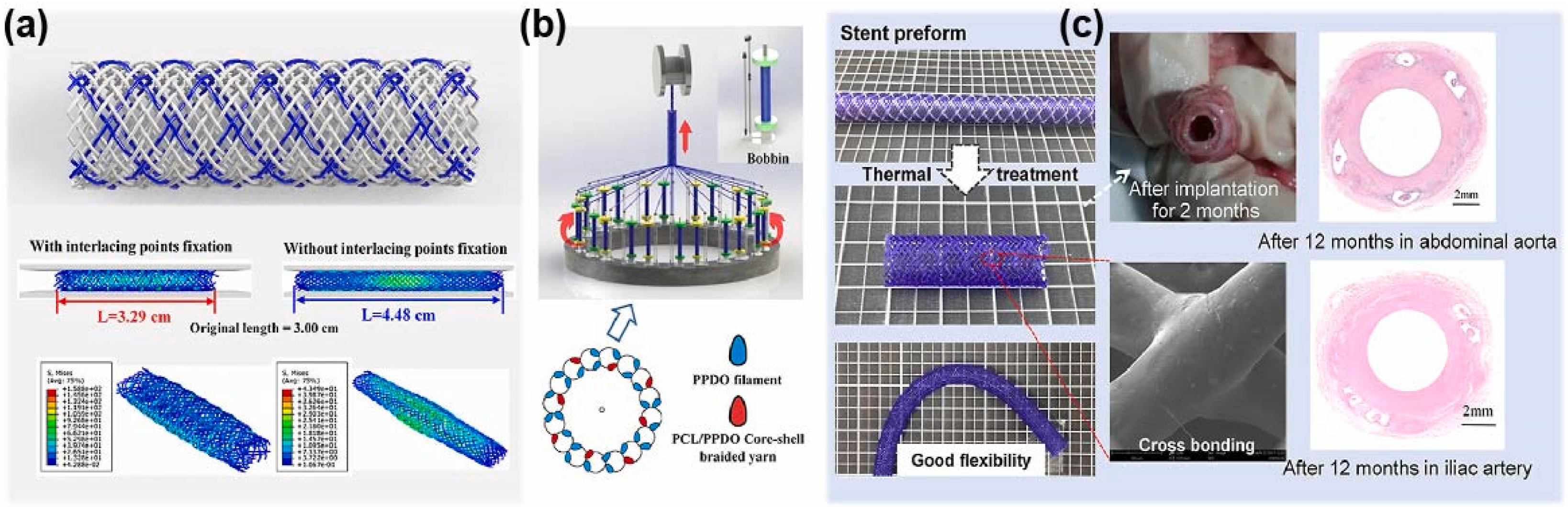
2.3. Weaving
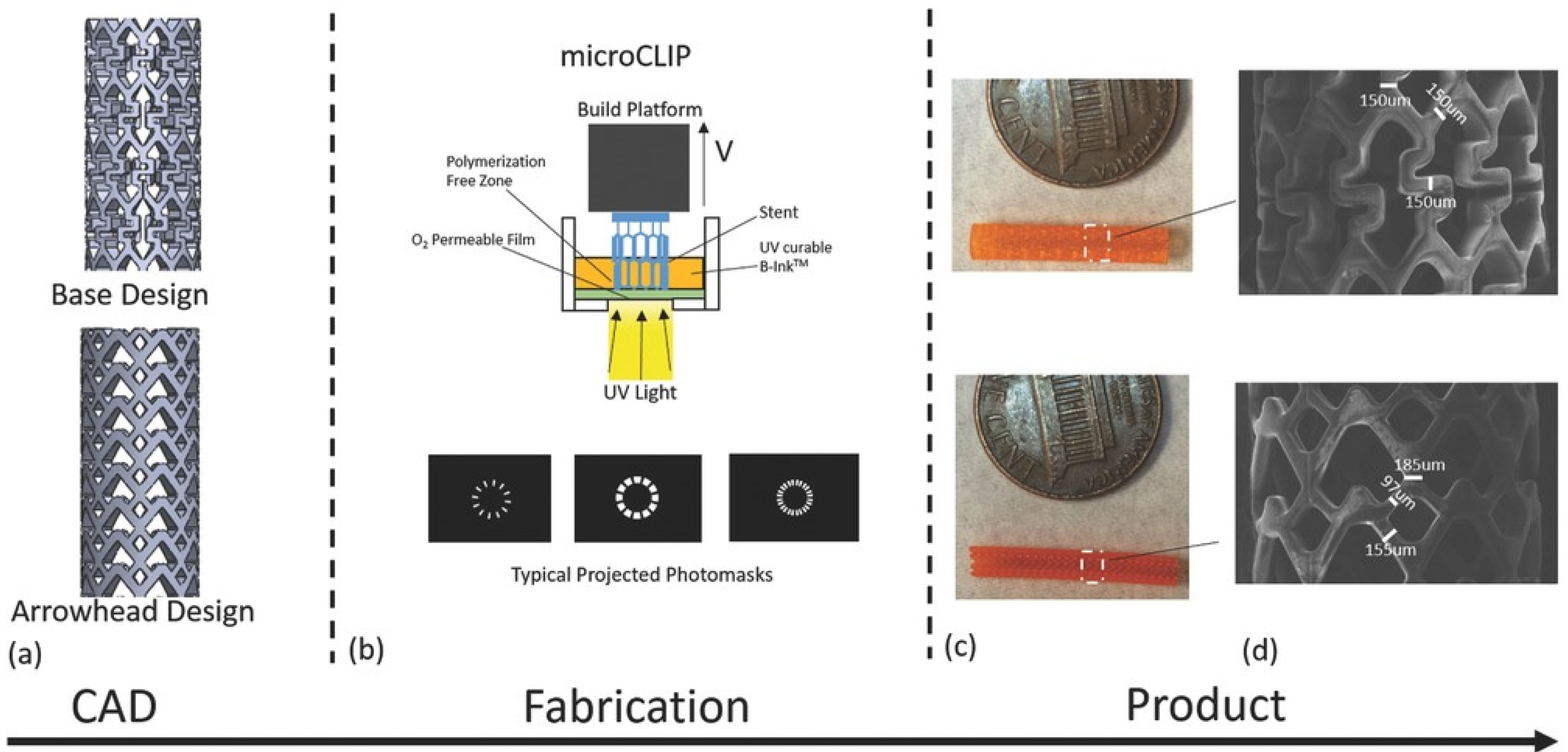
2.4. 3D Printing
3. Functionalization of Biodegradable Polymeric Stents
3.1. Stent Visualization
3.2. Biological Functional Modification of Polymeric Stents
3.2.1. The Anticoagulant and Endothelialization-Promoting Modification of Polymeric Stents
3.2.2. The Anti-Inflammatory Modification of Polymeric Stents
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Okrainec, K.; Banerjee, D.K.; Eisenberg, M.J. Coronary artery disease in the developing world. Am. Heart J. 2004, 148, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Barton, M.; Grüntzig, J.; Husmann, M.; Rösch, J. Balloon angioplasty—The legacy of Andreas Grüntzig, MD (1939–1985). Front. Cardiovasc. Med. 2014, 1, 15. [Google Scholar] [CrossRef]
- Grüntzig, A.R.; Senning, Å.; Siegenthaler, W.E. Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1979, 301, 61–68. [Google Scholar] [CrossRef]
- Hanawa, T. Materials for metallic stents. J. Artif. Organs 2009, 12, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, W.S. The pathophysiology and burden of restenosis. Am. J. Cardiol. 2007, 100, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Colombo, A.; Stefanadis, C. Inflammation and restenosis after percutaneous coronary interventions. Eur. Heart J. 2004, 25, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yang, K.; Cheng, R.; Xiang, Y.; Yuan, T.; Cheng, Y.; Sarmento, B.; Cui, W. The current status of biodegradable stent to treat benign luminal disease. Mater. Today 2017, 20, 516–529. [Google Scholar] [CrossRef]
- Commandeur, S.; van Beusekom, H.M.; van der Giessen, W.J. Polymers, drug release, and drug-eluting stents. J. Interv. Cardiol. 2006, 19, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Pauck, R.; Reddy, B. Computational analysis of the radial mechanical performance of PLLA coronary artery stents. Med. Eng. Phys. 2015, 37, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-D.; Li, Z.; Pan, Y.; Sabir, M.; Zheng, Y.-F.; Li, L. A review on biodegradable materials for cardiovascular stent application. Front. Mater. Sci. 2016, 10, 238–259. [Google Scholar] [CrossRef]
- Momma, C.; Knop, U.; Nolte, S. Laser cutting of slotted tube coronary stents—State-of-the-art and future developments. Prog. Biomed. Res. 1999, 4, 39–44. [Google Scholar]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Laasch, H.-U. Current designs of self-expanding stents. In Self-Expandable Stents in the Gastrointestinal Tract; Springer: New York, NY, USA, 2013; pp. 51–69. [Google Scholar]
- Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. [Google Scholar] [CrossRef]
- Polanec, B.; Kramberger, J.; Glodež, S. A review of production technologies and materials for manufacturing of cardiovascular stents. Adv. Prod. Eng. Manag. 2020, 15, 390–402. [Google Scholar] [CrossRef]
- Li, H.; Liu, K.; Zhao, D.; Wang, M.; Li, Q.; Hou, J. Multi-objective optimizations for microinjection molding process parameters of biodegradable polymer stent. Materials 2018, 11, 2322. [Google Scholar] [CrossRef]
- Liu, S.J.; Chiang, F.J.; Hsiao, C.Y.; Kau, Y.C.; Liu, K.S. Fabrication of balloon-expandable self-lock drug-eluting polycaprolactone stents using micro-injection molding and spray coating techniques. Ann. Biomed. Eng. 2010, 38, 3185–3194. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, C.J.; Liu, S.J.; Hsiao, C.Y.; Chen, J.K. The development of novel biodegradable bifurcation stents for the sustainable release of anti-proliferative sirolimus. Ann. Biomed. Eng. 2012, 40, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, W.; Zhou, T.; Wang, L.; Qiu, T. A Review on Manufacturing and Post-Processing Technology of Vascular Stents. Micromachines 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chen, C.S.; Chen, Y.S.; Ma, S.F. Developing in Biodegradable Stents Technology. Key Eng. Mater. 2014, 625, 483–488. [Google Scholar] [CrossRef]
- Tseng, S.-F.; Hung, T.-Y.; Chang, C.-M. Mechanical and microstructural properties of additively manufactured Ti–6Al–4 V stents with CO2 laser postannealing treatment. Int. J. Adv. Manuf. Technol. 2022, 119, 6571–6581. [Google Scholar] [CrossRef]
- Alefelder, J.; Philipp, J.; Engelmann, U.; Senge, T. Stented laser-welded vasovasostomy in the rat: Comparison of Nd: YAG and CO2 lasers. J. Reconstr. Microsurg. 1991, 7, 317–320. [Google Scholar] [CrossRef]
- Meng, H.; Liao, J.; Zhou, Y.; Zhang, Q. Laser micro-processing of cardiovascular stent with fiber laser cutting system. Opt. Laser Technol. 2009, 41, 300–302. [Google Scholar] [CrossRef]
- Badr, S.; Ben-Dor, I.; Dvir, D.; Barbash, I.M.; Kitabata, H.; Pendyala, L.K.; Loh, J.P.; Torguson, R.; Pichard, A.D.; Waksman, R. The state of the excimer laser for coronary intervention in the drug-eluting stent era. Cardiovasc. Revasc. Med. 2013, 14, 93–98. [Google Scholar] [CrossRef]
- Hendricks, F.; Patel, R.; Matylitsky, V. Micromachining of bio-absorbable stents with ultra-short pulse lasers. In Proceedings of the Frontiers in Ultrafast Optics: Biomedical, Scientific, and Industrial Applications XV, San Francisco, CA, USA, 7–12 February 2015; p. 935502. [Google Scholar]
- Stępak, B.; Antończak, A.J.; Bartkowiak-Jowsa, M.; Filipiak, J.; Pezowicz, C.; Abramski, K.M. Fabrication of a polymer-based biodegradable stent using a CO2 laser. Arch. Civ. Mech. Eng. 2014, 14, 317–326. [Google Scholar] [CrossRef]
- Guerra, A.J.; Farjas, J.; Ciurana, J. Fibre laser cutting of polycaprolactone sheet for stents manufacturing: A feasibility study. Opt. Laser Technol. 2017, 95, 113–123. [Google Scholar] [CrossRef]
- McClean, D.R.; Eigler, N.L. Stent design: Implications for restenosis. Rev. Cardiovasc. Med. 2003, 3, S16–S22. [Google Scholar]
- Kelly, N.; McGrath, D.J.; Sweeney, C.A.; Kurtenbach, K.; Grogan, J.A.; Jockenhoevel, S.; O’Brien, B.J.; Bruzzi, M.; McHugh, P.E. Comparison of computational modelling techniques for braided stent analysis. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Boey, F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874. [Google Scholar]
- Zhao, F.; Xue, W.; Wang, F.; Yu, C.; Xu, H.; Hao, Y.; Wang, L. A new approach to improve the local compressive properties of PPDO self-expandable stent. J. Mech. Behav. Biomed. Mater. 2017, 68, 318–326. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, J.; Xue, W.; Wang, F.; King, M.W.; Yu, C.; Jiao, Y.; Sun, K.; Wang, L. Development of a polycaprolactone/poly(p-dioxanone) bioresorbable stent with mechanically self-reinforced structure for congenital heart disease treatment. Bioact. Mater. 2021, 6, 2969–2982. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lou, C.W.; Lin, J.Y.; Lin, T.A.; Chen, Y.S.; Lin, J.H. Biodegradable Polyvinyl Alcohol Vascular Stents: Structural Model and Mechanical and Biological Property Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 404–413. [Google Scholar] [CrossRef]
- King, M.W.; Gupta, B.S.; Guidoin, R. Biotextiles as Medical Implants; Elsevier: Cambridge, UK, 2013. [Google Scholar]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Application of textile technology in tissue engineering: A review. Acta Biomater. 2021, 128, 60–76. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Dudek, P. FDM 3D printing technology in manufacturing composite elements. Arch. Metall. Mater. 2013, 58, 1415–1418. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Wei, Y.; Huang, C. Crushing behavior of a thin-walled circular tube with internal gradient grooves fabricated by SLM 3D printing. Thin-Walled Struct. 2017, 111, 1–8. [Google Scholar] [CrossRef]
- Ma, X.L. Research on application of SLA technology in the 3D printing technology. Appl. Mech. Mater. 2013, 401–403, 938–941. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Mitsouras, D.; Yoo, S.-J.; Liu, P.P.; Chatzizisis, Y.S.; Rybicki, F.J. Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ostadhossein, F.; Babu, R.; Kus, J.; Tankasala, D.; Sutrisno, A.; Walsh, K.A.; Bromfield, C.R.; Pan, D. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv. Healthc. Mater. 2017, 6, 1700008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhou, R.; Sun, J.; Li, H.; Jin, Y. Experimental study of polymeric stent fabrication using homemade 3D printing system. Polym. Eng. Sci. 2019, 59, 1122–1131. [Google Scholar] [CrossRef]
- Jia, H.; Gu, S.-Y.; Chang, K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv. Polym. Technol. 2018, 37, 3222–3228. [Google Scholar] [CrossRef]
- van Lith, R.; Baker, E.; Ware, H.; Yang, J.; Farsheed, A.C.; Sun, C.; Ameer, G. 3D-Printing Strong High-Resolution Antioxidant Bioresorbable Vascular Stents. Adv. Mater. Technol. 2016, 1, 1600138. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Attia, M.F.; Brummel, B.R.; Lex, T.R.; Van Horn, B.A.; Whitehead, D.C.; Alexis, F. Recent Advances in Polyesters for Biomedical Imaging. Adv. Healthc. Mater. 2018, 7, 1800798. [Google Scholar] [CrossRef]
- Waksman, R. Update on bioabsorbable stents: From bench to clinical. J. Interv. Cardiol. 2006, 19, 414–421. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Serruys, P.W.; Regar, E.; Dudek, D.; Thuesen, L.; Webster, M.W.; Onuma, Y.; Garcia-Garcia, H.M.; McGreevy, R.; Veldhof, S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): A prospective open-label trial. Lancet 2008, 371, 899–907. [Google Scholar] [CrossRef]
- Chevalier, B.; Abizaid, A.; Carrié, D.; Frey, N.; Lutz, M.; Weber-Albers, J.; Dudek, D.; Weng, S.-C.; Akodad, M.; Anderson, J. Clinical and angiographic outcomes with a novel radiopaque sirolimus-eluting bioresorbable vascular scaffold: The FANTOM II study. Circ. Cardiovasc. Interv. 2019, 12, e007283. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, Y.; Ge, L.; Zhang, Y.; Wang, Q.; Qian, J.; Qiu, Z.; Ge, J. A head to head comparison of XINSORB bioresorbable sirolimus-eluting scaffold versus metallic sirolimus-eluting stent: 180 days follow-up in a porcine model. Int. J. Cardiovasc. Imaging 2017, 33, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation 2012, 125, 2343–2353. [Google Scholar] [CrossRef]
- Abizaid, A.; Costa, R.A.; Schofer, J.; Ormiston, J.; Maeng, M.; Witzenbichler, B.; Botelho, R.V.; Costa, J.R.; Chamié, D.; Abizaid, A.S. Serial multimodality imaging and 2-year clinical outcomes of the novel DESolve novolimus-eluting bioresorbable coronary scaffold system for the treatment of single de novo coronary lesions. JACC Cardiovasc. Interv. 2016, 9, 565–574. [Google Scholar] [CrossRef]
- Han, Y.; Xu, B.; Fu, G.; Wang, X.; Xu, K.; Jin, C.; Tao, L.; Li, L.; Hou, Y.; Su, X. A randomized trial comparing the NeoVas sirolimus-eluting bioresorbable scaffold and metallic everolimus-eluting stents. JACC Cardiovasc. Interv. 2018, 11, 260–272. [Google Scholar] [CrossRef]
- Nagy, P. X-ray examination of integrated stent markers. IRBM 2015, 36, 15–19. [Google Scholar] [CrossRef]
- Nagy, P. X-ray Analysis of Stents and their Markers. Period. Polytech. Mech. Eng. 2015, 59, 30–34. [Google Scholar] [CrossRef]
- Hong, S.H.; Herman, A.M.; Stephenson, J.M.; Wu, T.; Bahadur, A.N.; Burns, A.R.; Marrelli, S.P.; Wythe, J.D. Development of barium-based low viscosity contrast agents for micro CT vascular casting: Application to 3D visualization of the adult mouse cerebrovasculature. J. Neurosci. Res. 2020, 98, 312–324. [Google Scholar] [CrossRef]
- Kashyap, D.; Kumar, P.K.; Kanagaraj, S. 4D printed porous radiopaque shape memory polyurethane for endovascular embolization. Addit. Manuf. 2018, 24, 687–695. [Google Scholar] [CrossRef]
- Ang, H.Y.; Toong, D.; Chow, W.S.; Seisilya, W.; Wu, W.; Wong, P.; Venkatraman, S.S.; Foin, N.; Huang, Y. Radiopaque Fully Degradable Nanocomposites for Coronary Stents. Sci. Rep. 2018, 8, 17409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van den Akker, N.M.; Molin, D.G.; Gagliardi, M.; van der Marel, C.; Lutz, M.; Knetsch, M.L.; Koole, L.H. A nontoxic additive to introduce x-ray contrast into poly(lactic acid). Implications for transient medical implants such as bioresorbable coronary vascular scaffolds. Adv. Healthc. Mater. 2014, 3, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hamideh, R.A.; Akbari, B.; Fathi, P.; Misra, S.K.; Sutrisno, A.; Lam, F.; Pan, D. Biodegradable MRI Visible Drug Eluting Stent Reinforced by Metal Organic Frameworks. Adv. Healthc. Mater. 2020, 9, e2000136. [Google Scholar] [CrossRef]
- Lee, H.I.; Heo, Y.; Baek, S.W.; Kim, D.S.; Song, D.H.; Han, D.K. Multifunctional Biodegradable Vascular PLLA Scaffold with Improved X-ray Opacity, Anti-Inflammation, and Re-Endothelization. Polymer 2021, 13, 1979. [Google Scholar] [CrossRef]
- Olsen, T.R.; Davis, L.L.; Nicolau, S.E.; Duncan, C.C.; Whitehead, D.C.; Van Horn, B.A.; Alexis, F. Non-invasive deep tissue imaging of iodine modified poly (caprolactone-co-1-4-oxepan-1, 5-dione) using X-ray. Acta Biomater. 2015, 20, 94–103. [Google Scholar] [CrossRef]
- Goodfriend, A.C.; Welch, T.R.; Nguyen, K.T.; Wang, J.; Johnson, R.F.; Nugent, A.; Forbess, J.M. Poly (gadodiamide fumaric acid): A bioresorbable, radiopaque, and MRI-visible polymer for biomedical applications. ACS Biomater. Sci. Eng. 2015, 1, 677–684. [Google Scholar] [CrossRef]
- Huang, J.; Lv, Z.; Wang, Y.; Wang, Z.; Gao, T.; Zhang, N.; Guo, M.; Zou, H.; Zhang, P. In Vivo MRI and X-Ray Bifunctional Imaging of Polymeric Composite Supplemented with GdPO4·H2O Nanobundles for Tracing Bone Implant and Bone Regeneration. Adv. Healthc. Mater. 2016, 5, 2182–2190. [Google Scholar] [CrossRef]
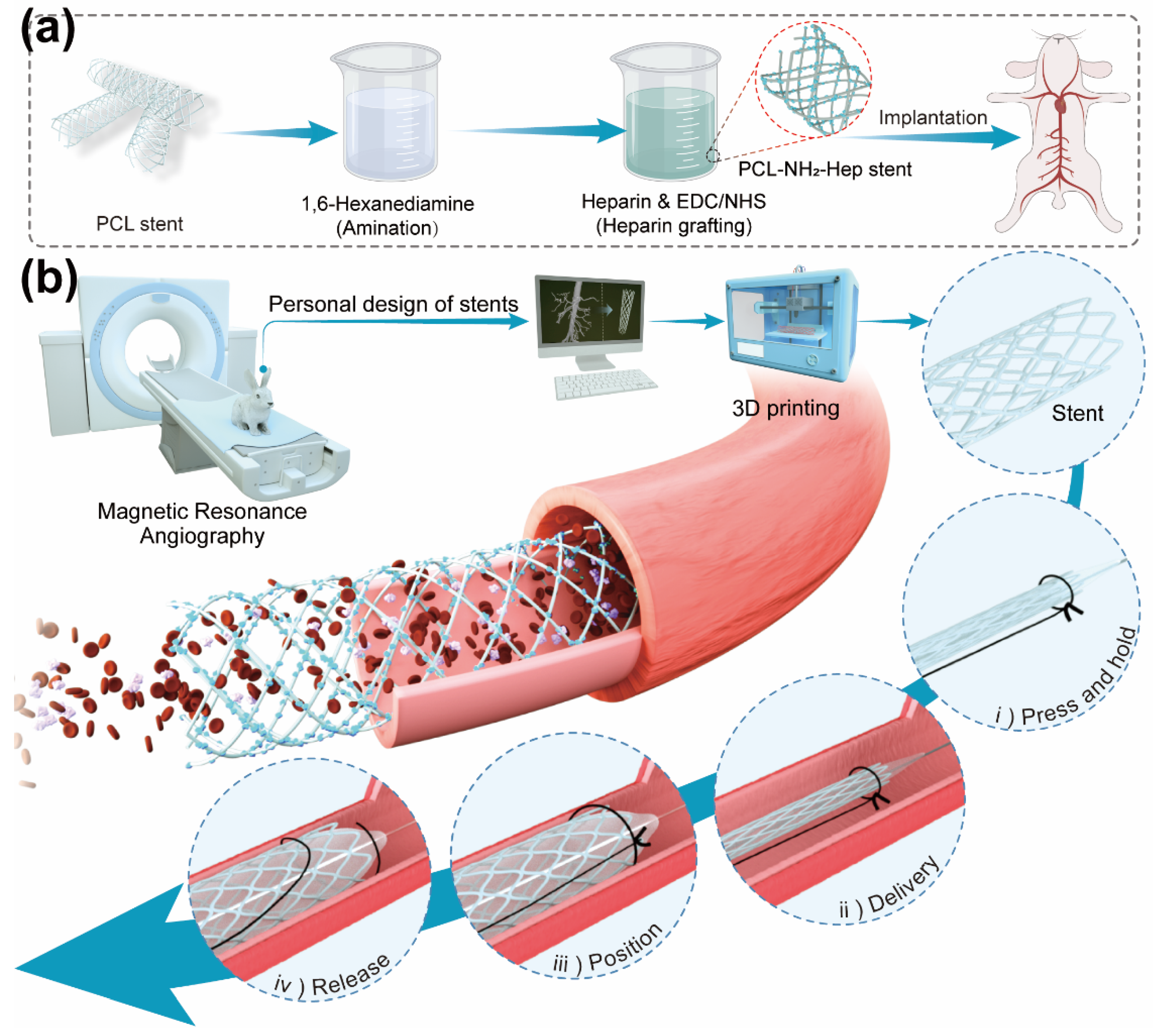
- Shen, Y.; Tang, C.; Sun, B.; Zhang, Y.; Sun, X.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; Gu, H.; Wang, W.; et al. 3D printed personalized, heparinized and biodegradable coronary artery stents for rabbit abdominal aorta implantation. Chem. Eng. J. 2022, 450, 138202. [Google Scholar] [CrossRef]
- Lyu, N.; Du, Z.; Qiu, H.; Gao, P.; Yao, Q.; Xiong, K.; Tu, Q.; Li, X.; Chen, B.; Wang, M.; et al. Mimicking the Nitric Oxide-Releasing and Glycocalyx Functions of Endothelium on Vascular Stent Surfaces. Adv. Sci. 2020, 7, 2002330. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)—Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Y.; Liu, J.; Hu, M.; Zhang, H.; Ren, K.; Wang, Y.; Ji, J. Hierarchical Capillary Coating to Biofunctionlize Drug-Eluting Stent for Improving Endothelium Regeneration. Research 2020, 2020, 1458090. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Tu, Q.; Gao, P.; Li, X.; Maitz, M.F.; Xiong, K.; Huang, N.; Yang, Z. Phenolic-amine chemistry mediated synergistic modification with polyphenols and thrombin inhibitor for combating the thrombosis and inflammation of cardiovascular stents. Biomaterials 2021, 269, 120626. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, R.; Hu, C.; Maitz, M.F.; Wu, H.; Liu, K.; Yang, L.; Luo, R.; Wang, Y. Epigallocatechin gallate mediated sandwich-like coating for mimicking endothelium with sustained therapeutic nitric oxide generation and heparin release. Biomaterials 2020, 269, 120418. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Liu, Y.; Xia, Q.; Yang, Y.; Chen, N.; Yang, M.; Luo, R.; Liu, G.; Wang, Y. A robust mussel-inspired zwitterionic coating on biodegradable poly(L-lactide) stent with enhanced anticoagulant, anti-inflammatory, and anti-hyperplasia properties. Chem. Eng. J. 2022, 427, 130910. [Google Scholar] [CrossRef]
- Jeong, Y.; Yao, Y.; Yim, E.K.F. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater. Sci. 2020, 8, 4383–4395. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef]
- Welt, F.G.; Rogers, C. Inflammation and restenosis in the stent era. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1769–1776. [Google Scholar] [CrossRef]
- Drachman, D.E.; Simon, D.I. Inflammation as a mechanism and therapeutic target for in-stent restenosis. Curr. Atheroscler. Rep. 2005, 7, 44–49. [Google Scholar] [CrossRef]





| Method | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Injection molding | Injecting thermoplastic materials or thermosetting materials into the molds |
|
|
| Weaving | Weaving the stent directly according to the shape and size via knitting, warp knitting, weft knitting, or other weaving technology. |
|
|
| Laser cutting | Carving the required structure on the polymer material via laser and cutting the stent from the tube. |
|
|
| 3D printing | Layer-by-layer printing based on digital model |
|
|
| Polymer | Fabrication Method | Brief Description | Advantages | Ref |
|---|---|---|---|---|
| PCL | Injection molding | Diameter 3–5 mm PLGA/paclitaxel coating Self-lock design | Ideal mechanical strength Sustainable release of paclitaxel | Liu et al. [21] |
| PCL | Injection molding | Bifurcation design PLGA/sirolimus coating | Design for bifurcation lesions Comparable mechanical properties with those of commercial stents SMCs proliferation↓ | Lee et al. [22] |
| PVA | Braiding warp knitting weft knitting | CS coating /Genipin chemical cross-linking | Good bending resistance property Antibacterial Satisfactory biological properties | Lin et al. [37] |
| PPDO/PCL | Braiding | Diameter 8 mm Consisted of PPDO monofilaments and PCL/PPDO core-shell composite yarns | Two-stage degradation Good structure stability Implantation in vivo in 12 months | Zhao et al. [36] |
| PLLA/PLGA | CO2 laser cutting | / | Applied for fabrication of some types of polymers Obtained a smooth and narrow cut in a 250 mm-thick polymer sheet | Stępak et al. [30] |
| PCL/PLA | Fiber laser cutting | / | High accuracy Less effect over the material properties | Guerra et al. [31] |
| PCL-graphene | 3D printing | Patient-specific stenting process Dual drug incorporation | Good mechanical properties Low toxicity Personal design | Misra et al. [46] |
| multifunctional polydiolcitrate (mPDC) | microCLIP | Polydiolcitrates can be engineered to be light-curable by incorporating methacrylate groups | Comparable mechanical strength of nitinol stents Customizable, compressed, and self-expanded within a clinically relevant time frame upon deployment in vitro | Robert et al. [49] |
| Stent | Company | Polymer | Marker Material Used | Visualization Method | Ref |
|---|---|---|---|---|---|
| REVA | Reva Medical | desaminotyrosine polycarbonate polymer (PTD-PC) | Iodized polymer | Iodine impregnation | [53] |
| Absorb 1.0 | Abbott | PLLA | Platinum | Marker fixation | [54] |
| Fantom | Reva Medical | PTD-PC | Iodized polymer | Iodine impregnation | [55] |
| XINSORB | Shandong Hua’an Biotechnology | PLLA | Platinum | Marker fixation | [56] |
| Igaki-Tamai | Kyoto Medical | PLLA | Gold | Marker fixation | [57] |
| DESolve | Elixir Medical | PLLA | Platinum | Marker fixation | [58] |
| NeoVas | Lepu Medical | PLLA | Platinum | Marker fixation | [59] |
| Biomaterial Category | Bioactive Cue | Biological Evaluation | Key Findings | Ref. |
|---|---|---|---|---|
| PCL | Heparin covalent grafting | In vitro and implantation | Platelet adhesion↓ SMCs proliferation↓ ECs proliferation↑ | Shen et al. [71] |
| Plasma polymeric allylamine (PPAm) | Cu-DOTA and HA coating | In vitro and implantation | Thrombus formation↓ SMCs migration↓ ECs adhesion↑ | Lyu et al. [72] |
| PLLA | hCOLIII and HA coating | In vitro and implantation | SMCs proliferation↓ ECs proliferation↑ Inflammation↓ | Yang et al. [73] |
| PLLA | Rapamycin-VEGF Hierarchical coating | In vitro and implantation | ECs proliferation↑ SMCs proliferation↑ | Wang et al. [74] |
| PPAm | TA and BVLD coating | In vitro and implantation | Inflammation↓ Thrombus formation↓ | Qiu et al. [75] |
| PLLA | CS/EGCG-Cu/Hep coating | In vitro and implantation | Thrombus formation↓ IL-1, IL-6, and TNF-α expression↓ | Zhang et al. [76] |
| PLLA | PCBDA coating | In vitro and implantation | Platelet adhesion↓ Fibrinogen adhesion↓ Inflammation↓ | Yang et al. [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. https://doi.org/10.3390/biom12091245
Shen Y, Yu X, Cui J, Yu F, Liu M, Chen Y, Wu J, Sun B, Mo X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules. 2022; 12(9):1245. https://doi.org/10.3390/biom12091245
Chicago/Turabian StyleShen, Yihong, Xiao Yu, Jie Cui, Fan Yu, Mingyue Liu, Yujie Chen, Jinglei Wu, Binbin Sun, and Xiumei Mo. 2022. "Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases" Biomolecules 12, no. 9: 1245. https://doi.org/10.3390/biom12091245
APA StyleShen, Y., Yu, X., Cui, J., Yu, F., Liu, M., Chen, Y., Wu, J., Sun, B., & Mo, X. (2022). Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules, 12(9), 1245. https://doi.org/10.3390/biom12091245








