The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies
Abstract
:1. Introduction
2. HPA Axis, Role of GABA, Actions of Allopregnanolone
Effect of Acute Stress on Allopregnanolone Levels in Female Animals
3. HPA Axis Dysregulation in Neuropsychiatric Diseases
Effect of Acute Stress on Allopregnanolone Levels in Females under Chronic Stress
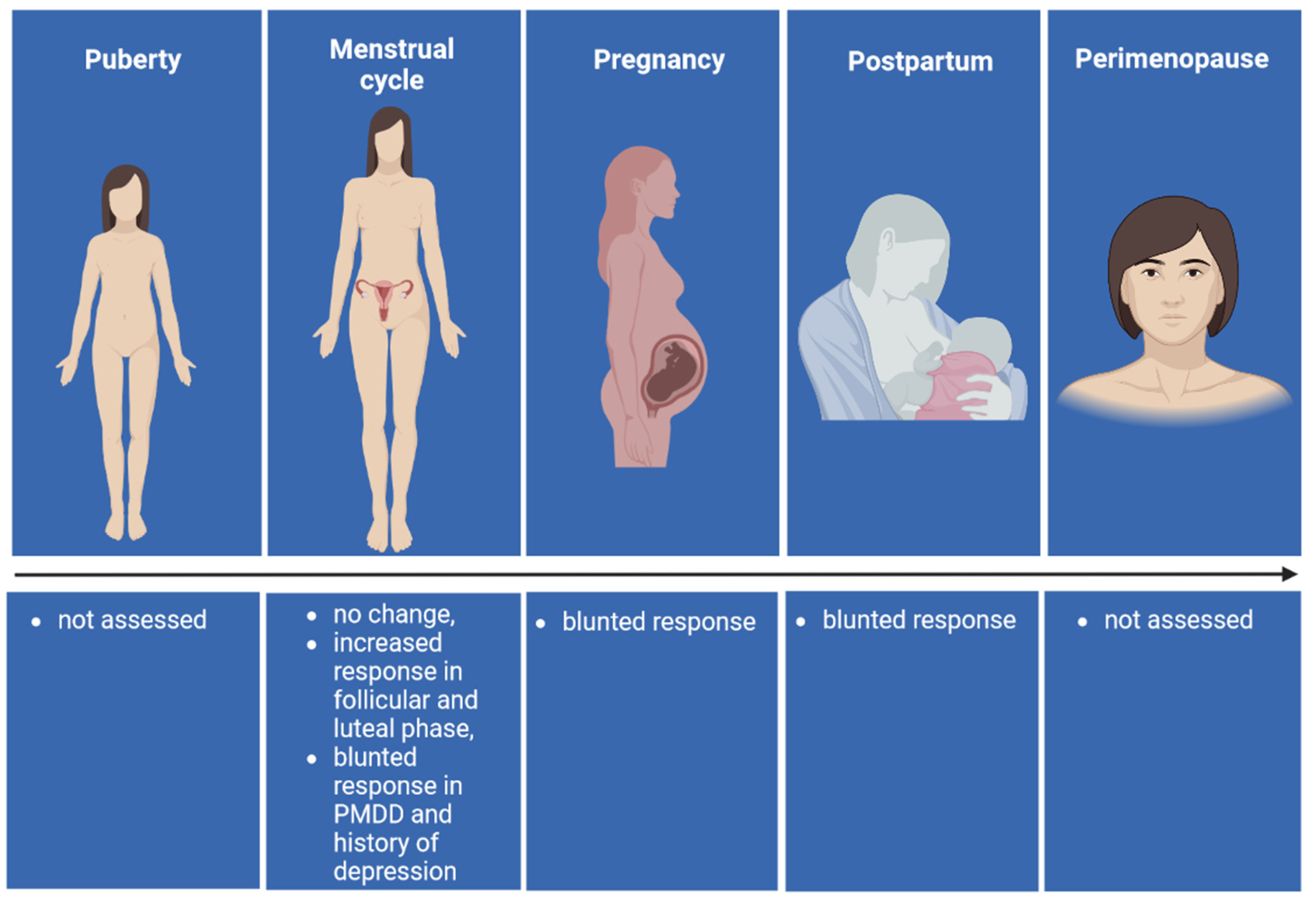
4. Allopregnanolone Levels Following Acute Stress in Women
4.1. Puberty
4.2. Menstrual Cycle
4.3. Pregnancy
4.4. Postpartum
4.5. Perimenopause
5. Hormonal Contraceptives and Allopregnanolone
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hantsoo, L.; Epperson, C.N. Anxiety disorders among women: A female lifespan approach. Focus (Am. Psychiatr. Publ.) 2017, 15, 162–172. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology 2022, 137, 105641. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Malcher-Lopes, R.; Cs Halmos, K.; Tasker, J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J. Neurosci. 2003, 23, 4850–4857. [Google Scholar] [CrossRef] [PubMed]
- Tasker, J.G.; Herman, J.P. Stress mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011, 14, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef]
- Kovács, K.J.; Miklós, I.H.; Bali, B. GABAergic mechanisms constraining the activity of the hypothalamo-pituitary-adrenocortical axis. Ann. N. Y. Acad. Sci. 2004, 1018, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Tasker, J.G.; Ziegler, D.R.; Cullinan, W.E. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002, 71, 457–468. [Google Scholar] [CrossRef]
- Cullinan, W.E.; Ziegler, D.R.; Herman, J.P. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef]
- Barbaccia, M.L.; Roscetti, G.; Trabucchi, M.; Mostallino, M.C.; Concas, A.; Purdy, R.H.; Biggio, G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology 1996, 63, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Barbaccia, M.L.; Roscetti, G.; Trabucchi, M.; Purdy, R.H.; Mostallino, M.C.; Concas, A.; Biggio, G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997, 120, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H.; Paul, S.M. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557. [Google Scholar] [CrossRef] [PubMed]
- Droogleever Fortuyn, H.A.; van Broekhoven, F.; Span, P.N.; Bäckström, T.; Zitman, F.G.; Verkes, R.J. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptor density. Psychoneuroendocrinology 2004, 29, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Patchev, V.K.; Shoaib, M.; Holsboer, F.; Almeida, O.F.X. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef]
- Patchev, V.K.; Hassan, A.H.S.; Holsboer, F.; Almeida, F.X. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 1996, 15, 533–540. [Google Scholar] [CrossRef]
- Biggio, G.; Concas, A.; Follesa, P.; Sanna, E.; Serra, M. Stress, ethanol, and neuroactive steroids. Pharmacol. Ther. 2007, 116, 140–171. [Google Scholar] [CrossRef]
- Crowley, S.K.; Girdler, S.S. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: What is the current state of knowledge in humans? Psychopharmacology 2014, 231, 3619–3634. [Google Scholar] [CrossRef]
- Porcu, P.; Morrow, A.L. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: Relevance for human studies. Psychopharmacology 2014, 231, 3257–3272. [Google Scholar] [CrossRef]
- Pisu, M.G.; Garau, A.; Boero, G.; Biggio, F.; Pibiri, V.; Dore, R.; Locci, V.; Paci, E.; Porcu, P.; Serra, M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience 2016, 320, 172–182. [Google Scholar] [CrossRef]
- Porcu, P.; Lallai, V.; Locci, A.; Catzeddu, S.; Serra, V.; Pisu, M.G.; Serra, M.; Dazzi, L.; Concas, A. Changes in stress-stimulated allopregnanolone levels induced by neonatal estradiol treatment are associated with enhanced dopamine release in adult female rats: Reversal by progesterone administration. Psychopharmacology 2017, 234, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Serra, M.; Concas, A. The brain as a target of hormonal contraceptives: Evidence from animal studies. Front. Neuroendocrinol. 2019, 55, 100799. [Google Scholar] [CrossRef] [PubMed]
- Sze, Y.; Gill, A.C.; Brunton, P.J. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J. Neuroendocrinol. 2018, 30, e12644. [Google Scholar] [CrossRef] [PubMed]
- Sze, Y.; Brunton, P.J. Effects of prenatal stress on neuroactive steroid responses to acute stress in adult male and female rats. J. Neuroendocrinol. 2021, 33, e12916. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.M.; Ruiz, E.; Ortega, E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochem. Res. 2001, 26, 555–558. [Google Scholar] [CrossRef]
- Cozzoli, D.K.; Tanchuck-Nipper, M.A.; Kaufman, M.N.; Horowitz, C.B.; Finn, D.A. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 2014, 48, 741–754. [Google Scholar] [CrossRef]
- Morrow, A.L.; Boero, G.; Porcu, P. A rationale for allopregnanolone treatment of alcohol use disorders: Basic and clinical studies. Alcohol. Clin. Exp. Res. 2020, 44, 320–339. [Google Scholar] [CrossRef]
- Misztal, T.; Młotkowska, P.; Marciniak, E.; Misztal, A. Allopregnanolone reduces neuroendocrine response to acute stressful stimuli in sheep. J. Endocrinol. 2020, 244, 201–211. [Google Scholar] [CrossRef]
- Bäckström, T.; Das, R.; Bixo, M. Positive GABAA receptor modulating steroids and their antagonists: Implications for clinical treatments. J. Neuroendocrinol. 2022, 34, e13013. [Google Scholar] [CrossRef]
- Boero, G.; Porcu, P.; Morrow, A.L. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol. Stress 2020, 12, 100203. [Google Scholar] [CrossRef]
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol. Stress 2020, 12, 100215. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Lightman, S.L.; Pariante, C.M. The interface of stress and the HPA axis in behavioural phenotypes of mental illness. In Behavioral Neurobiology of Stress-Related Disorders; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2014; Volume 18, pp. 13–24. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Borges Almeida, F.; Pinna, G.; Tannhauser Barros, H.M. The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Wolf, J.M.; Wolf, O.T. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2010, 35, 104–114. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- de Kloet, E.R. From receptor balance to rational glucocorticoid therapy. Endocrinology 2014, 155, 2754–2769. [Google Scholar] [CrossRef]
- Girdler, S.S.; Lindgren, M.; Porcu, P.; Rubinow, D.R.; Johnson, J.L.; Morrow, A.L. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 2012, 37, 543–553. [Google Scholar] [CrossRef]
- Pisu, M.G.; Serra, M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci. 2004, 74, 3181–3197. [Google Scholar] [CrossRef]
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatry 2006, 60, 704–713. [Google Scholar] [CrossRef]
- Romeo, E.; Brancati, A.; de Lorenzo, A.; Fucci, P.; Furnari, C.; Pompili, E.; Sasso, G.F.; Spalletta, G.; Troisi, A.; Pasini, A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin. Neuropharmacol. 1996, 19, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Schüle, C.; Nothdurfter, C.; Rupprecht, R. The role of allopregnanolone in depression and anxiety. Prog. Neurobiol. 2014, 113, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. USA 1998, 95, 3239–3244. [Google Scholar] [CrossRef]
- Wang, M.; Seippel, L.; Purdy, R.H.; Bäckström, T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnan-20-one. J. Clin. Endocrinol. Metab. 1996, 81, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Bixo, M.; Johansson, M.; Timby, E.; Michalski, L.; Bäckström, T. Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J. Neuroendocrinol. 2018, 30, e12553. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, A.M.; Marx, C.E.; Pineles, S.L.; Locci, A.; Scioli-Salter, E.R.; Nillni, Y.I.; Liang, J.J.; Pinna, G. Neuroactive steroids and PTSD treatment. Neurosci. Lett. 2017, 649, 156–163. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 KDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef]
- Hojo, Y.; Kawato, S. Neurosteroids in adult hippocampus of male and female rodents: Biosynthesis and actions of sex steroids. Front. Endocrinol. 2018, 9, 183. [Google Scholar] [CrossRef]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J. Neurochem. 2000, 75, 732–740. [Google Scholar] [CrossRef]
- Fone, K.C.F.; Porkess, M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008, 32, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Hawkley, L.C. Social isolation and health, with an emphasis on underlying mechanisms. Perspect. Biol. Med. 2003, 46, S39–S52. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Locci, A.; Pinna, G. Social isolation as a promising animal model of PTSD comorbid suicide: Neurosteroids and cannabinoids as possible treatment options. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2019, 92, 243–259. [Google Scholar] [CrossRef]
- Goel, N.; Bale, T.L. Examining the intersection of sex and stress in modeling neuropsychiatric disorders. J. Neuroendocrinol. 2009, 21, 415. [Google Scholar] [CrossRef] [PubMed]
- Barbaccia, M.L.; Concas, A.; Serra, M.; Biggio, G. Stress and neurosteroids in adult and aged rats. Exp. Gerontol. 1998, 33, 697–712. [Google Scholar] [CrossRef]
- Sundström Poromaa, I.; Smith, S.; Gulinello, M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch. Womens Ment. Health 2003, 6, 23–41. [Google Scholar] [CrossRef]
- Heim, C.; Newport, D.J.; Bonsall, R.; Miller, A.H.; Nemeroff, C.B. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 2001, 158, 575–581. [Google Scholar] [CrossRef]
- Klatzkin, R.R.; Morrow, A.L.; Light, K.C.; Pedersen, C.A.; Girdler, S.S. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol. Psychol. 2006, 71, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J.; Pletzer, B.; Kuhn, C. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Girdler, S.S.; Meltzer-Brody, S.E.; Stika, C.S.; Thurston, R.C.; Clark, C.T.; Prairie, B.A.; Moses-Kolko, E.; Joffe, H.; Wisner, K.L. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. Am. J. Psychiatry 2015, 172, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gilfarb, R.A.; Leuner, B. GABA system modifications during periods of hormonal flux across the female lifespan. Front. Behav. Neurosci. 2022, 16, 802530. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Serra, M.; Porcu, P. How hormonal contraceptives shape brain and behavior: A review of preclinical studies. Front. Neuroendocrinol. 2022, 66, 101017. [Google Scholar] [CrossRef]
- Romeo, R.D.; Lee, S.J.; McEwen, B.S. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 2004, 80, 387–393. [Google Scholar] [CrossRef]
- Grobin, C.A.; Morrow, A.L. 3Alpha-hydroxy-5alpha-pregnan-20-one levels and GABA(A) receptor-mediated 36Cl(-) flux across development in rat cerebral cortex. Brain Res. Dev. Brain Res. 2001, 131, 31–39. [Google Scholar] [CrossRef]
- Fadalti, M.; Petraglia, F.; Luisi, S.; Bernardi, F.; Casarosa, E.; Ferrari, E.; Luisi, M.; Saggese, G.; Genazzani, A.R.; Bernasconi, S. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr. Res. 1999, 46, 323–327. [Google Scholar] [CrossRef]
- Shen, H.; Gong, Q.H.; Aoki, C.; Yuan, M.; Ruderman, Y.; Dattilo, M.; Williams, K.; Smith, S.S. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 2007, 10, 469–477. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Petraglia, F.; Bernardi, F.; Casarosa, E.; Salvestroni, C.; Tonetti, A.; Nappi, R.E.; Luisi, S.; Palumbo, M.; Purdy, R.H.; et al. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. J. Clin. Endocrinol. Metab. 1998, 83, 2099–2103. [Google Scholar] [CrossRef]
- Corpéchot, C.; Collins, B.E.; Carey, M.P.; Tsouros, A.; Robel, P.; Fry, J.P. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997, 766, 276–280. [Google Scholar] [CrossRef]
- Locci, A.; Porcu, P.; Talani, G.; Santoru, F.; Berretti, R.; Giunti, E.; Licheri, V.; Sanna, E.; Concas, A. Neonatal estradiol exposure to female rats changes GABAA receptor expression and function, and spatial learning during adulthood. Horm. Behav. 2017, 87, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Bixo, M.; Johansson, M.; Nyberg, S.; Ossewaarde, L.; Ragagnin, G.; Savic, I.; Strömberg, J.; Timby, E.; van Broekhoven, F.; et al. Allopregnanolone and mood disorders. Prog. Neurobiol. 2014, 113, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Marinari, K.T.; Leshner, A.I.; Doyle, M.P. Menstrual cycle status and adrenocortical reactivity to psychological stress. Psychoneuroendocrinology 1976, 1, 213–218. [Google Scholar] [CrossRef]
- Tersman, Z.; Collins, A.; Eneroth, P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom. Med. 1991, 53, 185–197. [Google Scholar] [CrossRef]
- Ossewaarde, L.; Hermans, E.J.; van Wingen, G.A.; Kooijman, S.C.; Johansson, I.M.; Bäckström, T.; Fernández, G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 2010, 35, 47–55. [Google Scholar] [CrossRef]
- Meczekalski, B.; Tonetti, A.; Monteleone, P.; Bernardi, F.; Luisi, S.; Stomati, M.; Luisi, M.; Petraglia, F.; Genazzani, A.R. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. Eur. J. Endocrinol. 2000, 142, 280–285. [Google Scholar] [CrossRef]
- Lombardi, I.; Luisi, S.; Quirici, B.; Monteleone, P.; Bernardi, F.; Liut, M.; Casarosa, E.; Palumbo, M.; Petraglia, F.; Genazzani, A.R. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol. Endocrinol. 2004, 18, 79–87. [Google Scholar] [CrossRef]
- Altemus, M.; Redwine, L.S.; Leong, Y.M.; Frye, C.A.; Porges, S.W.; Carter, C.S. Responses to laboratory psychosocial stress in postpartum women. Psychosom. Med. 2001, 63, 814–821. [Google Scholar] [CrossRef]
- Childs, E.; Dlugos, A.; de Wit, H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology 2010, 47, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Girdler, S.S.; Mechlin, M.B.; Light, K.C.; Morrow, A.L. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology 2006, 43, 331–336. [Google Scholar] [CrossRef]
- Girdler, S.S.; Straneva, P.A.; Light, K.C.; Pedersen, C.A.; Morrow, A.L. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol. Psychiatry 2001, 49, 788–797. [Google Scholar] [CrossRef]
- Concas, A.; Mostallino, M.C.; Porcu, P.; Follesa, P.; Barbaccia, M.L.; Trabucchi, M.; Purdy, R.H.; Grisenti, P.; Biggio, G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. USA 1998, 95, 13284–13289. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Petraglia, F.; Benedetto, C.; Nappi, R.E.; Bernardi, F.; Fadalti, M.; Reis, F.M.; Luisi, M.; Genazzani, A.R. Serum allopregnanolone levels in pregnant women: Changes during pregnancy, at delivery, and in hypertensive patients. J. Clin. Endocrinol. Metab. 2000, 85, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Johnstone, H.A.; Hatzinger, M.; Liebsch, G.; Shipston, M.; Russell, J.A.; Landgraf, R.; Douglas, A.J. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J. Physiol. 1998, 508, 289–300. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress 2011, 14, 6–12. [Google Scholar] [CrossRef]
- Crowley, S.K.; O’Buckley, T.K.; Schiller, C.E.; Stuebe, A.; Morrow, A.L.; Girdler, S.S. Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: A pilot study. Psychopharmacology 2016, 233, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Duthie, L.; Reynolds, R.M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 2013, 98, 106–115. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: The neurosteroid opioid connection. J. Physiol. 2008, 586, 369–375. [Google Scholar] [CrossRef]
- Brunton, P.J.; McKay, A.J.; Ochȩdalski, T.; Piastowska, A.; Rȩbas, E.; Lachowicz, A.; Russell, J.A. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J. Neurosci. 2009, 29, 6449–6460. [Google Scholar] [CrossRef]
- Concas, A.; Follesa, P.; Barbaccia, M.L.; Purdy, R.H.; Biggio, G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur. J. Pharmacol. 1999, 375, 225–235. [Google Scholar] [CrossRef]
- Maguire, J.; Mody, I. GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Kanes, S.J. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol. Stress 2020, 12, 100212. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- Boero, G.; Biggio, F.; Pisu, M.G.; Locci, V.; Porcu, P.; Serra, M. Combined effect of gestational stress and postpartum stress on maternal care in rats. Physiol. Behav. 2018, 184, 172–178. [Google Scholar] [CrossRef]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Kimball, A.; Dichtel, L.E.; Nyer, M.B.; Mischoulon, D.; Fisher, L.B.; Cusin, C.; Dording, C.M.; Trinh, N.H.; Yeung, A.; Haines, M.S.; et al. The allopregnanolone to progesterone ratio across the menstrual cycle and in menopause. Psychoneuroendocrinology 2020, 112, 104512. [Google Scholar] [CrossRef]
- United Nations. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). Department of Economic and Social Affairs, Population Division. 2019. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf (accessed on 31 August 2022).
- Levin, E.R.; Vitek, W.S.; Hammes, S.R. Estrogens, progestins, and the female reproductive tract. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw Hill: New York, NY, USA, 2018; pp. 803–831. [Google Scholar]
- Kulkarni, J. Depression as a side effect of the contraceptive pill. Expert Opin. Drug Saf. 2007, 6, 371–374. [Google Scholar] [CrossRef]
- Kurshan, N.; Epperson, C.N. Oral contraceptives and mood in women with and without premenstrual dysphoria: A theoretical model. Arch. Womens Ment. Health 2006, 9, 1–14. [Google Scholar] [CrossRef]
- Böttcher, B.; Radenbach, K.; Wildt, L.; Hinney, B. Hormonal contraception and depression: A survey of the present state of knowledge. Arch. Gynecol. Obstet. 2012, 286, 231–236. [Google Scholar] [CrossRef]
- Cheslack-Postava, K.; Keyes, K.M.; Lowe, S.R.; Koenen, K.C. Oral contraceptive use and psychiatric disorders in a nationally representative sample of women. Arch. Womens Ment. Health 2015, 1, 103–111. [Google Scholar] [CrossRef]
- Hall, K.S.; White, K.O.C.; Rickert, V.I.; Reame, N.; Westhoff, C. Influence of depressed mood and psychological stress symptoms on perceived oral contraceptive side effects and discontinuation in young minority women. Contraception 2012, 86, 518–525. [Google Scholar] [CrossRef]
- Lewis, C.A.; Kimmig, A.-C.S.; Zsido, R.G.; Jank, A.; Derntl, B.; Sacher, J. Effects of hormonal contraceptives on mood: A focus on emotion recognition and reactivity, reward processing, and stress response. Curr. Psychiatry Rep. 2019, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Lundin, C.; Danielsson, K.G.; Bixo, M.; Moby, L.; Bengtsdotter, H.; Jawad, I.; Marions, L.; Brynhildsen, J.; Malmborg, A.; Lindh, I.; et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle—A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology 2017, 76, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, A.J.; Morgan, M.; Sogliano, C.; Biggio, G.; Concas, A. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertil. Steril. 2006, 85, 1371–1378. [Google Scholar] [CrossRef]
- Schaffir, J.; Worly, B.L.; Gur, T.L. Combined hormonal contraception and its effects on mood: A critical review. Eur. J. Contracept. Reprod. Health Care 2016, 21, 347–355. [Google Scholar] [CrossRef]
- Skovlund, C.W.; Mørch, L.S.; Kessing, V.L.; Lidegaard, Ø. Association of hormonal contraception with depression. JAMA Psychiatry 2016, 73, 1154–1162. [Google Scholar] [CrossRef]
- Sundström Poromaa, I.; Segebladh, B. Adverse mood symptoms with oral contraceptives. Acta Obstet. Gynecol. Scand. 2012, 91, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Toffol, E.; Heikinheimo, O.; Koponen, P.; Luoto, R.; Partonen, T. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception 2012, 86, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Anderl, C.; Li, G.; Chen, F.S. Oral contraceptive use in adolescence predicts lasting vulnerability to depression in adulthood. J. Child Psychol. Psychiatry 2020, 61, 148–156. [Google Scholar] [CrossRef]
- Anderl, C.; de Wit, A.E.; Giltay, E.J.; Oldehinkel, A.J.; Chen, F.S. Association between adolescent oral contraceptive use and future major depressive disorder: A prospective cohort study. J. Child Psychol. Psychiatry 2022, 63, 333–341. [Google Scholar] [CrossRef]
- de Wit, A.E.; Booij, S.H.; Giltay, E.J.; Joffe, H.; Schoevers, R.A.; Oldehinkel, A.J. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry 2020, 77, 52–59. [Google Scholar] [CrossRef]
- Skovlund, C.W.; Mørch, L.S.; Kessing, L.V.; Lange, T.; Lidegaard, Ø. Association of hormonal contraception with suicide attempts and suicides. Am. J. Psychiatry 2018, 175, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Porcu, P.; Sogliano, C.; Cinus, M.; Biggio, F.; Mancuso, L.; Mostallino, M.C.; Paoletti, A.M.; Purdy, R.H.; Biggio, G.; et al. Changes in GABAA receptor gamma2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology 2002, 42, 325–336. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Lello, S.; Fratta, S.; Orrù, M.; Ranuzzi, F.; Sogliano, C.; Concas, A.; Biggio, G.; Melis, G.B. Psychological effect of the oral contraceptive formulation containing 3 mg of prospirenone plus 30 microg of ethinyl estradiol. Fertil. Steril. 2004, 81, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Mostallino, M.C.; Sogliano, C.; Santoru, F.; Berretti, R.; Concas, A. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharmacol. Biochem. Behav. 2012, 102, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Santoru, F.; Berretti, R.; Locci, A.; Porcu, P.; Concas, A. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology 2014, 231, 3351–3364. [Google Scholar] [CrossRef]
- Sassoè-Pognetto, M.; Follesa, P.; Panzanelli, P.; Perazzini, A.Z.; Porcu, P.; Sogliano, C.; Cherchi, C.; Concas, A. Fluctuations in brain concentrations of neurosteroids are not associated to changes in gephyrin levels. Brain Res. 2007, 1169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Kudielka, B.M.; Gaab, J.; Schommer, N.C.; Hellhammer, D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Hii, H.L.; Pennell, C.E.; McKeague, I.W.; de Kloet, E.R.; Lye, S.; Stanley, F.J.; Mattes, E.; Foster, J.K. Analysis of baseline hypothalamic-pituitary-adrenal activity in late adolescence reveals gender specific sensitivity of the stress axis. Psychoneuroendocrinology 2013, 38, 1271–1280. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology 1995, 20, 509–514. [Google Scholar] [CrossRef]
- Roche, D.J.O.; King, A.C.; Cohoon, A.J.; Lovallo, W.R. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol. Biochem. Behav. 2013, 109, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Smith, S.A.; Boukina, N.; Dordari, A.; Mistry, A.; Taylor, B.C.; Felix, N.; Cameron, A.; Fang, Z.; Smith, A.; et al. Use of the birth control pill affects stress reactivity and brain structure and function. Horm. Behav. 2020, 124, 104783. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.J.; Odom, M.J.; Word, R.A.; Carr, B.R. Effect of oral contraceptives on adrenocorticotropin and growth hormone secretion following CRH and GHRH administration. Contraception 1989, 40, 691–699. [Google Scholar] [CrossRef]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O’Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Viau, V.; Meaney, M.J. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 1991, 129, 2503–2511. [Google Scholar] [CrossRef]
- van Leeuwen, N.; Bellingrath, S.; de Kloet, E.R.; Zitman, F.G.; Derijk, R.H.; Kudielka, B.M.; Wüst, S. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: Implication for the stress response. Psychoneuroendocrinology 2011, 36, 699–709. [Google Scholar] [CrossRef]
- Hamstra, D.A.; de Kloet, E.R.; van Hemert, A.M.; de Rijk, R.H.; van der Does, A.J.W. Mineralocorticoid receptor haplotype, oral contraceptives and emotional information processing. Neuroscience 2015, 286, 412–422. [Google Scholar] [CrossRef]
- Hamstra, D.A.; de Kloet, E.R.; Quataert, I.; Jansen, M.; van der Does, W. Mineralocorticoid receptor haplotype, estradiol, progesterone and emotional information processing. Psychoneuroendocrinology 2017, 76, 162–173. [Google Scholar] [CrossRef]
- Boero, G.; Tyler, R.E.; Todd, C.A.; O’Buckley, T.K.; Balan, I.; Besheer, J.; Morrow, A.L. (3α,5α)3-Hydroxypregnan-20-one (3α,5α-THP) regulation of hypothalamic and extrahypothalamic corticotropin releasing factor (CRF): Sexual dimorphism and brain region specificity in Sprague Dawley rats. Neuropharmacology 2021, 186, 108463. [Google Scholar] [CrossRef]
- Boero, G.; Tyler, R.E.; O’Buckley, T.K.; Balan, I.; Besheer, J.; Morrow, A.L. (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) regulation of the HPA axis in the context of different stressors and sex. Biomolecules 2022, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
| Experimental Group | Foot Shock Stress | Restraint Stress |
|---|---|---|
| Allopregnanolone | ||
| Group-housed | 14.3 ± 1.2 | 15.7 ± 2.0 |
| Group-housed + acute stress | 14.9 ± 1.8 | 25.8 ± 3.4 a |
| Socially isolated | 9.8 ± 0.8 a | 10.2 ± 1.3 b |
| Socially isolated + acute stress | 10.0 ± 1.1 | 25.7± 3.6 c |
| Corticosterone | ||
| Group-housed | 132.1 ± 12.0 | 151.2 ± 9.9 |
| Group-housed + acute stress | 190.2 ± 17.3 b | 1343.9 ± 140.3 a |
| Socially isolated | 70.1 ± 8.8 b | 72.0 ± 11.2 b |
| Socially isolated + acute stress | 324.6 ± 42.0 de | 1770.1 ± 261.1 de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisu, M.G.; Concas, L.; Siddi, C.; Serra, M.; Porcu, P. The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies. Biomolecules 2022, 12, 1262. https://doi.org/10.3390/biom12091262
Pisu MG, Concas L, Siddi C, Serra M, Porcu P. The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies. Biomolecules. 2022; 12(9):1262. https://doi.org/10.3390/biom12091262
Chicago/Turabian StylePisu, Maria Giuseppina, Luca Concas, Carlotta Siddi, Mariangela Serra, and Patrizia Porcu. 2022. "The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies" Biomolecules 12, no. 9: 1262. https://doi.org/10.3390/biom12091262
APA StylePisu, M. G., Concas, L., Siddi, C., Serra, M., & Porcu, P. (2022). The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies. Biomolecules, 12(9), 1262. https://doi.org/10.3390/biom12091262







