Circulating Fibroblast Growth Factor-23 Levels Can Predict Rapid Kidney Function Decline in a Healthy Population: A Community-Based Study
Abstract
:1. Background
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Information and Data Collection
2.3. Definitions of Covariates
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
3.2. Analysis of Factors Associated with the Possibility of RKFD
3.3. FGF-23 Could Predict RKFD in the Healthy Population
3.4. Subgroup and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, K.U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Kottgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.K.; Li, P.K. Chronic kidney disease epidemic: How do we deal with it? Nephrology 2018, 23 (Suppl. 4), 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwasongwe, S.; Min, Y.I.; Booth, J.N., 3rd; Katz, R.; Sims, M.; Correa, A.; Young, B.; Muntner, P. Masked hypertension and kidney function decline: The jackson heart study. J. Hypertens 2018, 36, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Wang, P.-H.; Lu, K.-Y.; Ko, C.-C.; She, Y.-H.; Lee, C.-C.; Wu, I.-W.; Sun, C.-Y.; Hsu, H.-J.; Pan, H.-C. Relationships between metabolic body composition status and rapid kidney function decline in a community-based population: A prospective observational study. Front. Public Health 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, D.E.; Shlipak, M.G.; Katz, R.; Fried, L.F.; Siscovick, D.; Chonchol, M.; Newman, A.B.; Sarnak, M.J. Rapid kidney function decline and mortality risk in older adults. Arch. Intern. Med. 2008, 168, 2212–2218. [Google Scholar] [CrossRef] [Green Version]
- Young, B.A.; Katz, R.; Boulware, L.E.; Kestenbaum, B.; de Boer, I.H.; Wang, W.; Fülöp, T.; Bansal, N.; Robinson-Cohen, C.; Griswold, M.; et al. Risk factors for rapid kidney function decline among african americans: The jackson heart study (jhs). Am. J. Kidney Dis. 2016, 68, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Meng, Q.; Yang, C.; Wang, Y.; Kong, G.; Zhao, Y.; Wang, F.; Zhang, L. Association between pulse pressure, systolic blood pressure and the risk of rapid decline of kidney function among general population without hypertension: Results from the china health and retirement longitudinal study (charls). J. Transl. Med. 2021, 19, 512. [Google Scholar] [CrossRef]
- Wahba, I.M.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Shlipak, M.G.; Wang, X.; Coresh, J.; Greene, T.; Stevens, L.; Kusek, J.W.; Beck, G.J.; Collins, A.J.; Levey, A.S. Cystatin c as a risk factor for outcomes in chronic kidney disease. Ann. Intern. Med. 2007, 147, 19–27. [Google Scholar] [CrossRef]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of c-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lerman, L.O. The metabolic syndrome and chronic kidney disease. Transl. Res 2017, 183, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Garcia, J.A.; Fernandez-Velasco, M.; Delgado, C.; Delgado, J.F.; Kuro, O.M.; Ruilope, L.M.; Ruiz-Hurtado, G. Pth, vitamin d, and the fgf-23-klotho axis and heart: Going beyond the confines of nephrology. Eur. J. Clin. Investig. 2018, 48, e12902. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Battaglia, Y. Clinical significance of fgf-23 in patients with ckd. Int. J. Nephrol. 2011, 2011, 364890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral. Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef] [Green Version]
- White, K.E.; Evans, W.E.; O’Riordan, J.L.; Speer, M.C.; Econs, M.J.; Lorenz-Depiereux, B.; Grabowski, M.; Meitinger, T.; Strom, T.M. Autosomal dominant hypophosphataemic rickets is associated with mutations in fgf23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef]
- .Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of fgf23 demonstrates an essential physiological role of fgf23 in phosphate and vitamin d metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Bergwitz, C.; Juppner, H. Regulation of phosphate homeostasis by pth, vitamin d, and fgf23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (ckd-mbd): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef]
- Blau, J.E.; Collins, M.T. The pth-vitamin d-fgf23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Larsson, T.; Nisbeth, U.; Ljunggren, Ö.; Jüppner, H.; Jonsson, K.B. Circulating concentration of fgf-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003, 64, 2272–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Venrooij, N.A.; Pereira, R.C.; Tintut, Y.; Fishbein, M.C.; Tumber, N.; Demer, L.L.; Salusky, I.B.; Wesseling-Perry, K. Fgf23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol. Dial. Transplant. 2014, 29, 1525–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, M.A.; Eisenga, M.F.; van Ballegooijen, A.J.; Beulens, J.W.J.; Vervloet, M.G.; Navis, G.; Gansevoort, R.T.; Bakker, S.J.L.; De Borst, M.H. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: The prevention of renal and vascular endstage disease (prevend) study. Nephrol. Dial. Transpl. 2021, 36, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M. New creatinine-and cystatin c–based equations to estimate gfr without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: A consensus statement. JAMA Netw Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yang, W.; Parikh, R.V.; Anderson, A.H.; Chen, T.K.; Cohen, D.L.; He, J.; Mohanty, M.J.; Lash, J.P.; Mills, K.T.; et al. Race, genetic ancestry, and estimating kidney function in ckd. N. Engl. J. Med. 2021, 385, 1750–1760. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V. A unifying approach for gfr estimation: Recommendations of the nkf-asn task force on reassessing the inclusion of race in diagnosing kidney disease. Am. J. Kidney Dis. 2021, 79, 268–288.e1. [Google Scholar] [CrossRef]
- Fu, E.L.; Coresh, J.; Grams, M.E.; Clase, C.M.; Elinder, C.G.; Paik, J.; Ramspek, C.; Inker, L.A.; Levey, A.S.; Dekker, F.W.; et al. Removing race from the ckd-epi equation and its impact on prognosis in a predominantly white european population. Nephrol. Dial. Transpl. 2022, gfac197. [Google Scholar] [CrossRef]
- Lee, M.J.; Hsu, H.J.; Wu, I.W.; Sun, C.Y.; Ting, M.K.; Lee, C.C. Vitamin d deficiency in northern taiwan: A community-based cohort study. BMC Public Health 2019, 19, 337. [Google Scholar] [CrossRef]
- Lipsy, R.J. The national cholesterol education program adult treatment panel iii guidelines. J. Manag. Care Pharm. 2003, 9, 2–5. [Google Scholar] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Neves, F.M.; Araujo, C.B.; de Freitas, D.F.; Arruda, B.F.T.; de Macedo Filho, L.J.M.; Salles, V.B.; Meneses, G.C.; Martins, A.M.C.; Liborio, A.B. Fibroblast growth factor 23, endothelium biomarkers and acute kidney injury in critically-ill patients. J. Transl. Med. 2019, 17, 121. [Google Scholar] [CrossRef]
- Pan, H.-C.; Huang, T.-M.; Sun, C.-Y.; Chou, N.-K.; Tsao, C.-H.; Yeh, F.-Y.; Lai, T.-S.; Chen, Y.-M.; Wu, V.-C. Predialysis serum lactate levels could predict dialysis withdrawal in type 1 cardiorenal syndrome patients. EClinicalMedicine 2022, 44, 101232. [Google Scholar] [CrossRef]
- Pan, H.-C.; Huang, T.T.-M.; Huang, C.-T.; Sun, C.-Y.; Chen, Y.-M.; Wu, V.-C. Urinary biomarkers can predict weaning from acute dialysis therapy in critically ill patients. Arch. Pathol. Lab. Med. 2022, 146, 1353–1363. [Google Scholar] [CrossRef]
- Hin, L.Y.; Lau, T.K.; Rogers, M.S.; Chang, A.M. Dichotomization of continuous measurements using generalized additive modelling--application in predicting intrapartum caesarean delivery. Stat. Med. 1999, 18, 1101–1110. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sun, C.Y.; Lee, C.C.; Wu, I.W.; Chen, Y.C.; Lin, Y.H.; Fang, W.C.; Pan, H.C. Ketoanalogue supplements reduce mortality in patients with pre-dialysis advanced diabetic kidney disease: A nationwide population-based study. Clin. Nutr. 2021, 40, 4149–4160. [Google Scholar] [CrossRef]
- Shu, K.H.; Wang, C.H.; Wu, C.H.; Huang, T.M.; Wu, P.C.; Lai, C.H.; Tseng, L.J.; Tsai, P.R.; Connolly, R.; Wu, V.C. Urinary pi-glutathione s-transferase predicts advanced acute kidney injury following cardiovascular surgery. Sci. Rep. 2016, 6, 26335. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.C.; Shiao, C.C.; Chi, N.H.; Wang, C.H.; Chueh, S.J.; Liou, H.H.; Spapen, H.D.; Honore, P.M.; Chu, T.S. Outcome prediction of acute kidney injury biomarkers at initiation of dialysis in critical units. J. Clin. Med. 2018, 7, 202. [Google Scholar] [CrossRef]
- Wang, J.J.; Chi, N.H.; Huang, T.M.; Connolly, R.; Chen, L.W.; Chueh, S.J.; Kan, W.C.; Lai, C.C.; Wu, V.C.; Fang, J.T.; et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care 2018, 22, 108. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inf. Decis Mak 2008, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Jhee, J.H.; Nam, K.H.; An, S.Y.; Cha, M.-U.; Lee, M.; Park, S.; Kim, H.; Yun, H.-R.; Kee, Y.K.; Park, J.T. Severe vitamin d deficiency is a risk factor for renal hyperfiltration. Am. J. Clin. Nutr. 2018, 108, 1342–1351. [Google Scholar] [CrossRef] [Green Version]
- Mac Way, F.; Lessard, M.; Lafage-Proust, M.H. Pathophysiology of chronic kidney disease-mineral and bone disorder. Jt. Bone Spine 2012, 79, 544–549. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nagano, N.; Urakawa, I.; Yamazaki, Y.; Iijima, K.; Fujita, T.; Yamashita, T.; Fukumoto, S.; Shimada, T. Direct evidence for a causative role of fgf23 in the abnormal renal phosphate handling and vitamin d metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010, 78, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, C.A.; Foller, M. Phosphate homeostasis, inflammation and the regulation of fgf-23. Kidney Blood Press Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kendrick, J. Vascular calcification in chronic kidney disease: Role of disordered mineral metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar] [CrossRef] [Green Version]
- Dunkler, D.; Gao, P.; Lee, S.F.; Heinze, G.; Clase, C.M.; Tobe, S.; Teo, K.K.; Gerstein, H.; Mann, J.F.; Oberbauer, R.; et al. Risk prediction for early ckd in type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2015, 10, 1371–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.-C.; Chou, K.-M.; Sun, C.-Y.; Lee, C.-C.; Wu, I.-W.; Chen, Y.-C.; Pan, H.-C. Thermal perception abnormalities can predict diabetic kidney disease in type 2 diabetes mellitus patients. Kidney Blood Press. Res. 2020, 45, 926–938. [Google Scholar] [CrossRef]
- Atkins, R.C. The epidemiology of chronic kidney disease. Kidney Int. Suppl. 2005, 67, S14–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Hora Passos, R.; Ramos, J.G.R.; Gobatto, A.; Mendonça, E.J.B.; Miranda, E.A.; Dutra, F.R.D.; Coelho, M.F.R.; Pedroza, A.C.; Batista, P.B.P.; Dutra, M.M.D. Lactate clearance is associated with mortality in septic patients with acute kidney injury requiring continuous renal replacement therapy: A cohort study. Medicine 2016, 95, e5112. [Google Scholar]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef] [PubMed]

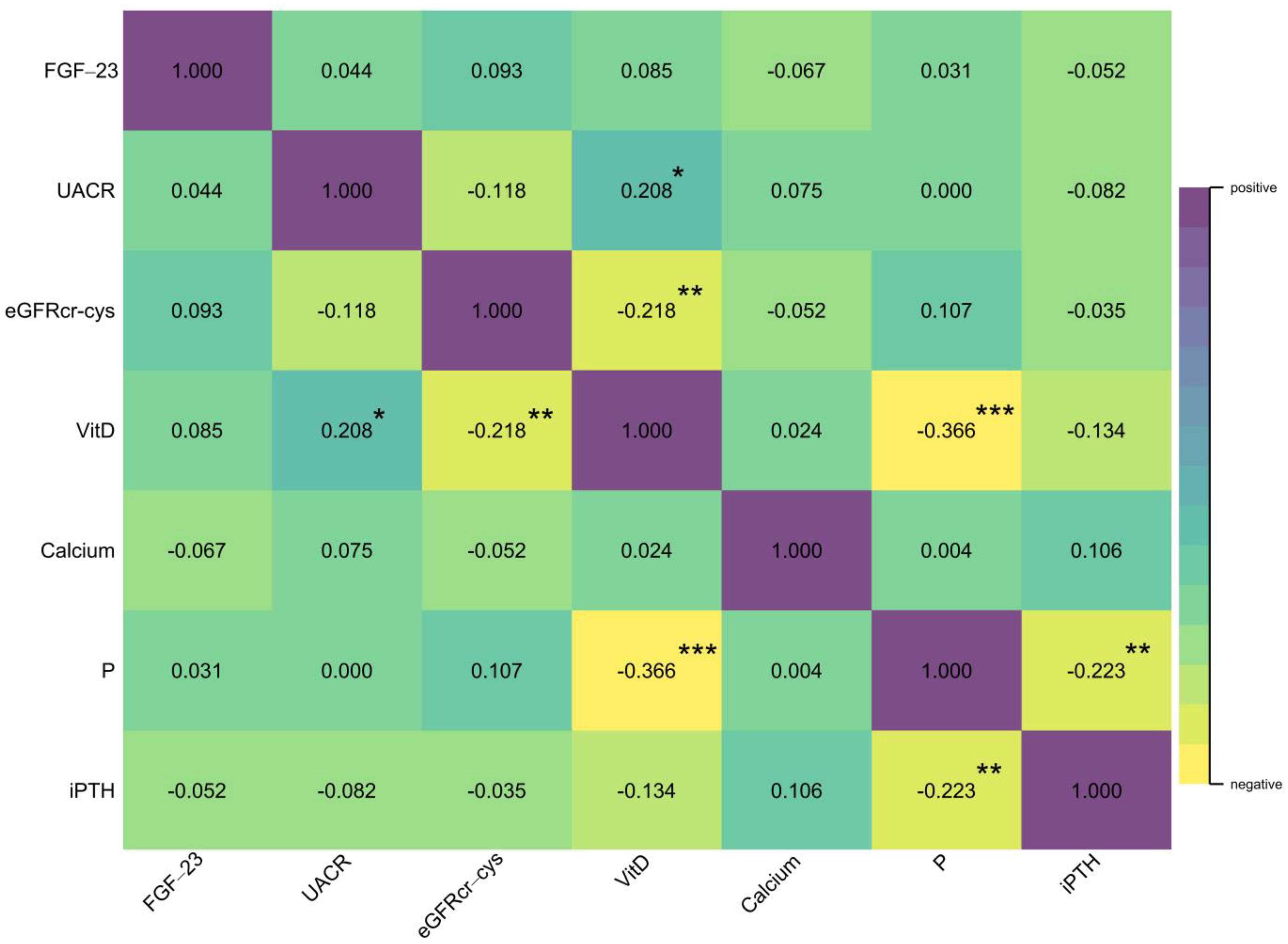

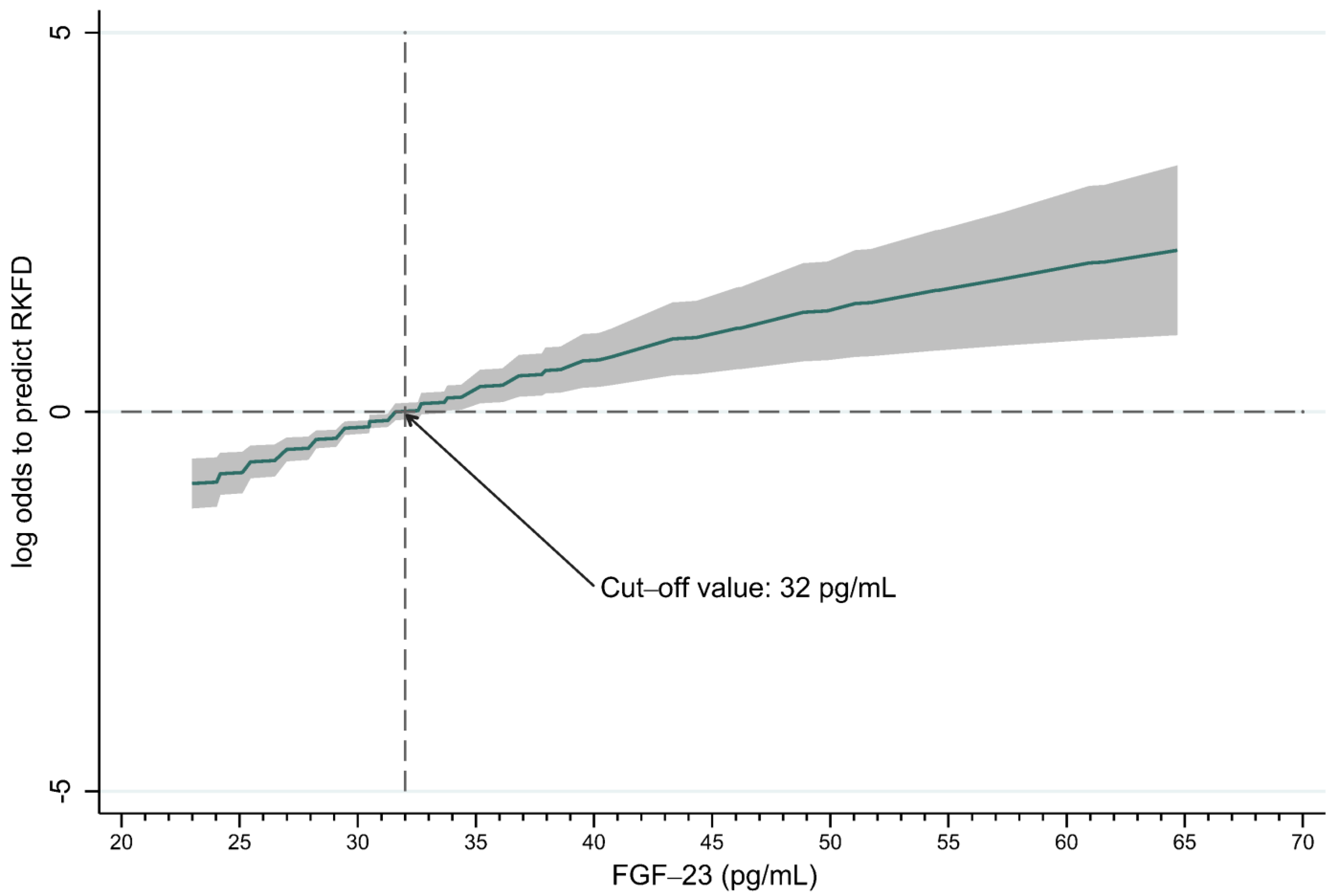

| Total (n = 220) | RKFD (n = 110) | No RKFD (n = 110) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 58.8 (10.8) | 59.3 (10.8) | 58.3 (10.9) | 0.52 |
| Male gender, n | 46 (20.9%) | 22 (20.0%) | 24 (21.8%) | 0.74 |
| Hypertension, n | 56 (25.5%) | 34 (30.9%) | 22 (20.0%) | 0.063 |
| DM, n | 25 (11.4%) | 17 (15.5%) | 8 (7.3%) | 0.056 |
| CKD, n | 6 (2.7%) | 4 (3.6%) | 2 (1.8%) | 0.41 |
| Cardiovascular disease, n | 17 (7.7%) | 10 (9.1%) | 7 (6.4%) | 0.45 |
| CVA, n | 3 (1.4%) | 3 (2.7%) | 0 (0.0%) | 0.081 |
| HBV, n | 28 (12.7%) | 14 (12.7%) | 14 (12.7%) | 1.00 |
| HCV, n | 5 (2.3%) | 2 (1.8%) | 3 (2.7%) | 0.65 |
| Gout, n | 7 (3.2%) | 5 (4.5%) | 2 (1.8%) | 0.25 |
| Autoimmune disease, n | 2 (0.9%) | 2 (1.8%) | 0 (0.0%) | 0.16 |
| Kidney stone | 4 (1.8%) | 1 (0.9%) | 3 (2.7%) | 0.31 |
| Mental disorder | 3 (1.4%) | 2 (1.8%) | 1 (0.9%) | 0.56 |
| Endocrine disorder | 2 (0.9%) | 0 (0.0%) | 2 (1.8%) | 0.16 |
| Metabolic syndrome | 63 (28.6%) | 43 (39.1%) | 20 (18.2%) | <0.001 |
| Biochemical and physiological profiles | ||||
| SBP, mmHg | 129.50 (118.00–141.00) | 129.50 (118.00–143.00) | 129.50 (119.00–137.00) | 0.89 |
| BMI, kg/m2 | 23.95 (22.22–26.47) | 24.23 (22.22–26.25) | 23.72 (22.21–26.67) | 0.71 |
| BMI > 24, n | 108 (49.1%) | 61 (55.5%) | 47 (42.7%) | 0.059 |
| Central obesity, n | 95 (43.2%) | 56 (50.9%) | 39 (35.5%) | 0.021 |
| Hgb, g/dL | 13.70 (12.80–14.60) | 13.70 (12.60–14.60) | 13.60 (13.00–14.60) | 0.93 |
| Total cholesterol, mg/dL | 207.50 (189.50–230.00) | 199.50 (187.00–223.00) | 213.50 (195.00–235.00) | 0.011 |
| LDL cholesterol, mg/dL | 121.40 (105.05–144.70) | 117.80 (100.40–138.10) | 129.90 (109.90–150.60) | 0.016 |
| HDL cholesterol, mg/dL | 56.55 (47.75–66.95) | 54.70 (46.60–64.70) | 57.50 (48.90–71.80) | 0.084 |
| Triglyceride, mg/dL | 92.00 (69.00–132.00) | 96.00 (69.00–155.00) | 90.00 (69.00–127.00) | 0.33 |
| BUN, mg/dL | 12.00 (10.00–15.00) | 12.00 (10.00–16.00) | 12.00 (10.00–15.00) | 0.97 |
| Creatinine, mg/dL | 0.63 (0.55–0.77) | 0.61 (0.54–0.75) | 0.67 (0.55–0.78) | 0.049 |
| Alk-P, mg/dL | 64.5 (55.00–79.00) | 65.0 (54.00–80.00) | 64.0 (56.00–76.00) | 0.82 |
| eGFRcr-cys, mL/min/1.73 m2 | 101.89 (82.68–114.96) | 108.13 (90.75–121.69) | 99.32 (80.85–110.07) | <0.001 |
| Uric acid, mg/dL | 5.10 (4.30–6.10) | 5.10 (4.20–6.10) | 5.10 (4.40–6.10) | 0.93 |
| Albumin, g/dL | 4.69 (0.26) | 4.69 (0.27) | 4.69 (0.25) | 0.84 |
| GPT, U/L | 21.00 (17.00–30.00) | 22.00 (17.00–31.00) | 21.00 (17.00–28.00) | 0.27 |
| UACR, mg/g | 5.90 (3.85–9.50) | 6.50 (4.10–11.20) | 5.30 (3.70–8.00) | 0.020 |
| Fasting glucose, mg/dL | 96.00 (91.50–104.00) | 97.00 (93.00–108.00) | 95.00 (91.00–102.00) | 0.038 |
| HbA1C, % | 5.60 (5.40–5.95) | 5.60 (5.40–6.10) | 5.60 (5.40–5.90) | 0.29 |
| Insulin, μIU/mL | 5.85 (4.21–9.20) | 6.16 (4.68–9.92) | 5.25 (3.89–8.58) | 0.048 |
| HOMA-IR | 1.45 (0.95–2.38) | 1.54 (1.18–2.58) | 1.31 (0.91–2.02) | 0.013 |
| FGF-23, pg/mL | 29.69 (27.10–32.43) | 30.73 (30.42–34.34) | 27.15 (23.63–29.04) | <0.001 |
| Total Vitamin D, ng/mL | 24.54 (19.44–31.70) | 25.30 (20.16–32.81) | 23.98 (19.10–30.77) | 0.31 |
| iPTH, mg/dL | 44.10 (34.20–58.60) | 43.85 (31.60–53.40) | 44.60 (35.60–62.30) | 0.12 |
| P, mg/dL | 3.88 (0.53) | 3.90 (0.48) | 3.87 (0.58) | 0.62 |
| Ca, mg/dL | 9.30 (9.15–9.50) | 9.30 (9.20–9.60) | 9.30 (9.10–9.50) | 0.39 |
| Medication use | ||||
| OHA, n | 24 (11.1%) | 17 (15.9%) | 7 (6.4%) | 0.027 |
| Anti-hypertensives, n | 51 (23.5%) | 31 (29.0%) | 20 (18.2%) | 0.061 |
| Pain killer, n | 30 (14.4%) | 20 (19.6%) | 10 (9.4%) | 0.037 |
| Variables | Beta Coefficient | Standard Error | Odds Ratios (95% CI) | p-Value |
|---|---|---|---|---|
| Univariable analysis | ||||
| Age, per 10 years | 0.08 | 0.13 | 1.08 (0.85, 1.39) | 0.517 |
| Male | −0.11 | 0.33 | 0.90 (0.47, 1.72) | 0.740 |
| Hypertension | 0.58 | 0.32 | 1.79 (0.96, 3.32) | 0.065 |
| DM | 0.85 | 0.45 | 2.33 (0.96, 5.65) | 0.061 |
| CKD | 0.71 | 0.88 | 2.04 (0.37, 11.36) | 0.417 |
| Cardiovascular disease | 0.39 | 0.51 | 1.47 (0.54, 4.02) | 0.451 |
| Gout | 0.94 | 0.85 | 2.57 (0.49, 13.55) | 0.265 |
| Metabolic syndrome | 1.06 | 0.32 | 2.89 (1.56, 5.36) | 0.001 |
| BMI, per 1 kg/m2 | 0.51 | 0.27 | 1.67 (0.98, 2.84) | 0.060 |
| Central obesity | 0.64 | 0.28 | 1.89 (1.10, 3.24) | 0.021 |
| Hgb, per 1 g/dL | −0.02 | 0.10 | 0.98 (0.81, 1.19) | 0.830 |
| Total cholesterol, per 10 mg/dL | −0.09 | 0.04 | 0.92 (0.85, 0.99) | 0.029 |
| LDL cholesterol, per 10 mg/dL | −0.09 | 0.05 | 0.91 (0.83, 1.00) | 0.043 |
| HDL cholesterol, per 10 mg/dL | −0.17 | 0.09 | 0.85 (0.71, 1.02) | 0.074 |
| Triglyceride, per 10 mg/dL | 0.03 | 0.02 | 1.03 (0.99, 1.07) | 0.175 |
| BUN, per 1 mg/dL | −0.00 | 0.03 | 1.00 (0.93, 1.07) | 0.890 |
| Creatinine, per 1 mg/dL | −1.59 | 0.90 | 0.20 (0.04, 1.19) | 0.077 |
| eGFRcr-cys, per 10 mL/min/1.73 m2 | 0.24 | 0.07 | 1.27 (1.11, 1.45) | <0.001 |
| Uric acid, per 1 mg/dL | 0.03 | 0.11 | 1.03 (0.83, 1.28) | 0.804 |
| Albumin, per 1 g/dL | 0.11 | 0.52 | 1.11 (0.40, 3.06) | 0.836 |
| GPT, per 10 U/L | 0.03 | 0.06 | 1.03 (0.92, 1.16) | 0.613 |
| UACR, per 1 mg/g | 0.07 | 0.03 | 1.07 (1.02, 1.14) | 0.012 |
| Fasting glucose, per 10 mg/dL | 0.17 | 0.08 | 1.19 (1.02, 1.39) | 0.025 |
| HbA1C, per 1 % | 0.45 | 0.21 | 1.56 (1.04, 2.35) | 0.030 |
| Insulin, per 10 μIU/mL | 0.48 | 0.27 | 1.62 (0.95, 2.75) | 0.077 |
| HOMA-IR | 0.19 | 0.10 | 1.21 (0.99, 1.46) | 0.056 |
| FGF-23, per 10 pg/mL | 0.80 | 0.22 | 2.23 (1.43, 3.45) | <0.001 |
| Total Vitamin D, per 1 ng/mL | 0.02 | 0.02 | 1.02 (0.98, 1.05) | 0.338 |
| iPTH, per 1 mg/dL | −0.00 | 0.01 | 1.00 (0.99, 1.01) | 0.634 |
| P, per 1 mg/dL | 0.13 | 0.26 | 1.14 (0.69, 1.87) | 0.619 |
| Ca, per 1 mg/dL | 0.29 | 0.39 | 1.34 (0.62, 2.89) | 0.460 |
| OHA use | 1.01 | 0.47 | 2.75 (1.09, 6.94) | 0.032 |
| Anti-hypertensives | 0.61 | 0.33 | 1.84 (0.97, 3.48) | 0.063 |
| Pain killer use | 0.85 | 0.42 | 2.34 (1.04, 5.29) | 0.041 |
| Vegetarian | 0.02 | 0.36 | 1.02 (0.51, 2.06) | 0.950 |
| Multivariable analysis * | ||||
| Central obesity | 1.63 | 0.76 | 5.09 (1.15, 22.60) | 0.032 |
| eGFRcr-cys, per 10 mL/min/1.73 m2 | 0.92 | 0.25 | 2.50 (1.54, 4.05) | <0.001 |
| UACR, per 1 mg/g | 0.15 | 0.06 | 1.16 (1.03, 1.32) | 0.017 |
| FGF-23, per 10 pg/mL | 1.06 | 0.34 | 2.87 (1.47, 5.62) | 0.002 |
| High FGF-23 ≥32 pg/mL (n = 57) | Low FGF-23 <32 pg/mL (n = 163) | p-Value | |
|---|---|---|---|
| RKFD | 40 (70.2%) | 70 (42.9%) | <0.001 |
| Demographics | |||
| Age, years | 55.9 (11.0) | 59.8 (10.6) | 0.020 |
| Male gender, n | 15 (26.3%) | 31 (19.0%) | 0.24 |
| Hypertension, n | 11 (19.3%) | 45 (27.6%) | 0.22 |
| DM, n | 7 (12.3%) | 18 (11.0%) | 0.80 |
| CKD, n | 3 (5.3%) | 3 (1.8%) | 0.17 |
| Cardiovascular disease, n | 6 (10.5%) | 11 (6.7%) | 0.36 |
| CVA, n | 1 (1.8%) | 2 (1.2%) | 0.77 |
| HBV, n | 9 (15.8%) | 19 (11.7%) | 0.42 |
| HCV, n | 0 (0.0%) | 5 (3.1%) | 0.18 |
| Gout, n | 4 (7.0%) | 3 (1.8%) | 0.055 |
| Autoimmune disease, n | 1 (1.8%) | 1 (0.6%) | 0.43 |
| Metabolic syndrome | 14 (24.6%) | 49 (30.1%) | 0.43 |
| SBP, mmHg | 126.00 (116.00–136.00) | 131.00 (119.00–143.00) | 0.041 |
| BMI, kg/m2 | 23.12 (21.75–25.44) | 24.16 (22.28–26.94) | 0.039 |
| BMI > 24, n | 25 (43.9%) | 83 (50.9%) | 0.36 |
| Central obesity, n | 18 (31.6%) | 77 (47.2%) | 0.040 |
| Hgb, g/dL | 13.80 (13.10–14.60) | 13.60 (12.70–14.60) | 0.41 |
| Total cholesterol, mg/dL | 206.00 (187.00–235.00) | 208.00 (191.00–230.00) | 0.50 |
| LDL cholesterol, mg/dL | 117.20 (93.70–144.80) | 123.70 (108.40–144.60) | 0.15 |
| HDL cholesterol, mg/dL | 56.40 (46.70–69.40) | 57.00 (48.60–66.90) | 0.82 |
| Triglyceride, mg/dL | 86.00 (61.00–138.00) | 97.00 (71.00–132.00) | 0.24 |
| BUN, mg/dL | 12.00 (10.00–15.00) | 12.00 (10.00–15.00) | 0.77 |
| Creatinine, mg/dL | 0.61 (0.54–0.73) | 0.64 (0.55–0.77) | 0.31 |
| eGFR-cys, mL/min/1.73 m2 | 109.81 (95.51–121.47) | 100.96 (80.85–113.33) | 0.009 |
| Uric acid, mg/dL | 5.00 (4.30–6.00) | 5.10 (4.40–6.20) | 0.31 |
| Albumin, g/dL | 4.66 (0.28) | 4.70 (0.26) | 0.39 |
| GPT, U/L | 22.00 (17.00–31.00) | 21.00 (17.00–29.00) | 0.95 |
| UACR, mg/g | 5.40 (3.50–8.20) | 6.40 (4.10–9.90) | 0.074 |
| Fasting glucose, mg/dL | 95.00 (90.00–101.00) | 97.00 (92.00–104.00) | 0.064 |
| HbA1C, % | 5.60 (5.30–5.80) | 5.70 (5.40–6.00) | 0.13 |
| Insulin, μIU/mL | 5.82 (3.16–8.43) | 5.86 (4.48–9.56) | 0.12 |
| HOMA-IR | 1.45 (0.76–2.10) | 1.45 (1.05–2.39) | 0.11 |
| FGF-23, pg/mL | 39.70 (34.34–54.48) | 27.57 (24.52–30.54) | <0.001 |
| Total Vitamin D, ng/mL | 24.80 (19.28–30.98) | 24.29 (19.46–31.70) | 0.66 |
| iPTH, mg/dL | 40.10 (32.70–51.30) | 45.20 (34.80–60.10) | 0.16 |
| P, mg/dL | 3.88 (0.55) | 3.89 (0.53) | 0.89 |
| Ca, mg/dL | 9.30 (9.10–9.50) | 9.30 (9.20–9.60) | 0.74 |
| Medication use | |||
| OHA, n | 7 (13.0%) | 17 (10.5%) | 0.62 |
| Anti-hypertensives, n | 9 (16.4%) | 42 (25.9%) | 0.15 |
| Pain killer, n | 9 (16.7%) | 21 (13.6%) | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Fang, W.-C.; Chu, S.-C.; Wang, P.-H.; Lee, C.-C.; Wu, I.-W.; Sun, C.-Y.; Hsu, H.-J.; Chen, C.-Y.; Chen, Y.-C.; et al. Circulating Fibroblast Growth Factor-23 Levels Can Predict Rapid Kidney Function Decline in a Healthy Population: A Community-Based Study. Biomolecules 2023, 13, 31. https://doi.org/10.3390/biom13010031
Chen H-Y, Fang W-C, Chu S-C, Wang P-H, Lee C-C, Wu I-W, Sun C-Y, Hsu H-J, Chen C-Y, Chen Y-C, et al. Circulating Fibroblast Growth Factor-23 Levels Can Predict Rapid Kidney Function Decline in a Healthy Population: A Community-Based Study. Biomolecules. 2023; 13(1):31. https://doi.org/10.3390/biom13010031
Chicago/Turabian StyleChen, Hsing-Yu, Wei-Ching Fang, Shao-Chi Chu, Po-Hsi Wang, Chin-Chan Lee, I-Wen Wu, Chiao-Yin Sun, Heng-Jung Hsu, Chun-Yu Chen, Yung-Chang Chen, and et al. 2023. "Circulating Fibroblast Growth Factor-23 Levels Can Predict Rapid Kidney Function Decline in a Healthy Population: A Community-Based Study" Biomolecules 13, no. 1: 31. https://doi.org/10.3390/biom13010031







