The Photobiomodulation of MAO-A Affects the Contractile Activity of Smooth Muscle Gastric Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Isolation of the Smooth Muscle Tissues and Registration of the Contractile Activity
2.4. Wet-Organ Bath and In Vitro Irradiation of the Tissues
2.5. Light Sources
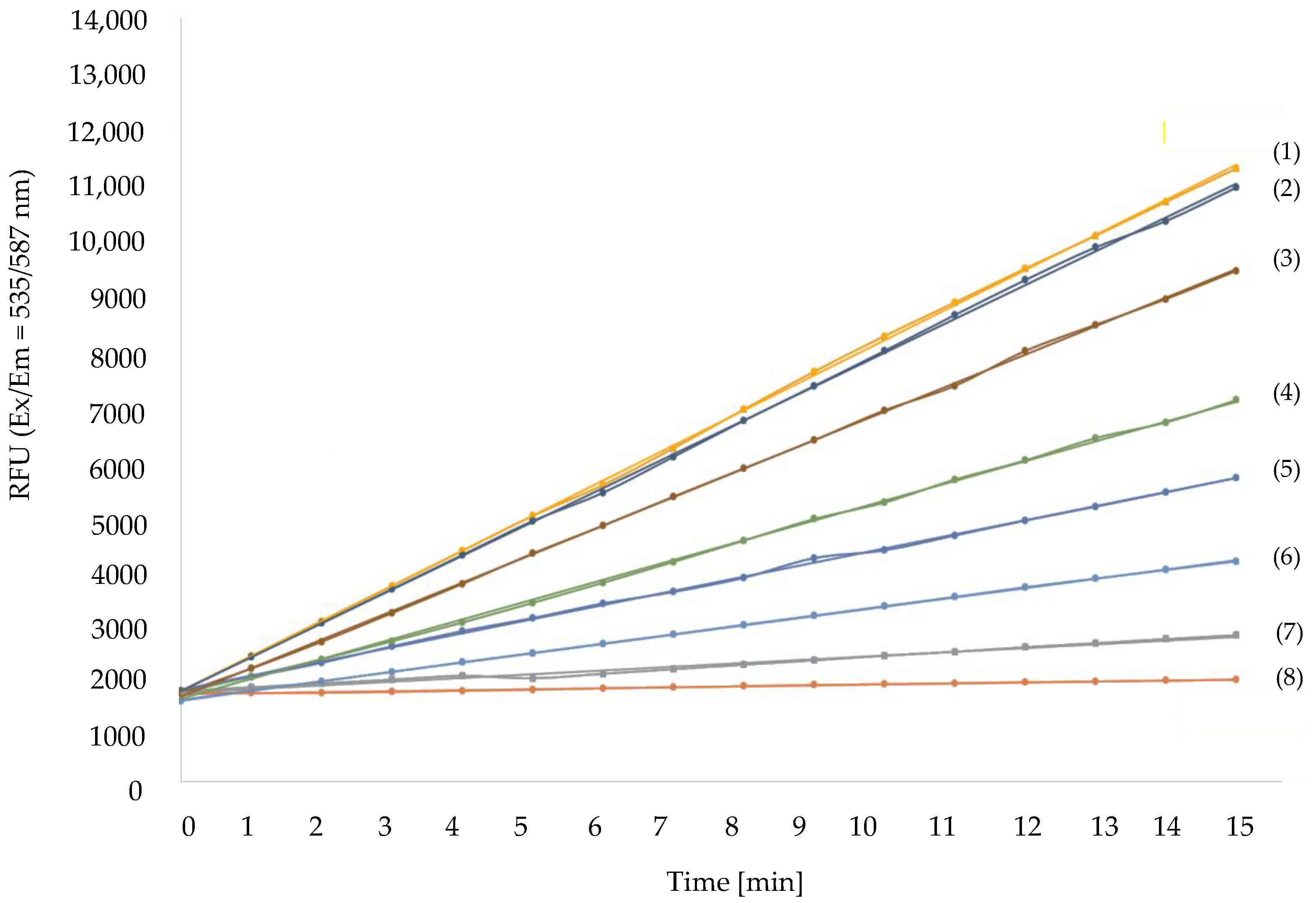
2.6. Evaluation of the MAO-A Activity
2.7. Expression of the MAOA Gene in Response to ER Exposure
2.8. Statistical Analysis of the Obtained Results
3. Results
3.1. Effects of In Vitro ER on SCA of Isolated Gastric SMT
3.2. Different ER Affect MAO-A Enzymatic Activity Differently
3.3. Investigation of the Alterations in MAOA Gene Expression under the Influence of ER
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Opel, D.R.; Hagstrom, E.; Pace, A.K.; Sisto, K.; Hirano-Ali, S.A.; Desai, S.; Swan, J. Light-emitting Diodes: A Brief Review and Clinical Experience. J. Clin. Aesthet. Dermatol. 2015, 8, 36–44. [Google Scholar]
- Hu, C.; Zuo, H.; Li, Y. Effects of Radiofrequency Electromagnetic Radiation on Neurotransmitters in the Brain. Front. Public Health 2021, 9, 691880. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Nagakura, Y.; Kiso, T.; Miyata, K.; Ito, H.; Iwaoka, K.; Yamaguchi, T. The effect of the selective 5-HT3 receptor agonist on ferret gut motility. Life Sci. 2002, 71, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. 2007, 32, 394–399. [Google Scholar]
- Li, H.-J.; Peng, R.-Y.; Wang, C.-Z.; Qiao, S.-M.; Yong, Z.; Gao, Y.-B.; Xu, X.-P.; Wang, S.-X.; Dong, J.; Zuo, H.-Y. Alterations of cognitive function and 5-HT system in rats after long term microwave exposure. Physiol. Behav. 2015, 140, 236–246. [Google Scholar] [CrossRef]
- Francois, A.; Ksas, B.; Gourmelon, P.; Griffiths, N.M. Changes in 5-HT-mediated pathways in radiation-induced attenuation and recovery of ion transport in rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G75–G82. [Google Scholar] [CrossRef] [Green Version]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S287–S296. [Google Scholar] [CrossRef] [Green Version]
- Mentis, A.A.; Dardiotis, E.; Katsouni, E.; Chrousos, G.P. From warrior genes to translational solutions: Novel insights into monoamine oxidases (MAOs) and aggression. Transl. Psychiatry 2021, 11, 130. [Google Scholar] [CrossRef]
- Finberg, J.P.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggiorani, D.; Manzella, N.; Edmondson, D.E.; Mattevi, A.; Parini, A.; Binda, C.; Mialet-Perez, J. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxidative Med. Cell. Longev. 2017, 2017, 3017947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spies, M.; James, G.M.; Vraka, C.; Philippe, C.; Hienert, M.; Gryglewski, G.; Komorowski, A.; Kautzky, A.; Silberbauer, L.; Pichler, V.; et al. Brain monoamine oxidase A in seasonal affective disorder and treatment with bright light therapy. Transl. Psychiatry 2018, 8, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xenodochidis, C.; Draganova, M.; Georgieva, M.; Miloshev, G.; Zagorchev, P. In vitro irradiation of smooth muscle tissues with LED red and laser near-infrared light modulates neurotransmission pathways. J. Chem. Technol. Metall. 2022; in press. [Google Scholar]
- Zagorchev, P.; Xenodochidis, C.; Georgieva, M.; Miloshev, G.; Andonov, B.; Dimitrova, S.; Draganova, M. LED system optimization for photobiomodulation of biological tissues. J. Chem. Technol. Metall. 2021, 56, 1156–1161. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [Green Version]
- Wunsch, A.; Matuschka, K. A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed. Laser Surg. 2014, 32, 93–100. [Google Scholar] [CrossRef]
- Hack, S.J.; Kinsey, L.J.; Beane, W.S. An Open Question: Is Non-Ionizing Radiation a Tool for Controlling Apoptosis-Induced Proliferation? Int. J. Mol. Sci. 2021, 22, 11159. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Shechter, A. Light Therapy. In Encyclopedia of the Neurological Sciences; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 886–889. [Google Scholar]
- Liu, Y.-L.; Gong, S.-Y.; Xia, S.-T.; Wang, Y.-L.; Peng, H.; Shen, Y.; Liu, C.-F. Light therapy: A new option for neurodegenerative diseases. Chin. Med. J. 2020, 134, 634–645. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Yang, Y.; Tucker, D.; Lu, Y.; Xin, N.; Zhang, G.; Yang, L.; Li, J.; Du, X. Low-Level Laser Irradiation Improves Depression-Like Behaviors in Mice. Mol. Neurobiol. 2017, 54, 4551–4559. [Google Scholar] [CrossRef]
- Tomaz de Magalhaes, M.; Núñez, S.C.; Kato, I.T.; Simões Ribeiro, M. Light therapy modulates serotonin levels and blood flow in women with headache. A preliminary study. Exp. Biol. Med. 2016, 241, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Seno, S.; Tomura, S.; Miyazaki, H.; Sato, S.; Saitoh, D. Effects of Selective Serotonin Reuptake Inhibitors on Depression-Like Behavior in a Laser-Induced Shock Wave Model. Front. Neurol. 2021, 12, 602038. [Google Scholar] [CrossRef] [PubMed]
- Tahkamo, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.L.; Tamarkin, L.; Sunderland, T.; Garrick, N.A.; Cohen, R.M. Human plasma melatonin is elevated during treatment with the monoamine oxidase inhibitors clorgyline and tranylcypromine but not deprenyl. Psychiatry Res. 1986, 17, 119–127. [Google Scholar] [CrossRef]
- Wang, J.; Edmondson, D.E. High-level expression and purification of rat monoamine oxidase A (MAO A) in Pichia pastoris: Comparison with human MAO A. Protein Expr. Purif. 2010, 70, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Gambichler, T.; Bader, A.; Vojvodic, M.; Bechara, F.G.; Sauermann, K.; Altmeyer, P.; Hoffmann, K. Impact of UVA exposure on psychological parameters and circulating serotonin and melatonin. BMC Dermatol. 2002, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Ming, Q.; Zhong, X.; Dong, D.; Li, C.; Xiong, G.; Cheng, C.; Cao, W.; He, J.; Wang, X.; et al. The MAOA Gene Influences the Neural Response to Psychosocial Stress in the Human Brain. Front. Behav. Neurosci. 2020, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Gehrman, P.; Martin, J.L.; Shochat, T.; Marler, M.; Corey-Bloom, J.; Levi, L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav. Sleep Med. 2003, 1, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.K.; Freelance, C.B.; Willis, G.L. The effect of light exposure on insomnia and nocturnal movement in Parkinson’s disease: An open label, retrospective, longitudinal study. Sleep Med. 2018, 44, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Endoscopic near infrared photoimmunotherapy using a fiber optic diffuser for peritoneal dissemination of gastric cancer. Cancer Sci. 2018, 109, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Soyer, T.; Aliefendioğlu, D.; Aktuna, Z.; Çağlayan, O.; Reşat Aydos, T.; Çakmak, M. Effect of phototherapy on gastrointestinal smooth muscle activity and oxidative stress. Pediatr. Surg. Int. 2011, 27, 1197–1202. [Google Scholar] [CrossRef] [PubMed]

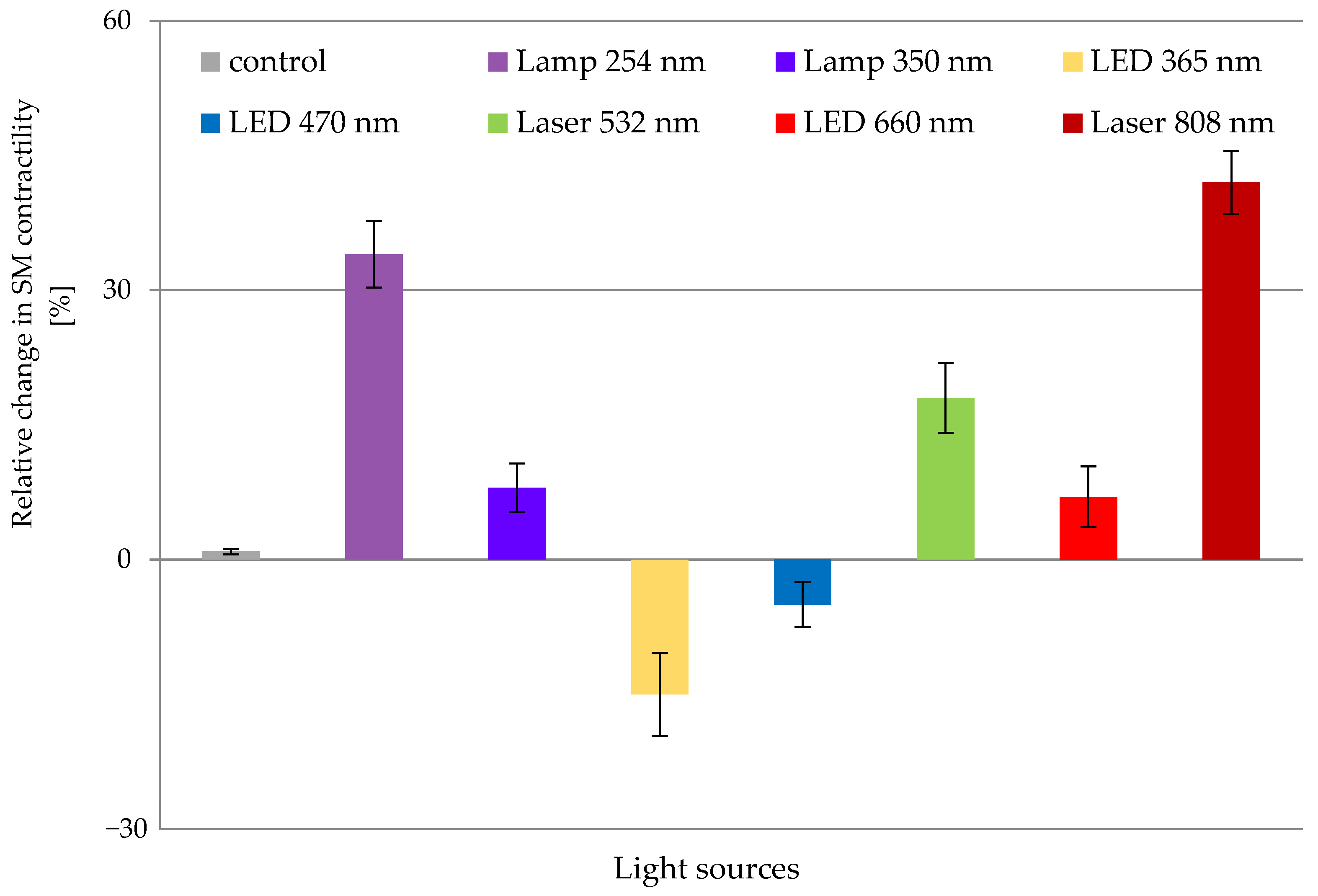


| Light Sources | Spectrum Range | λmax [nm] | P [W] | PD [mW/cm2] | F [J/cm2] |
|---|---|---|---|---|---|
| LEDs | UV Visible, Blue Visible, Red | 365 | 3 | 4 | 0.24 |
| 470 | 3 | 4 | 0.24 | ||
| 660 | 3 | 4 | 0.24 | ||
| Lasers | Visible, Green Near-Infrared | 532 | 0.2 | 4 | 0.24 |
| 808 | 0.5 | 4 | 0.24 | ||
| Lamps | UVA/B UVC | 350 | 6 | 4 | 0.24 |
| 254 | 6 | 4 | 0.24 |
| Oligomer Name | Sequence 5’–3’ | Amplicon Length, bp |
|---|---|---|
| RatHPRT1_For | GTTGGATACAGGCCAGACTTTGT | 70 |
| RatHPRT1_Rev | AGTCAAGGGCATATCCAACAACA | |
| RatMAOA_For | CACAGTGGAGTGGCTACATGG | 76 |
| RatMAOA_Rev | CCTAGAGCATTCAACACCTCTCT |
| ER Sources | Exposure Time to ER [min] | Relative MAO-A Activity [%] | Clorgyline [μM] |
|---|---|---|---|
| Lamp 254 nm | 1 2 | 51.75 ± 0.15 35.2 ± 0.5 | 10 |
| Lamp 350 nm | 1 2 | 93.55 ± 0.45 73.9 ± 1.1 | 100 |
| LED 365 nm | 1 2 | 128.85 ± 0.25 133.45 ± 0.45 | 1 |
| LED 470 nm | 1 2 | 105.65 ± 0.45 108.25 ± 0.15 | 100 |
| Laser 532 nm | 1 2 | 76.85 ± 0.15 75.65 ± 0.25 | 1 |
| LED 660 nm | 1 2 | 95.40 ± 0.3 91.85 ± 0.6 | 100 |
| Laser 808 nm | 1 2 | 87.15 ± 0.5 70.55 ± 0.6 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenodochidis, C.; Staneva, D.; Vasileva, B.; Draganova, M.; Miloshev, G.; Georgieva, M.; Zagorchev, P. The Photobiomodulation of MAO-A Affects the Contractile Activity of Smooth Muscle Gastric Tissues. Biomolecules 2023, 13, 32. https://doi.org/10.3390/biom13010032
Xenodochidis C, Staneva D, Vasileva B, Draganova M, Miloshev G, Georgieva M, Zagorchev P. The Photobiomodulation of MAO-A Affects the Contractile Activity of Smooth Muscle Gastric Tissues. Biomolecules. 2023; 13(1):32. https://doi.org/10.3390/biom13010032
Chicago/Turabian StyleXenodochidis, Charilaos, Dessislava Staneva, Bela Vasileva, Milena Draganova, George Miloshev, Milena Georgieva, and Plamen Zagorchev. 2023. "The Photobiomodulation of MAO-A Affects the Contractile Activity of Smooth Muscle Gastric Tissues" Biomolecules 13, no. 1: 32. https://doi.org/10.3390/biom13010032









