PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds
Abstract
1. Introduction
2. PI3K–AKT Signaling in Breast Cancer
2.1. Overview of the PI3K–AKT Signaling Pathway
2.2. The Role of PI3K–AKT Signaling in Breast Cancer
3. Research Status of Inhibitors Targeting PI3K–AKT Pathway in Breast Cancer
3.1. PI3K Inhibitors
3.2. AKT Inhibitors
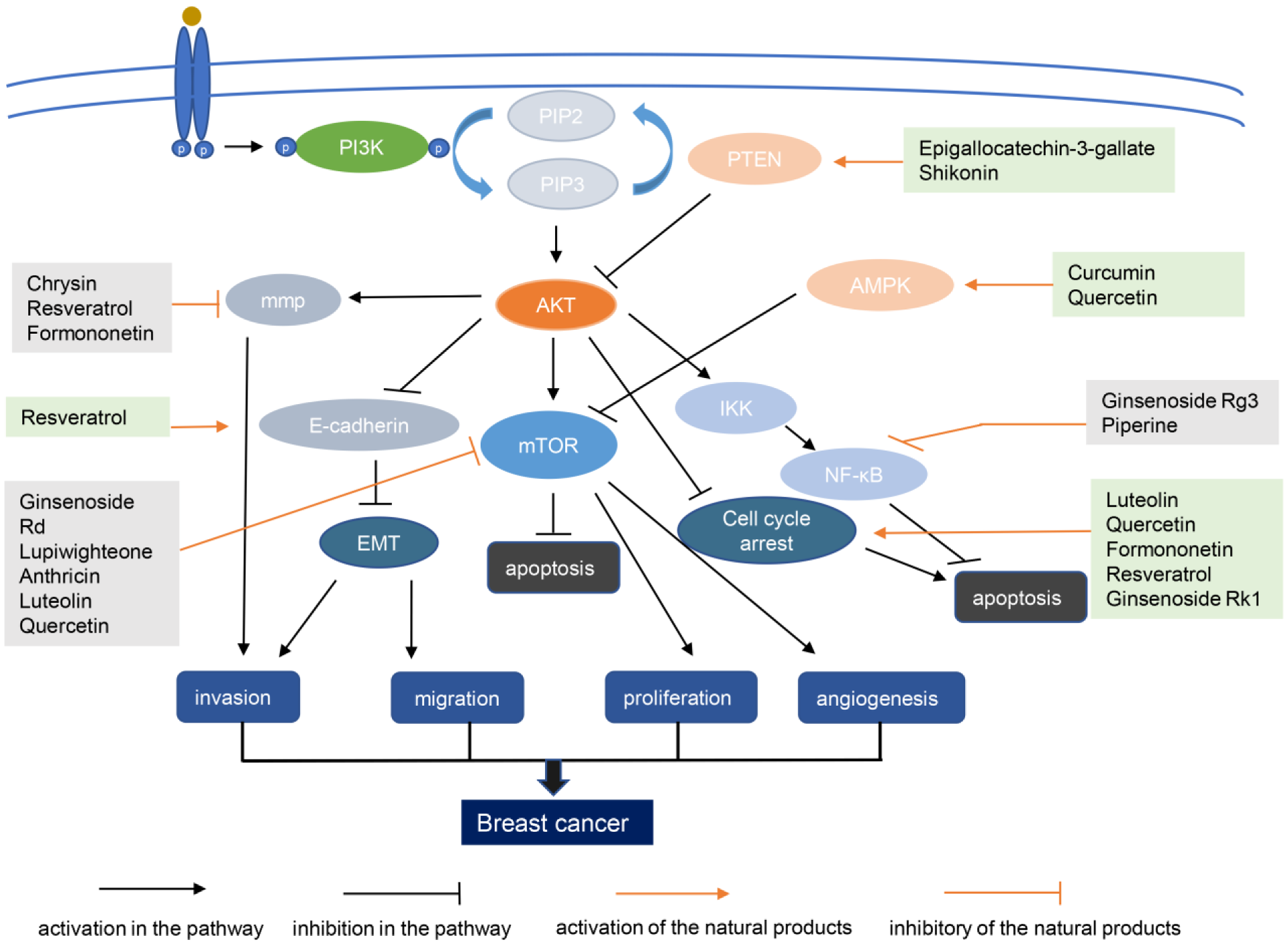
4. Natural Products and Synthetic Analogs for PI3K–AKT-Targeting Breast Cancer Treatments
4.1. Flavonoids
4.1.1. Curcumin
4.1.2. Quercetin
4.1.3. Formononetin
4.1.4. Saponins
4.2. Non-Flavonoid Polyphenols
Resveratrol
4.3. Others
5. Limitations of Conventional Breast Cancer Treatment and Potential Biomarkers
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Abel, M.K.; Brabham, C.E.; Guo, R.; Fahrner-Scott, K.; Wong, J.; Alvarado, M.; Ewing, C.; Esserman, L.J.; Mukhtar, R.A. Breast conservation therapy versus mastectomy in the surgical management of invasive lobular carcinoma measuring 4 cm or greater. Am. J. Surg. 2020, 221, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Siddiqui, S.A. Breast cancer management: Past, present and evolving. Indian J. Cancer 2012, 49, 277. [Google Scholar] [CrossRef]
- Mollon, L.; Aguilar, A.; Anderson, E.; Dean, J.; Davis, L.; Warholak, T.; Aizer, A.A.; Platt, E.; Bardiya, A.; Tang, D. A systematic literature review of the prevalence of pik3ca mutations and mutation hotspots in hr+/her2-metastatic breast cancer. Cancer Res. 2018, 78, 1207. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Tuberous sclerosis complex: From molecular biology to novel therapeutic approaches. IUBMB Life 2016, 68, 955–962. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef]
- Franke, T.F.; Yang, S.-I.; Chan, T.; Datta, K.; Kazlauskas, A.; Morrison, D.K.; Kaplan, D.R.; Tsichlis, P.N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995, 81, 727–736. [Google Scholar] [CrossRef]
- Kohn, A.D.; Kovacina, K.S.; Roth, R.A. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995, 14, 4288–4295. [Google Scholar] [CrossRef]
- Burgering, B.M.T.; Coffer, P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.P.; Cardone, C.; Martini, G.; Ciardiello, D.; Belli, V.; Matrone, N.; Barra, G.; Napolitano, S.; Della Corte, C.; Turano, M.; et al. Receptor tyrosine kinase-dependent PI3K activation is an escape mechanism to vertical suppression of the EGFR/RAS/MAPK pathway in KRAS-mutated human colorectal cancer cell lines. J. Exp. Clin. Cancer Res. 2019, 38, 41. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Akinleye, A.; Avvaru, P.; Furqan, M.; Song, Y.; Liu, D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013, 6, 88. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Manning, B.D. Insulin Signaling: Inositol Phosphates Get into the Akt. Cell 2010, 143, 861–863. [Google Scholar] [CrossRef]
- Chalhoub, N.; Baker, S.J. Pten and the pi3-kinase pathway in cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 127–150. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Z.; Zhang, S.; Liu, P.; Zhang, L.; Lee, S.H.; Zhang, J.; Signoretti, S.; Loda, M.; Roberts, T.M. Essential roles of pi (3) k–p110β in cell growth, metabolism and tumorigenesis. Nature 2008, 454, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Duggal, S.; Jailkhani, N.; Midha, M.K.; Agrawal, N.; Rao, K.V.S.; Kumar, A. Defining the Akt1 interactome and its role in regulating the cell cycle. Sci. Rep. 2018, 8, 1303. [Google Scholar] [CrossRef] [PubMed]
- Matheny, R.W., Jr.; Geddis, A.V.; Abdalla, M.N.; Leandry, L.A.; Ford, M.; McClung, H.L.; Pasiakos, S.M. Akt 2 is the predominant akt isoform expressed in human skeletal muscle. Physiol. Rep. 2018, 6, e13652. [Google Scholar] [CrossRef]
- Alcantara, D.; Timms, A.E.; Gripp, K.; Baker, L.; Park, K.; Collins, S.; Cheng, C.; Stewart, F.; Mehta, S.G.; Saggar, A.; et al. Mutations of AKT3 are associated with a wide spectrum of developmental disorders including extreme megalencephaly. Brain 2017, 140, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. Mtor at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Ravichandran, L.V.; Chen, H.; Li, Y.; Quon, M.J. Phosphorylation of ptp1b at ser50 by akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 2001, 15, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1999, 1, E33–E35. [Google Scholar] [CrossRef] [PubMed]
- del Peso, L.; González-García, M.; Page, C.; Herrera, R.; Nuñez, G. Interleukin-3-Induced Phosphorylation of BAD Through the Protein Kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of nfκb and the essentialness of nfκb for the oncogenicity of pi3k and akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Burgering, B.M.T.; Medema, R.H. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J. Leukoc. Biol. 2003, 73, 689–701. [Google Scholar] [CrossRef]
- Nidai Ozes, O.; Mayo, L.D.; Gustin, J.A.; Pfeffer, S.R.; Pfeffer, L.M.; Donner, D.B. Nf-κb activation by tumour necrosis factor requires the akt serine–threonine kinase. Nature 1999, 401, 82–85. [Google Scholar] [CrossRef]
- Rössig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-Dependent Phosphorylation of p21 Cip1 Regulates PCNA Binding and Proliferation of Endothelial Cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Environmental exposure, and other behavioral risk factors in breast cancer. Curr. Cancer Ther. Rev. 2006, 2, 3–21. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C.; Easton, D.F.; Sherman, M.E.; Garcia-Closas, M.; Dowsett, M.; et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, S.J. Will molecular classification replace traditional breast pathology? Int. J. Surg. Pathol. 2010, 18, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mukohara, T. PI3K mutations in breast cancer: Prognostic and therapeutic implications. Breast Cancer Targets Ther. 2015, 7, 111–123. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Koren, S.; Reavie, L.; Couto, J.P.; De Silva, D.; Stadler, M.B.; Roloff, T.; Britschgi, A.; Eichlisberger, T.; Kohler, H.; Aina, O.; et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature 2015, 525, 114–118. [Google Scholar] [CrossRef]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef]
- Campbell, R.A.; Bhat-Nakshatri, P.; Patel, N.M.; Constantinidou, D.; Ali, S.; Nakshatri, H. Phosphatidylinositol 3-kinase/akt-mediated activation of estrogen receptor α: A new model for anti-estrogen resistance. J. Biol. Chem. 2001, 276, 9817–9824. [Google Scholar] [CrossRef]
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, C.M.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. PIK3CA and PIK3CB Inhibition Produce Synthetic Lethality when Combined with Estrogen Deprivation in Estrogen Receptor–Positive Breast Cancer. Cancer Res. 2009, 69, 3955–3962. [Google Scholar] [CrossRef]
- Creighton, C.J.; Fu, X.; Hennessy, B.T.; Casa, A.J.; Zhang, Y.; Gonzalez-Angulo, A.M.; Lluch, A.; Gray, J.W.; Brown, P.H.; Hilsenbeck, S.G.; et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Forbes, J.T.; Shah, C.; Wyatt, S.K.; Manning, H.C.; Olivares, M.G.; Sanchez, V.; Dugger, T.C.; Granja, N.D.M.; Narasanna, A.; et al. Inhibition of Mammalian Target of Rapamycin Is Required for Optimal Antitumor Effect of HER2 Inhibitors against HER2-Overexpressing Cancer Cells. Clin. Cancer Res. 2009, 15, 7266–7276. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Sánchez, V.; Kuba, M.G.; Rinehart, C.; Arteaga, C.L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl. Acad. Sci. USA 2011, 109, 2718–2723. [Google Scholar] [CrossRef]
- Hu, L.; Hofmann, J.; Lu, Y.; Mills, G.B.; Jaffe, R.B. Inhibition of phosphatidylinositol 3’-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002, 62, 1087–1092. [Google Scholar]
- Ssw, N.; Tsao, M.; Chow, S.; Hedley, D. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000, 60, 5451–5455. [Google Scholar]
- Dong, J.; Peng, J.; Zhang, H.; Mondesire, W.H.; Jian, W.; Mills, G.B.; Hung, M.-C.; Meric-Bernstam, F. Role of Glycogen Synthase Kinase 3β in Rapamycin-Mediated Cell Cycle Regulation and Chemosensitivity. Cancer Res. 2005, 65, 1961–1972. [Google Scholar] [CrossRef]
- A Trial of bkm120 (a pi3k Inhibitor) in Patients with Triple Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCCT2eTripleI3K&coCancerreast+Cancer&drBreast&raa (accessed on 15 May 2022).
- Safety and Efficacy of Bkm120 and Lapatinib in Her2+/Pi3k-Activated, Trastuzumab-Resistant Advanced Breast Cancer (pikher2). Available online: https://clinicaltrials.gov/ct2/show/NCT01589861?term=PI3K&cond=Breast+Cancer&draw=2&rank=4 (accessed on 15 May 2022).
- Neophoebe: Neoadjuvant Trastuzumab + Bkm120 in Combination with Weekly Paclitaxel in Her2-Positive Primary Breast Cancer (neophoebe). Available online: https://clinicaltrials.gov/ct2/show/NCT01816594?term=PI3K&cond=Breast+Cancer&draw=4&rank=14 (accessed on 15 May 2022).
- Bkm120 and Fulvestrant for Treating Postmenopausal Patients with Estrogen Receptor-Positive Stage Iv Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01339442?term=PI3K&cond=Breast+Cancer&draw=4&rank=17 (accessed on 15 May 2022).
- Trial of Bkm120/Tamoxifen-Combination in Patients with Hr-Pos, Her2-Neg Breast Cancer (Piktam). Available online: https://clinicaltrials.gov/ct2/show/NCT02404844?term=PI3K&cond=Breast+Cancer&draw=4&rank=20 (accessed on 15 May 2022).
- Phase ib, Dose Escalation Study of Oral Lde225 in Combination with Bkm120 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01576666?term=PI3K&cond=Breast+Cancer&draw=6&rank=202 (accessed on 15 May 2022).
- Efficacy and Safety of Tenalisib (rp6530), a Pi3k δ/γ and Sik3 Inhibitor, in Patients with Locally Advanced or Metastatic Breast cance. Available online: https://clinicaltrials.gov/ct2/show/NCT05021900?term=PI3K&cond=Breast+Cancer&draw=2&rank=11 (accessed on 15 May 2022).
- Taselisib and Enzalutamide in Treating Patients with Androgen Receptor Positive Triple-Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02457910?term=PI3K&cond=Breast+Cancer&draw=2&rank=51 (accessed on 15 May 2022).
- Phosphatidylinositol 3-Kinase (pi3k) Alpha Inhibition in Advanced Breast Cancer (Piknic). Available online: https://clinicaltrials.gov/ct2/show/NCT02506556?term=PI3K&cond=Breast+Cancer&draw=2&rank=2 (accessed on 15 May 2022).
- Byl719 and Letrozole In Post-Menopausal Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01791478?term=PI3K&cond=Breast+Cancer&draw=2&rank=10 (accessed on 15 May 2022).
- Byl719 and Nab-Paclitaxel in Locally Recurrent or Metastatic Her-2 Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02379247?term=PI3K&cond=Breast+Cancer&draw=2&rank=79 (accessed on 15 May 2022).
- Gdc-0941 and Cisplatin in Treating Patients with Androgen Receptor-Negative Triple Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01918306?term=PI3K&cond=Breast+Cancer&draw=2&rank=32 (accessed on 15 May 2022).
- A Study of Pi3-Kinase Inhibitor gdc-0941 in Combination with Paclitaxel, with and without Bevacizumab or Trastuzumab, and with Letrozole, in Participants with Locally Recurrent or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00960960?term=PI3K&cond=Breast+Cancer&draw=2 (accessed on 15 May 2022).
- Gdc-0084 in Combination with Trastuzumab for Patients with Her2-Positive Breast Cancer Brain Metastases. Available online: https://clinicaltrials.gov/ct2/show/NCT03765983?term=PI3K&cond=Breast+Cancer&draw=3&rank=143 (accessed on 15 May 2022).
- A study of pf-05212384 in Combination with Other Anti-Tumor Agents and in Combination with Cisplatin in Patients with Triple Negative Breast Cancer in an Expansion Arm (tnbc). Available online: https://clinicaltrials.gov/ct2/show/NCT01920061?term=PI3K&cond=Breast+Cancer&draw=2&rank=74 (accessed on 15 May 2022).
- Dose Finding Study of pf-05212384 with Paclitaxel and Carboplatin in Patients with Advanced Solid Tumor (iosi-ndu-001). Available online: https://clinicaltrials.gov/ct2/show/NCT02069158?term=PI3K&cond=Breast+Cancer&draw=6&rank=221 (accessed on 15 May 2022).
- Pi3kbeta Inhibitor Azd8186 and Docetaxel in Treating Patients Advanced Solid Tumors with Pten or Pik3cb Mutations that Are Metastatic or Cannot Be Removed by Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT03218826?term=PI3K&cond=Breast+Cancer&draw=3&rank=117 (accessed on 15 May 2022).
- A Phase 1b/2 Study of Serabelisib in Combination with Canagliflozin in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04073680?term=PI3K&cond=Breast+Cancer&draw=3&rank=148 (accessed on 15 May 2022).
- Ipatasertib + Pertuzumab +Trastuzumab in Advanced Her2+ Pi3kca-Mutant Breast Cancer Patients (Ipather). Available online: https://clinicaltrials.gov/ct2/show/NCT04253561?term=PI3K&cond=Breast+Cancer&draw=3 (accessed on 15 May 2022).
- Akt Inhibitor Mk2206 in Combination with Lapatinib Ditosylate in Patients with Advanced or Metastatic Solid Tumors or Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01245205?term=PI3K&cond=Breast+Cancer&draw=3&rank=184 (accessed on 15 May 2022).
- Mk2206 and Paclitaxel in Treating Patients with Locally Advanced or Metastatic Solid Tumors or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01263145?term=PI3K&cond=Breast+Cancer&draw=3&rank=165 (accessed on 15 May 2022).
- Akt Inhibitor Mk2206 in Treating Patients with Advanced Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01277757?term=PI3K&cond=Breast+Cancer&draw=3&rank=104 (accessed on 15 May 2022).
- Akt Inhibitor Mk-2206 and Anastrozole with or without Goserelin Acetate in Treating Patients with Stage ii-iii Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01776008?term=PI3K&cond=Breast+Cancer&draw=3 (accessed on 15 May 2022).
- Leevers, S.J.; Vanhaesebroeck, B.; Waterfield, M.D. Signalling Through Phosphoinositide 3-Kinases: The Lipids Take Centre Stage. Curr. Opin. Cell Biol. 1999, 11, 219–225. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Immunity, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.M. The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem. J. 2008, 410, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L. Phase 2 study of buparlisib (bkm120), a pan-class i pi3k inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, W.; Wang, X.; Chen, L.; Wang, S. BKM120 sensitizes BRCA-proficient triple negative breast cancer cells to olaparib through regulating FOXM1 and Exo1 expression. Sci. Rep. 2021, 11, 4774. [Google Scholar] [CrossRef]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of Tenalisib (Rp6530) in Combination with Romidepsin in Patients with RELAPSED/refractory T-Cell Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03770000 (accessed on 15 May 2022).
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H. A phase ib study of alpelisib (byl719), a pi3kα-specific inhibitor, with letrozole in er+/her2− metastatic breast cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef]
- Sharma, P.; Abramson, V.G.; O’Dea, A.P.; Nye, L.; Mayer, I.A.; Pathak, H.B.; Hoffmann, M.; Stecklein, S.R.; Elia, M.; Lewis, S.; et al. Clinical and Biomarker Results from Phase I/II Study of PI3K Inhibitor Alpelisib plus Nab-paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3896–3904. [Google Scholar] [CrossRef]
- Folkes, A.J.; Ahmadi, K.; Alderton, W.K.; Alix, S.; Baker, S.J.; Box, G.; Chuckowree, I.S.; Clarke, P.A.; Depledge, P.; Eccles, S.A. The identification of 2-(1 h-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno [3, 2-d] pyrimidine (gdc-0941) as a potent, selective, orally bioavailable inhibitor of class i pi3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef]
- Vuylsteke, P.; Huizing, M.; Petrakova, K.; Roylance, R.; Laing, R.; Chan, S.; Abell, F.; Gendreau, S.; Rooney, I.; Apt, D.; et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016, 27, 2059–2066. [Google Scholar] [CrossRef]
- Schöffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar] [CrossRef]
- Salphati, L.; Alicke, B.; Heffron, T.P.; Shahidi-Latham, S.; Nishimura, M.; Cao, T.; Carano, R.A.; Cheong, J.; Greve, J.; Koeppen, H.; et al. Brain Distribution and Efficacy of the Brain Penetrant PI3K Inhibitor GDC-0084 in Orthotopic Mouse Models of Human Glioblastoma. Drug Metab. Dispos. 2016, 44, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Ippen, F.M.; Alvarez-Breckenridge, C.A.; Kuter, B.M.; Fink, A.L.; Bihun, I.V.; Lastrapes, M.; Penson, T.; Schmidt, S.P.; Wojtkiewicz, G.R.; Ning, J.; et al. The Dual PI3K/mTOR Pathway Inhibitor GDC-0084 Achieves Antitumor Activity in PIK3CA-Mutant Breast Cancer Brain Metastases. Clin. Cancer Res. 2019, 25, 3374–3383. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Dehnhardt, C.M.; Delos Santos, E.; Chen, Z.; Dos Santos, O.; Ayral-Kaloustian, S.; Khafizova, G.; Brooijmans, N.; Mallon, R.; Hollander, I. Bis (morpholino-1, 3, 5-triazine) derivatives: Potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (pki-587), a highly efficacious dual inhibitor. J. Med. Chem. 2010, 53, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.M.; Chen, Z.; Dos Santos, O.; Dehnhardt, C.; Santos, E.D.; Ayral-Kaloustian, S.; Mallon, R.; Hollander, I.; Feldberg, L.; Lucas, J. Pki-179: An orally efficacious dual phosphatidylinositol-3-kinase (pi3k)/mammalian target of rapamycin (mtor) inhibitor. Bioorganic Med. Chem. Lett. 2010, 20, 5869–5873. [Google Scholar] [CrossRef] [PubMed]
- Hancox, U.; Cosulich, S.; Hanson, L.; Trigwell, C.; Lenaghan, C.; Ellston, R.; Dry, H.; Crafter, C.; Barlaam, B.; Fitzek, M.; et al. Inhibition of PI3Kβ Signaling with AZD8186 Inhibits Growth of PTEN-Deficient Breast and Prostate Tumors Alone and in Combination with Docetaxel. Mol. Cancer Ther. 2015, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Williams, K.A.; Krie, A.K.; De, P.; Dey, N.; Leyland-Jones, B.; Starks, D.; Rojas-Espaillat, L.A. Results of a phase Ib trial evaluating the safety and clinical activity of sapanisertib (TAK 228) in combination with serabelisib (TAK 117) and paclitaxel in patients with advanced ovarian, endometrial, or breast cancer. J. Clin. Oncol. 2020, 38, 3604. [Google Scholar] [CrossRef]
- Chu, N.; Viennet, T.; Bae, H.; Salguero, A.; Boeszoermenyi, A.; Arthanari, H.; Cole, P.A. The structural determinants of PH domain-mediated regulation of Akt revealed by segmental labeling. Elife 2020, 9, e59151. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Z.; Wei, W. Phosphorylation of Akt at the C-terminal tail triggers Akt Activation. Cell Cycle 2014, 13, 2162–2164. [Google Scholar] [CrossRef]
- Liu, P.; Begley, M.; Michowski, W.; Inuzuka, H.; Ginzberg, M.; Gao, D.; Tsou, P.; Gan, W.; Papa, A.; Kim, B.M.; et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 2014, 508, 541–545. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. Pi3k and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Lin, N.U.; Maurer, M.A.; Chen, H.; Mahvash, A.; Sahin, A.; Akcakanat, A.; Li, Y.; Abramson, V.; Litton, J.; et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21, 78. [Google Scholar] [CrossRef]
- Ma, C.X.; Suman, V.; Goetz, M.P.; Northfelt, D.; Burkard, M.E.; Ademuyiwa, F.; Naughton, M.; Margenthaler, J.; Aft, R.; Gray, R. A phase ii trial of neoadjuvant mk-2206, an akt inhibitor, with anastrozole in clinical stage ii or iii pik3ca-mutant er-positive and her2-negative breast cancer. Clin. Cancer Res. 2017, 23, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Krop, I.; Akcakanat, A.; Chen, H.; Liu, S.; Li, Y.; Culotta, K.S.; Tarco, E.; Piha-Paul, S.; Moulder-Thompson, S.; et al. SU2C Phase Ib Study of Paclitaxel and MK-2206 in Advanced Solid Tumors and Metastatic Breast Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju493. [Google Scholar] [CrossRef]
- Wisinski, K.B.; Tevaarwerk, A.J.; Burkard, M.E.; Rampurwala, M.; Eickhoff, J.; Bell, M.C.; Kolesar, J.M.; Flynn, C.; Liu, G. Phase I Study of an AKT Inhibitor (MK-2206) Combined with Lapatinib in Adult Solid Tumors Followed by Dose Expansion in Advanced HER2+ Breast Cancer. Clin. Cancer Res. 2016, 22, 2659–2667. [Google Scholar] [CrossRef]
- Takeda, T.; Wang, Y.; Bryant, S.H. Structural insights of a PI3K/mTOR dual inhibitor with the morpholino-triazine scaffold. J. Comput.-Aided Mol. Des. 2016, 30, 323–330. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mtor for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Sangai, T.; Akcakanat, A.; Chen, H.; Tarco, E.; Wu, Y.; Do, K.-A.; Miller, T.W.; Arteaga, C.L.; Mills, G.B.; Gonzalez-Angulo, A.M. Biomarkers of response to akt inhibitor mk-2206 in breast cancerantitumor activity of mk-2206. Clin. Cancer Res. 2012, 18, 5816–5828. [Google Scholar] [CrossRef]
- Davies, B.R.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.-H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J. Preclinical pharmacology of azd5363, an inhibitor of akt: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic backgroundazd5363, an oral inhibitor of akt. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef]
- Lin, J.; Sampath, D.; Nannini, M.A.; Lee, B.B.; Degtyarev, M.; Oeh, J.; Savage, H.; Guan, Z.; Hong, R.; Kassees, R. Targeting activated akt with gdc-0068, a novel selective akt inhibitor that is efficacious in multiple tumor modelsgdc-0068, a novel selective atp-competitive akt inhibitor. Clin. Cancer Res. 2013, 19, 1760–1772. [Google Scholar] [CrossRef]
- Han, H.S.; Swanton, C.; Janjigian, Y.Y.; Sutherland, S.C.; Chandarlapaty, S.; Lehman, R.; Hamilton, N.; Knowles, J.; Lee, R.; Yan, L.; et al. A phase I study of the AKT inhibitor (MK-2206) with concurrent trastuzumab and lapatinib in patients with HER2-positive solid tumors. J. Clin. Oncol. 2011, 29, 3028. [Google Scholar] [CrossRef]
- Krop, I.E.; Saura, C.; Ahnert, J.R.; Becerra, C.; Britten, C.D.; Isakoff, S.J.; Demanse, D.; Hackl, W.; Quadt, C.; Silva, A.P.; et al. A phase I/IB dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer. J. Clin. Oncol. 2012, 30, 508. [Google Scholar] [CrossRef]
- Shimizu, T.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Smith, L.S.; Gunn, S.; Smetzer, L.; Mays, T.A.; Kaiser, B. The clinical effect of the dual-targeting strategy involving pi3k/akt/mtor and ras/mek/erk pathways in patients with advanced cancerclinical effect of dual pi3k and mapk pathways inhibitions. Clin. Cancer Res. 2012, 18, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.L.; Pollack, S.F.; Liu, M.; Li, X. Cdk 4/6 inhibitors sensitize pik3ca mutant breast cancer to pi3k inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, J.; Saleh, N.; Chen, S.-C.; Ayers, G.; Abramson, V.; Mayer, I.; Richmond, A. Inhibition of the PI3K/mTOR Pathway in Breast Cancer to Enhance Response to Immune Checkpoint Inhibitors in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 5207. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2015, 25, 41–59. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Luo, X.; Huang, J.; Luo, F.; Li, H.; Li, H.; Ren, G. Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and pi3k/akt signaling pathway. J. Appl. Toxicol. 2014, 34, 105–112. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (cd44+/cd24−) by inhibiting the pi3k/akt/mtor-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, J.; Xin, M.; Huang, W.; Chen, X. Formononetin induces cell cycle arrest of human breast cancer cells via igf1/pi3k/akt pathways in vitro and in vivo. Horm. Metab. Res. 2011, 43, 681–686. [Google Scholar] [CrossRef]
- Guan, F.; Ding, Y.; Zhang, Y.; Zhou, Y.; Li, M.; Wang, C. Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS ONE 2016, 11, e0146553. [Google Scholar] [CrossRef]
- Squires, M.S.; Hudson, E.; Howells, L.; Sale, S.; Houghton, C.E.; Jones, J.; Fox, L.H.; Dickens, M.; Prigent, S.A.; Manson, M.M. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 2002, 65, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Liu, T.; Qian, L.; Xiao, C.; Zhou, X.; Ai, H.; Wang, J.; Fan, W.; Pan, J. Shikonin inhibits migration and invasion of triple-negative breast cancer cells by suppressing epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. J. Cancer 2021, 12, 76–88. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, H.; Ahn, J.; Jung, S.K.; Um, M.Y.; Son, K.-H.; Kim, T.W.; Ha, T.Y. Anthricin Isolated from Anthriscus sylvestris (L.) Hoffm. Inhibits the Growth of Breast Cancer Cells by Inhibiting Akt/mTOR Signaling, and Its Apoptotic Effects Are Enhanced by Autophagy Inhibition. Evidence-Based Complement. Altern. Med. 2013, 2013, 385219. [Google Scholar] [CrossRef]
- Won, Y.-S.; Seo, K.-I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. 2020, 135, 110863. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef]
- Zhang, E.; Shi, H.; Yang, L.; Wu, X.; Wang, Z. Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and suppresses angiogenesis and breast tumor growth. Oncol. Rep. 2017, 38, 359–367. [Google Scholar] [CrossRef]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Surh, Y.-J.; Na, H.-K. Ginsenoside rg3 inhibits constitutive activation of nf-κb signaling in human breast cancer (mda-mb-231) cells: Erk and akt as potential upstream targets. J. Cancer Prev. 2014, 19, 23. [Google Scholar] [CrossRef]
- Qin, H.-L.; Wang, X.-J.; Yang, B.-X.; Du, B.; Yun, X.-L. Notoginsenoside R1 attenuates breast cancer progression by targeting CCND2 and YBX3. Chin. Med. J. 2021, 134, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Yim-Im, W.; Sawatdichaikul, O.; Semsri, S.; Horata, N.; Mokmak, W.; Tongsima, S.; Suksamrarn, A.; Choowongkomon, K. Computational analyses of curcuminoid analogs against kinase domain of HER2. BMC Bioinform. 2014, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Loo, W.T.; Sze, S.; Tong, Y. Curcumin inhibits cell proliferation of mda-mb-231 and bt-483 breast cancer cells mediated by down-regulation of nfκb, cyclind and mmp-1 transcription. Phytomedicine 2009, 16, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Lai, Y.-A.; Lin, Y.-C.; Ma, J.-W.; Huang, L.-F.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Curcumin Suppresses Doxorubicin-Induced Epithelial–Mesenchymal Transition via the Inhibition of TGF-β and PI3K/AKT Signaling Pathways in Triple-Negative Breast Cancer Cells. J. Agric. Food Chem. 2013, 61, 11817–11824. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef]
- Xiao-Ai, L.; Bei, W.; Xiao-Hong, X.; Lei, P.; Bin, W.; Xiao-Xue, D.; Chen-Hui, Z.; Qi-Wei, D. Curcumin re-sensitizes multidrug resistant (MDR) breast cancer to cisplatin through inducing autophagy by decreasing CCAT1 expression. RSC Adv. 2017, 7, 33572–33579. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and Its Derivatives: Synthesis, Pharmacological Uses with Special Emphasis on Anti-Tumor Properties and Prodrug with Enhanced Bio-Availability. Anti-Cancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Y.; Ye, Z.; Lin, B.; Zeng, J.; Li, C.; Liang, T.; Zhou, K.; Li, J. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2018, 42, 1625–1636. [Google Scholar] [CrossRef]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through akt-mtor pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Safi, A.; Heidarian, E.; Ahmadi, R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell. Med. 2021, 10, 11–22. [Google Scholar] [CrossRef]
- LEMEŽIENĖ, N.; Padarauskas, A.; BUTKUTĖ, B.; CESEVIČIENĖ, J.; Taujenis, L.; NORKEVIČIENĖ, E.; MIKALIŪNIENĖ, J. The concentration of isolavones in red clover (trifolium pratense l.) at lowering stage. Zemdirb. -Agric. 2015, 102, 443–448. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L. Formononetin-induced Apoptosis by Activation of Ras/p38 Mitogen-activated Protein Kinase in Estrogen Receptor-positive Human Breast Cancer Cells. Horm. Metab. Res. 2012, 44, 943–948. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin Inhibits Migration and Invasion of MDA-MB-231 and 4T1 Breast Cancer Cells by Suppressing MMP-2 and MMP-9 Through PI3K/AKT Signaling Pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xu, H.; Wu, Z.F.; Chen, C.; Liu, J.Y.; Wu, G.N.; Yao, X.Q.; Liu, F.K.; Li, G.; Shen, L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget 2015, 6, 44563–44578. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Na, H.-K.; Surh, Y.-J. Ginsenoside Rg3 Induces Apoptosis of Human Breast Cancer (MDA-MB-231) Cells. J. Cancer Prev. 2013, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fan, D. Ginsenoside Rk1 induces cell death through ROS-mediated PTEN/PI3K/Akt/mTOR signaling pathway in MCF-7 cells. J. Funct. Foods 2019, 57, 255–265. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model. Food Funct. 2018, 9, 5513–5527. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for Breast Cancer Prevention and Therapy: Preclinical Evidence and Molecular Mechanisms; Academic Press: Cambridge, MA, USA, 2016; Volume 40–41, pp. 209–232. [Google Scholar] [CrossRef]
- Park, M.-A.; Hwang, K.-A.; Choi, K.-C. Diverse animal models to examine potential role (s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property? Lab. Anim. Res. 2011, 27, 265–273. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Zhu, J.; Orloff, M.; Eng, C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011, 301, 168–176. [Google Scholar] [CrossRef]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Sinha, C.; Ignatoski, K.; Lippman, M.E.; Ethier, S.P.; Chinnaiyan, A.M. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003, 63, 132–139. [Google Scholar] [PubMed]
- Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H.; Way, T.-D.; Chen, W.-J. 3, 5, 4′-trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of pi3k/akt and wnt/β-catenin signaling cascades and reversal of epithelial–mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Quax, W.J.; Kayser, O. Seasonal Variations in the Deoxypodophyllotoxin Content and Yield of Anthriscus sylvestris L. (Hoffm.) Grown in the Field and under Controlled Conditions. J. Agric. Food Chem. 2011, 59, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Alli, P.M.; Pinn, M.L.; Jaffee, E.M.; McFadden, J.M.; Kuhajda, F.P. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 2004, 24, 39–46. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Hubbard, R.A.; Miglioretti, D.L.; Geller, B.M.; Yankaskas, B.C.; Lehman, C.D.; Taplin, S.H.; Sickles, E.A.; Consortium, B.C.S. Comparative effectiveness of digital versus film-screen mammography in community practice in the united states: A cohort study. Ann. Intern. Med. 2011, 155, 493–502. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Dhankhar, R.; Vyas, S.P.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K. Advances in Novel Drug Delivery Strategies for Breast Cancer Therapy. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 230–249. [Google Scholar] [CrossRef]
- Moo, T.-A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-term effects of breast cancer surgery, treatment, and survivor care. J. Midwifery Women’s Health 2019, 64, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of Care and Promising New Agents for Triple Negative Metastatic Breast Cancer. Cancers 2014, 6, 2187–2223. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.; Akcakanat, A.; Liu, S.; Green, M.; Murray, J.; Chen, H.; Palla, S.; Koenig, K.; Brewster, A.; Valero, V.; et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancer. Ann. Oncol. 2014, 25, 1122–1127. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.; Sakko, A.J. The magnitude of androgen receptor positivity in breast cancer is critical for reliable prediction of disease outcomear robustly predicts outcome in breast cancer. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef]
- Chia, K.M.; Liu, J.; Francis, G.D.; Naderi, A. A Feedback Loop between Androgen Receptor and ERK Signaling in Estrogen Receptor-Negative Breast Cancer. Neoplasia 2011, 13, 154–166. [Google Scholar] [CrossRef]
- Yard, B.D.; Adams, D.J.; Chie, E.K.; Tamayo, P.; Battaglia, J.S.; Gopal, P.; Rogacki, K.; Pearson, B.E.; Phillips, J.; Raymond, D.P.; et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat. Commun. 2016, 7, 11428. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 4490. [Google Scholar] [CrossRef]
- Burstein, H.J. The Distinctive Nature of HER2-Positive Breast Cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef]
- Ruiz-Saenz, A.; Dreyer, C.; Campbell, M.R.; Steri, V.; Gulizia, N.; Moasser, M.M. Her2 amplification in tumors activates pi3k/akt signaling independent of her3her2-amplified tumors overcome the requirement for her3. Cancer Res. 2018, 78, 3645–3658. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; De Marchi, U.; Zoratti, M.; Paradisi, C. Ester-Based Precursors to Increase the Bioavailability of Quercetin. J. Med. Chem. 2006, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Mechanism | Combination with | Phase | Refs. |
|---|---|---|---|---|
| BKM120 | PI3K inhibitor | - Lapatinib Trastuzumab/Paclitaxel Fulvestrant Tamoxifen LDE225 | Phase II Phase I/II Phase II Phase I Phase II Phase I | [58] [59] [60] [61] [62] [63] |
| Tenalisib (RP6530) Taselisib (GDC-0032) | PI3K δ/γ inhibitor PI3K inhibitor | - Enzalutamide | Phase II Phase I/II | [64] [65] |
| BYL-719 (alpelisib) | PI3K α inhibitor | - Letrozole Nab-paclitaxel | Phase II Phase I Phase I/II | [66] [67] [68] |
| Pictilisib (GDC-0941) | PI3K inhibitor | Cisplatin Paclitaxel (with and without Bevacizumab or Trastuzumab) and Letrozole | Phase I/II Phase I | [69] [70] |
| GDC-0084 | PI3K inhibitor | Trastuzumab | Phase II | [71] |
| PF-05212384 (gedatolisib) | PI3K/mTOR inhibitor | Docetaxel/Cisplatin/Dacomitinib Paclitaxel and carboplatin | Phase I Phase I | [72] [73] |
| AZD8186 | PI3K β Inhibitor | Docetaxel | Phase I | [74] |
| Serabelisib | PI3K α inhibitor | Canagliflozin | Phase I/II | [75] |
| Ipatasertib | AKT inhibitor | Trastuzumab and pertuzumab | Phase I | [76] |
| MK2206 | AKT inhibitor | Lapatinib ditosylate Paclitaxel - Anastrozole (with or without goserelin acetate) | Phase I Phase I Phase II Phase II | [77] [78] [79] [80] |
| Drugs | Sources | In Vitro | In Vivo | Dose | Treatment Time | Mechanism | Refs. |
|---|---|---|---|---|---|---|---|
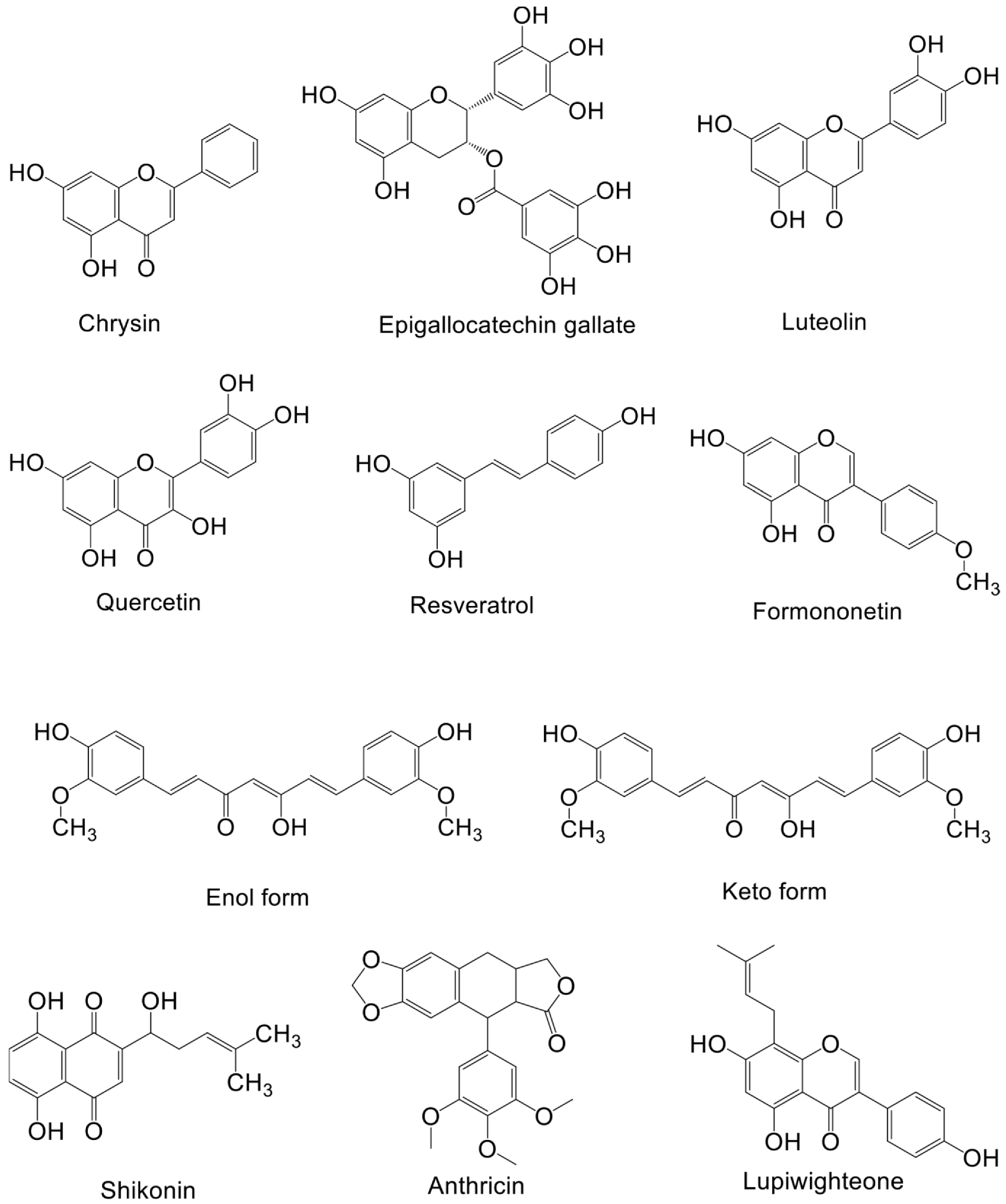
| Chrysin | Passiflora caerulea | MDA-MB-231 cell and BT-549 cell | - | 5, 10, 20 μM | 48 h | Inhibits p-AKT, vimentin and snail expression, inhibits metastasis | [121] |
| Epigallocatechin-3-gallate | Green tea | T47D cell and HFF cell | - | 20, 40, 80 μM | 72 h | Inhibits AKT and hTERT expression, induced apoptosis | [122] |
| Luteolin | Reseda luteola | MCF7-TamR cells | - | 20, 30 μM | 72 h | Inhibition of PI3K/AKT/mTOR, RAS expression, reverses tamoxifen-resistance in ER+ breast cancer cells | [123] |
| Quercetin | Capparis spinosa | MCF-7 cells and CD44+/CD24−CSCs | - | 50 μM | 24 h or 48 h | Inhibits PI3K/AKT/mTOR, promotes apoptosis, attenuates breast cancer stem cell cloning and mammary gland production | [124] |
| Resveratrol | Blueberries | MDA-MB-231, MDA-MB-453, MDA-MB-436, BT549 cells, | nude mice | 25, 50 μM cell and 40 mg/kg mice | 48 h or 72 h, 8 weeks | Inhibits PI3K/AKT/mTOR, decreases vimentin, slug, and MMP2, decreases cell viability and migration | [125] |
| Formononetin | Trifolium pratense | MCF-7 cells | nude mice | 40, 80 μM cell and 15, 30, 60 mg/kg/day | 48 h, 20 days | Inhibits cell proliferation, induces cell arrest in G0/G1 phase, reduce cyclin D1, p-IGF-1R, and p-AKT expression, inhibits local tumor growth in vivo | [126] |
| Curcumin | Curcuma longa | MDA-MB-231 | - | 25 μM | 3 h, 6 h, or 24 h | Reduces the content of AKT protein, accelerates AKT ubiquitination, and affects AKT aggregation, impairs cellular UPS function, participates in the activation of autophagy, Inhibits the growth and migration of breast cancer cells | [127] |
| Curcumin | Curcuma longa | MDA-MB-468 cells and HBL100 cells | - | 10 μM, 20 μM, 40 μM | 24 h or 48 h | Reduces the phosphorylation of AKT and EGFR, inhibits the activities of ERK1, ERK2, and JNK, induces cell arrest in S and G2/M phases, triggers apoptosis of breast cancer cells | [128] |
| Shikonin | Lithospermum erythrorhizon | MDA-MB-231 and BT549 cells | - | 1 μM, 5 μM | 24 h | Decreases the expression of p-Akt, downregulates miR-17-5p, inhibits TNBC cell migration, invasion, and EMT, miR-17-5p binds to the 3’UTR of PTEN to downregulate its expression | [129] |
| Anthricin | A. sylvestris (L.) Hoffm | MCF-7 cell and MDA-MB-231 cell | - | 25 μM, 50 μM | 12 h or 24 h | Decreases phosphorylation of Akt, p70S6K, and mTOR, induces cell arrest in the G2/M phase, increased protective autophagy and apoptosis | [130] |
| Lupiwighteone | Glycyrrhiza glabra | MCF-7 cell and MDA-MB-231 cell | - | 10 μM, 20 μM, 40 μM | 48 h | Inhibits PI3K/Akt/mTOR signaling pathway, inhibits cell proliferation, induces caspase-dependent cell death | [131] |
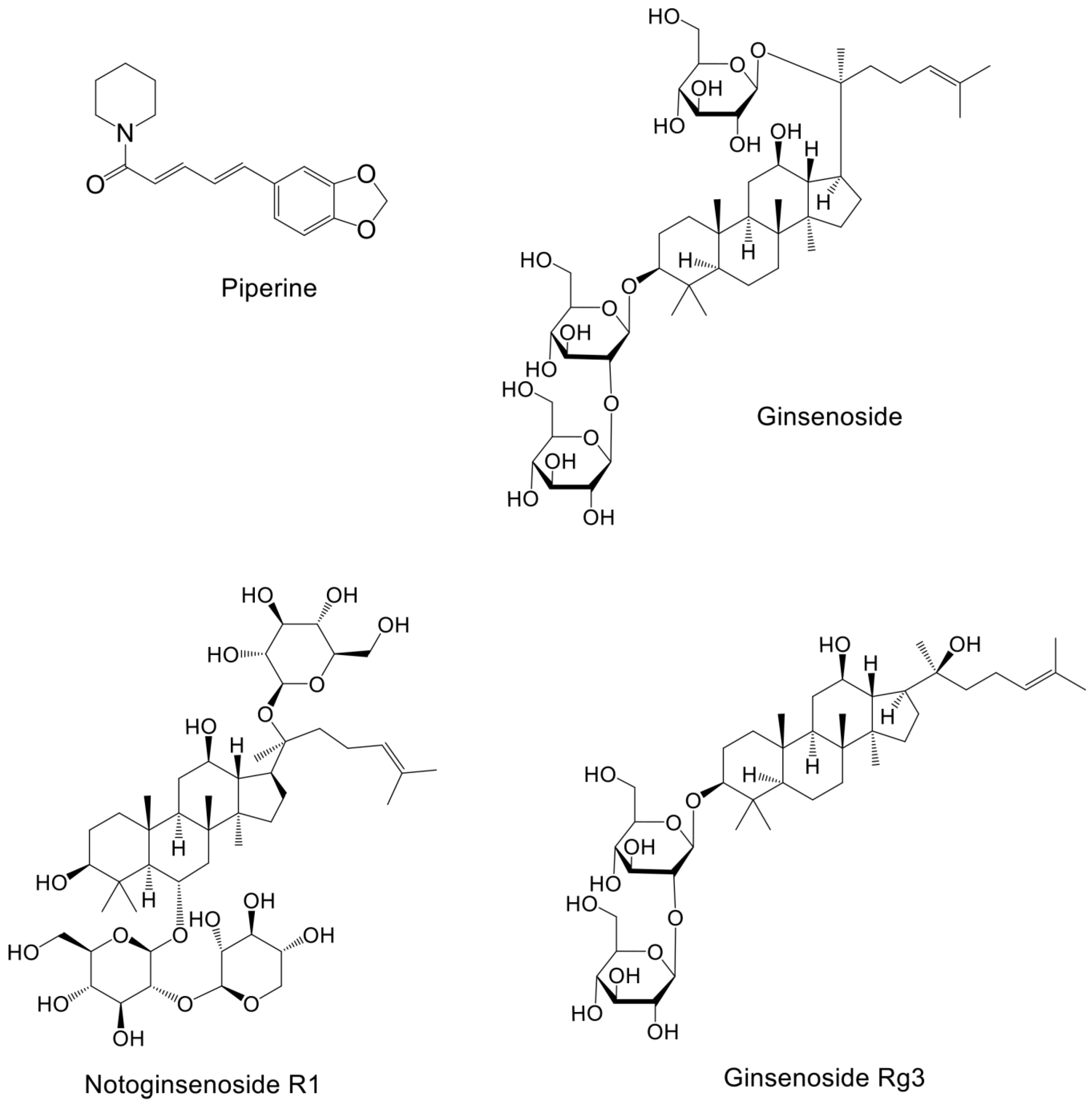
| Piperine | Piper nigrum Linn | SKBR3 cell and MCF-7 cells | - | 25 μM, 50 μM | 24 h or 48 h | Reduces the expression of p-Akt, p-p38, and mmp-9 induced by EGF, enhances the effect of paclitaxel on breast cancer cytotoxicity, downregulates the expression of SREBP-1, and FAS and HER2, inhibits the migration of breast cancer cells | [132] |
| Ginsenoside Rd | Panax ginseng | HUVECs cell and MDA-MB-231 cell, | xenograft mouse | 25 μM, 50 μM cell, 3 and 10 mg/kg/day mice | 48 h,28 day | Inhibit Akt/mTOR/p70S6K and HIF-1α activation, prevent VEGF-induced HUVECs migration, invasion, and formation of capillary-like structures, reduces VEGF-induced VEGFR2 activation in HUVECs | [133] |
| Ginsenoside Rg3 | Panax ginseng | MDA-MB-231 cell | - | 30 μM | 24 h | Inhibits the phosphorylation of ERK and Akt, reduces the transcriptional activity of NF-κB and the nuclear translocation of the p65 subunit, inhibits the degradation of IκBα and the catalytic activity of IKKβ, enhances the interaction between p53 and a negative regulator (Mdm2) | [134] |
| Notoginsenoside R1 | Panax notoginseng | MCF-7 cell and MDA-MB-231 cell | - | 75 μ, 150 uM | 24 h or 48 h | Reduces p-PI3K and p-AKT levels, downregulates the expression of CCND2 and YBX3, and increase the cells arrested in the G1 phase, decreases YBX3 and expression of KRAS, inhibit the proliferation, migration, invasion, and angiogenesis ability of breast cancer cells | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Long, H.; Zhou, Z.; Fu, Y.; Jiang, B. PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules 2023, 13, 93. https://doi.org/10.3390/biom13010093
Yuan Y, Long H, Zhou Z, Fu Y, Jiang B. PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules. 2023; 13(1):93. https://doi.org/10.3390/biom13010093
Chicago/Turabian StyleYuan, Yeqin, Huizhi Long, Ziwei Zhou, Yuting Fu, and Binyuan Jiang. 2023. "PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds" Biomolecules 13, no. 1: 93. https://doi.org/10.3390/biom13010093
APA StyleYuan, Y., Long, H., Zhou, Z., Fu, Y., & Jiang, B. (2023). PI3K–AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules, 13(1), 93. https://doi.org/10.3390/biom13010093





