LAM2: An Unusual Laminaran Structure for a Novel Plant Elicitor Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Structural Identification
2.3. Gene Expression Analysis
2.4. Resin Embedding and Immunofluorescence Labeling
2.5. Image Analysis and Statistical Test
3. Results
3.1. Structural Identification of LAM2 Isolated from L. hyperborea
3.2. Immunity Gene Expression Analysis
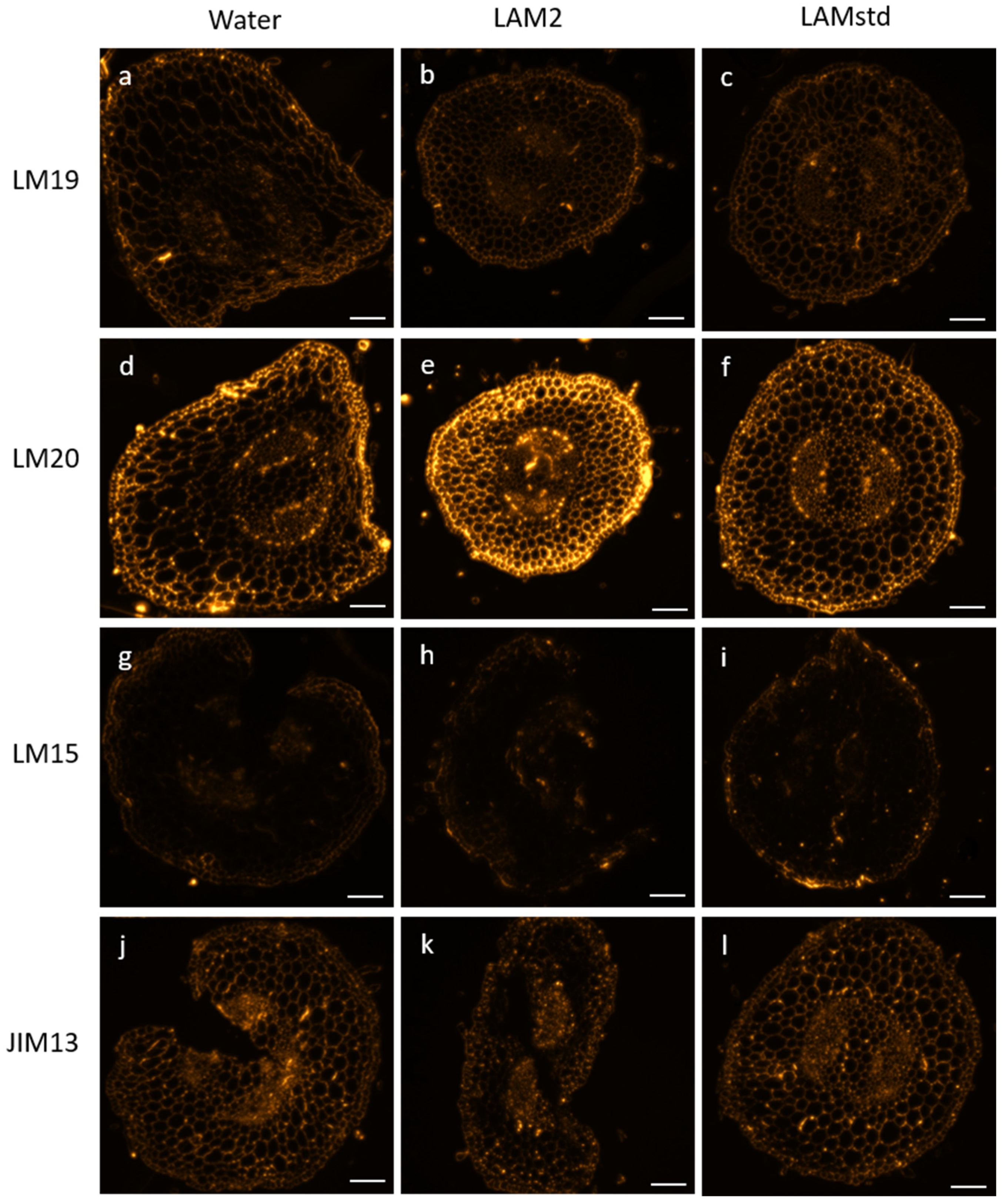
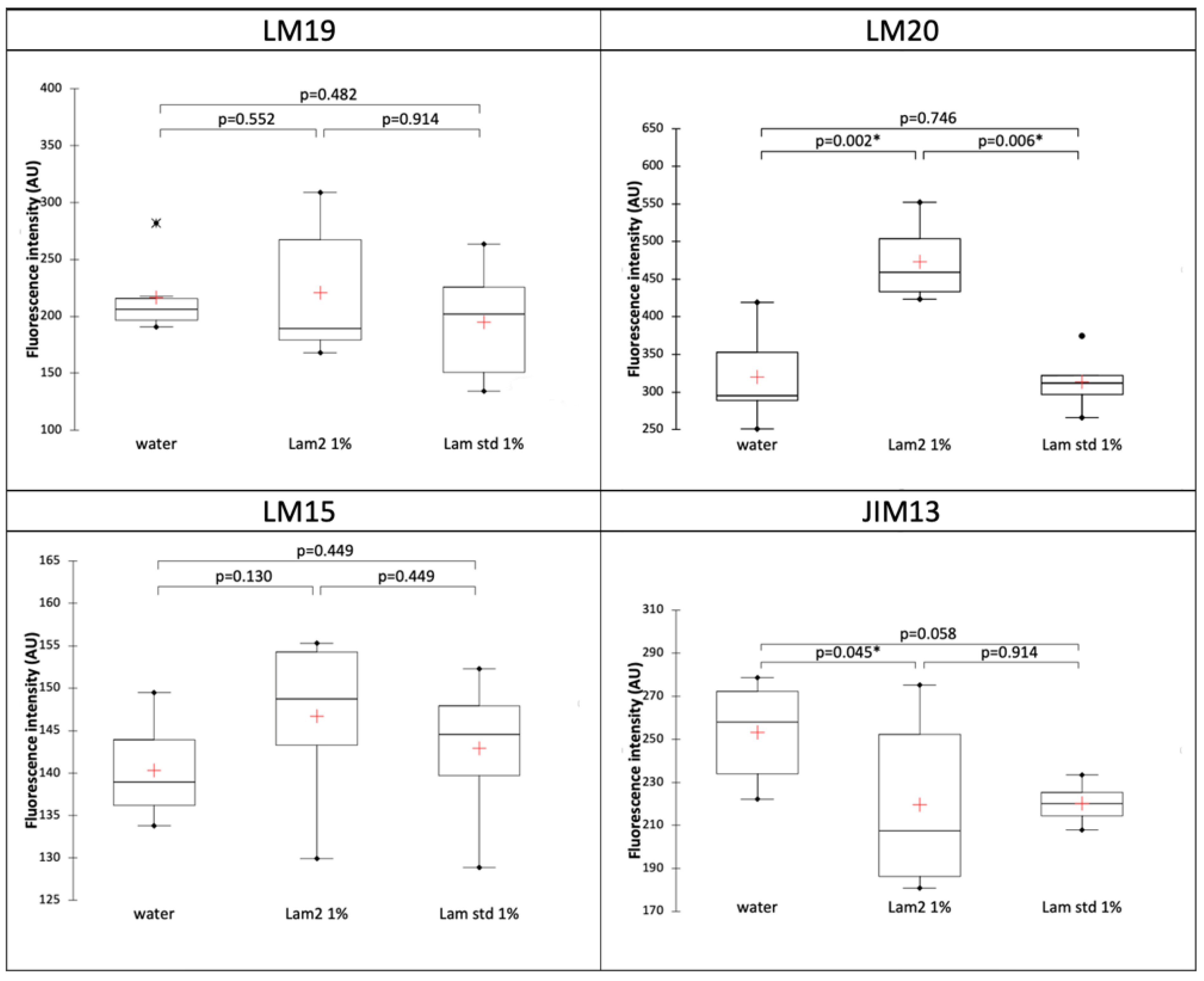
3.3. Immunocytochemistry
Characterization of Cell Wall Glycopolymers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Lane, C.E.; Mayes, C.; Druehl, L.D.; Saunders, G.W. A Multi-Gene Molecular Investigation of the Kelp (Laminariales, Phaeophyceae) Supports Substantial Taxonomic Re-Organization 1. J. Phycol. 2006, 42, 493–512. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The Effects of β-Glucan on Human Immune and Cancer Cells. J. Hematol. Oncol. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Yamaguchi, Y.; Hirano, S. Elicitor Actions of N-Acetylchitooligosaccharides and Laminarioligosaccharides for Chitinase and L-Phenylalanine Ammonia-Lyase Induction in Rice Suspension Culture. Biosci. Biotechnol. Biochem. 1997, 61, 975–978. [Google Scholar] [CrossRef]
- Ménard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.-C.; Kauffmann, S. Beta-1,3 Glucan Sulfate, but Not Beta-1,3 Glucan, Induces the Salicylic Acid Signaling Pathway in Tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 Glucans Are Elicitors of Defense Responses in Tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Cardinale, F.; Jonak, C.; Ligterink, W.; Niehaus, K.; Boller, T.; Hirt, H. Differential Activation of Four Specific MAPK Pathways by Distinct Elicitors. J. Biol. Chem. 2000, 275, 36734–36740. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis Cinerea and Plasmopara Viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Trouvelot, S.; Varnier, A.-L.; Allègre, M.; Mercier, L.; Baillieul, F.; Arnould, C.; Gianinazzi-Pearson, V.; Klarzynski, O.; Joubert, J.-M.; Pugin, A.; et al. A β-1,3 Glucan Sulfate Induces Resistance in Grapevine against Plasmopara Viticola Through Priming of Defense Responses, Including HR-like Cell Death. Mol. Plant-Microbe Interact. 2008, 21, 232–243. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A Disease Resistance Elicitor Laminarin Enhances Tea Defense against a Piercing Herbivore Empoasca (Matsumurasca) Onukii Matsuda. Sci. Rep. 2019, 9, 814. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown Seaweed Fucus Vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E.; Lewis, B.A. Separation and Characterization of the Soluble and Insoluble Components of Insoluble Laminaran. Carbohydr. Res. 1974, 33, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Reason, A.J.; Haslam, S.M.; McDowell, R.A.; Chizhov, O.S.; Usov, A.I. Structural Analysis of Laminarans by MALDI and FAB Mass Spectrometry. Carbohydr. Res. 1998, 310, 203–210. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Effect of Season on the Composition of Bioactive Polysaccharides from the Brown Seaweed Saccharina Longicruris. Phytochemistry 2009, 70, 1069–1075. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Structural Characterization of Laminaran and Galactofucan Extracted from the Brown Seaweed Saccharina Longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Roset, M.S.; Ciocchini, A.E.; Ugalde, R.A.; Iñón de Iannino, N. The Brucella Abortus Cyclic β-1,2-Glucan Virulence Factor Is Substituted with O-Ester-Linked Succinyl Residues. J. Bacteriol. 2006, 188, 5003–5013. [Google Scholar] [CrossRef] [PubMed]
- Bekkby, T.; Rinde, E.; Gundersen, H.; Norderhaug, K.; Gitmark, J.; Christie, H. Length, Strength and Water Flow: Relative Importance of Wave and Current Exposure on Morphology in Kelp Laminaria hyperborea. Mar. Ecol. Prog. Ser. 2014, 506, 61–70. [Google Scholar] [CrossRef]
- Liesner, D.; Shama, L.N.S.; Diehl, N.; Valentin, K.; Bartsch, I. Thermal Plasticity of the Kelp Laminaria digitata (Phaeophyceae) Across Life Cycle Stages Reveals the Importance of Cold Seasons for Marine Forests. Front. Mar. Sci. 2020, 7, 456. [Google Scholar] [CrossRef]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef]
- Rebaque, D.; del Hierro, I.; López, G.; Bacete, L.; Vilaplana, F.; Dallabernardina, P.; Pfrengle, F.; Jordá, L.; Sánchez-Vallet, A.; Pérez, R.; et al. Cell Wall-Derived Mixed-Linked β-1,3/1,4-Glucans Trigger Immune Responses and Disease Resistance in Plants. Plant J. 2021, 106, 601–615. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory Activities of Ascophyllum Nodosum Extract in Tomato and Sweet Pepper Crops in a Tropical Environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Cheong, J.J.; Birberg, W.; Fügedi, P.; Pilotti, A.; Garegg, P.J.; Hong, N.; Ogawa, T.; Hahn, M.G. Structure-Activity Relationships of Oligo-Beta-Glucoside Elicitors of Phytoalexin Accumulation in Soybean. Plant Cell 1991, 3, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yamada, A.; Hong, N.; Ogawa, T.; Ishii, T.; Shibuya, N. Differences in the Recognition of Glucan Elicitor Signals between Rice and Soybean: β-Glucan Fragments from the Rice Blast Disease Fungus Pyricularia Oryzae That Elicit Phytoalexin Biosynthesis in Suspension-Cultured Rice Cells. Plant Cell 2000, 12, 817–826. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, H.; Wang, W.; Wang, M.; Zhang, H.; Zhao, X.; Du, Y. β-1,3-Glucan with Different Degree of Polymerization Induced Different Defense Responses in Tobacco. Carbohydr. Polym. 2011, 86, 774–782. [Google Scholar] [CrossRef]
- Rupérez, P.; Gómez-Ordóñez, E.; Jiménez-Escrig, A. Biological Activity of Algal Sulfated and Nonsulfated Polysaccharides. In Bioactive Compounds from Marine Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 219–247. ISBN 978-1-118-41289-3. [Google Scholar]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Astha; Sekhon, P. S. Biochemical Basis of Systemic Acquired Resistance Induced by Different Systemic Acquired Resistance (SAR) Elicitors in Brassica Cultivars Challenge Inoculated with Downy Mildew Pathogen. J. Appl. Nat. Sci. 2021, 13, 301–307. [Google Scholar] [CrossRef]
- Wormit, A.; Usadel, B. The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef]
- Wydra, K.; Beri, H. Structural Changes of Homogalacturonan, Rhamnogalacturonan I and Arabinogalactan Protein in Xylem Cell Walls of Tomato Genotypes in Reaction to Ralstonia Solanacearum. Physiol. Mol. Plant Pathol. 2006, 68, 41–50. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl Esterification of Pectin Plays a Role during Plant–Pathogen Interactions and Affects Plant Resistance to Diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant Cell Wall-Mediated Immunity: Cell Wall Changes Trigger Disease Resistance Responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Swaminathan, S.; Lionetti, V.; Zabotina, O.A. Plant Cell Wall Integrity Perturbations and Priming for Defense. Plants 2022, 11, 3539. [Google Scholar] [CrossRef]
- Mareri, L.; Romi, M.; Cai, G. Arabinogalactan Proteins: Actors or Spectators during Abiotic and Biotic Stress in Plants? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2019, 153, 173–185. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An Extended Set of Monoclonal Antibodies to Pectic Homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic Homogalacturonan Masks Abundant Sets of Xyloglucan Epitopes in Plant Cell Walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally Regulated Epitopes of Cell Surface Arabinogalactan Proteins and Their Relation to Root Tissue Pattern Formation. Plant J. Cell Mol. Biol. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Valdor, J.F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of Carbohydrate Structural Features Recognized by Anti-Arabinogalactan-Protein Monoclonal Antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef]



| COOH | C1 | C3 | C5 | C2 | C4 | C6 | -CH2- | |
|---|---|---|---|---|---|---|---|---|
| 3-linked Glc | 102.53 | 84.15 | 75.58 | 73.25 | 68.08 | 60.66 | ||
| 3,6-linked Glc | 86.09 | |||||||
| 6-succinyl Glc | 102.53 | 84.15 | 74.36 | 70.79 | 69.21 | 63.17 | ||
| Succinate | 175.69 179.46 | 43.86 |
| LOX-D | PAL5 | PPO-D | Pti-5 | Worky28 | Worky70-80 | |
|---|---|---|---|---|---|---|
| LAM2 1%/water | 1.38 | 1.14 | 1.42 | 1.56 * | −1.68 * | −2.28 * |
| LAM std 1%/water | −1.00 | 1.16 | −1.06 | 1.14 | −2.32 * | −1.71 * |
| LAM2 1%/LAM std 1% | 1.38 | −1.02 | 1.50 * | 1.36 | −1.38 | −1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirande-Ney, C.; Arnaudin, Q.; Durambur, G.; Plasson, C.; Bernard, S.; Chamot, C.; Grivotte, J.; Mati-Baouche, N.; Driouich, A.; Brebion, J.; et al. LAM2: An Unusual Laminaran Structure for a Novel Plant Elicitor Candidate. Biomolecules 2023, 13, 1483. https://doi.org/10.3390/biom13101483
Mirande-Ney C, Arnaudin Q, Durambur G, Plasson C, Bernard S, Chamot C, Grivotte J, Mati-Baouche N, Driouich A, Brebion J, et al. LAM2: An Unusual Laminaran Structure for a Novel Plant Elicitor Candidate. Biomolecules. 2023; 13(10):1483. https://doi.org/10.3390/biom13101483
Chicago/Turabian StyleMirande-Ney, Cathleen, Quentin Arnaudin, Gaëlle Durambur, Carole Plasson, Sophie Bernard, Christophe Chamot, Julie Grivotte, Narimane Mati-Baouche, Azeddine Driouich, Jeremy Brebion, and et al. 2023. "LAM2: An Unusual Laminaran Structure for a Novel Plant Elicitor Candidate" Biomolecules 13, no. 10: 1483. https://doi.org/10.3390/biom13101483






