Factors Affecting the Bioproduction of Resveratrol by Grapevine Cell Cultures under Elicitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Elicitor Treatment
2.3. Determination of Stilbenoids
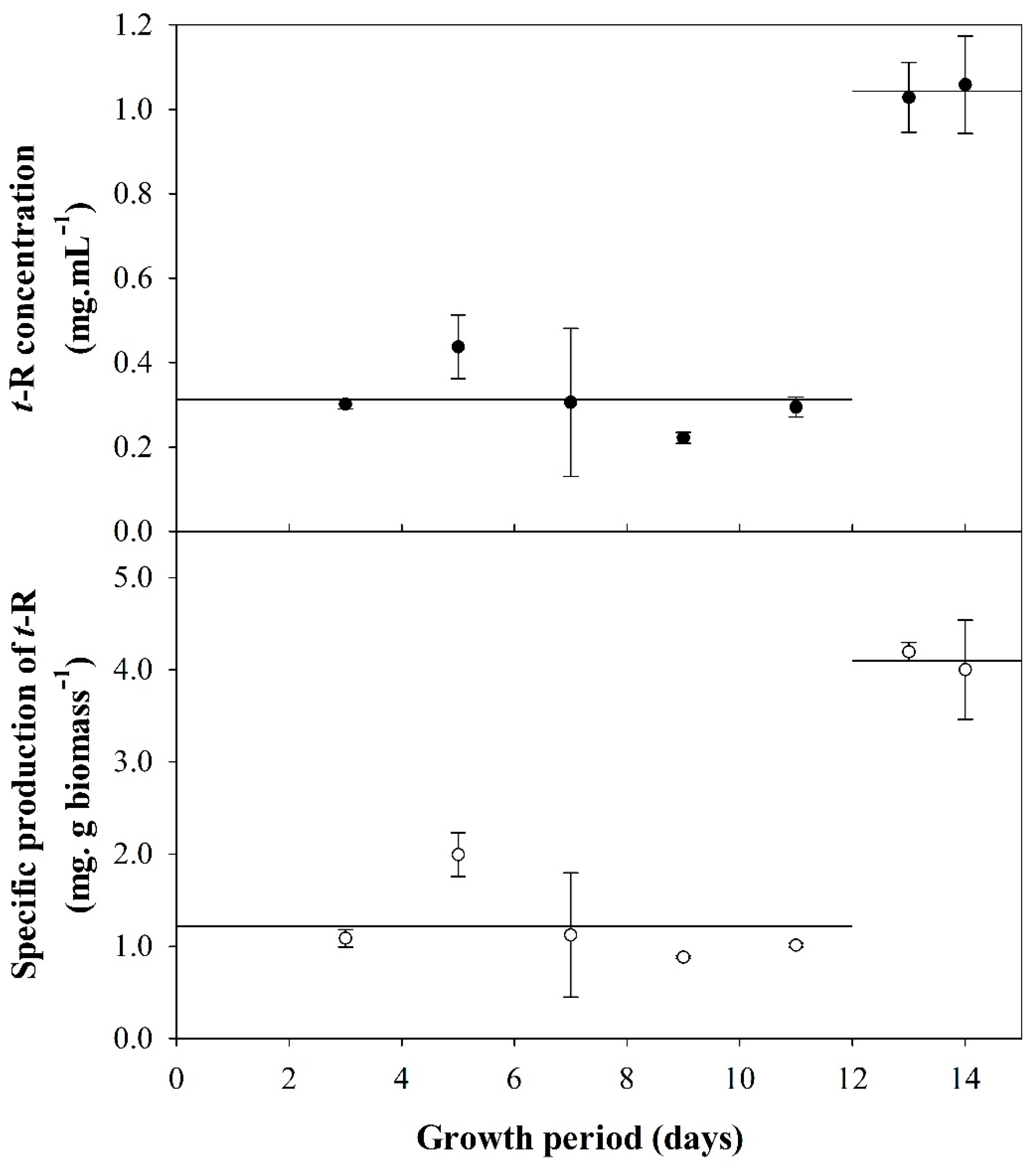
2.4. Effect of Growth Phase
2.5. Effect of DIMEB Concentration and Cell Density
2.6. Elicitor Mix Concentration Optimization
2.7. Effect of Order of Addition of Elicitors
2.8. Effect of Darkness and Ageing
2.9. Statistical Analysis
3. Results
3.1. Effect of the Cellular Physiological State (Growth Phase) on the Production of t-R by Elicitation with DIMEB
3.2. Effect of DIMEB Concentration and Cell Density on t-R Production by Elicitation with DIMEB
3.3. Elicitor Mix Concentration Optimization by Factorial Design 32
3.4. Effect of the Order of Addition of Elicitors to Cultures of Gamay Grapevine Cell Suspensions
3.5. Effect of Adaptation to the Darkness and Ageing of the Cell Suspension
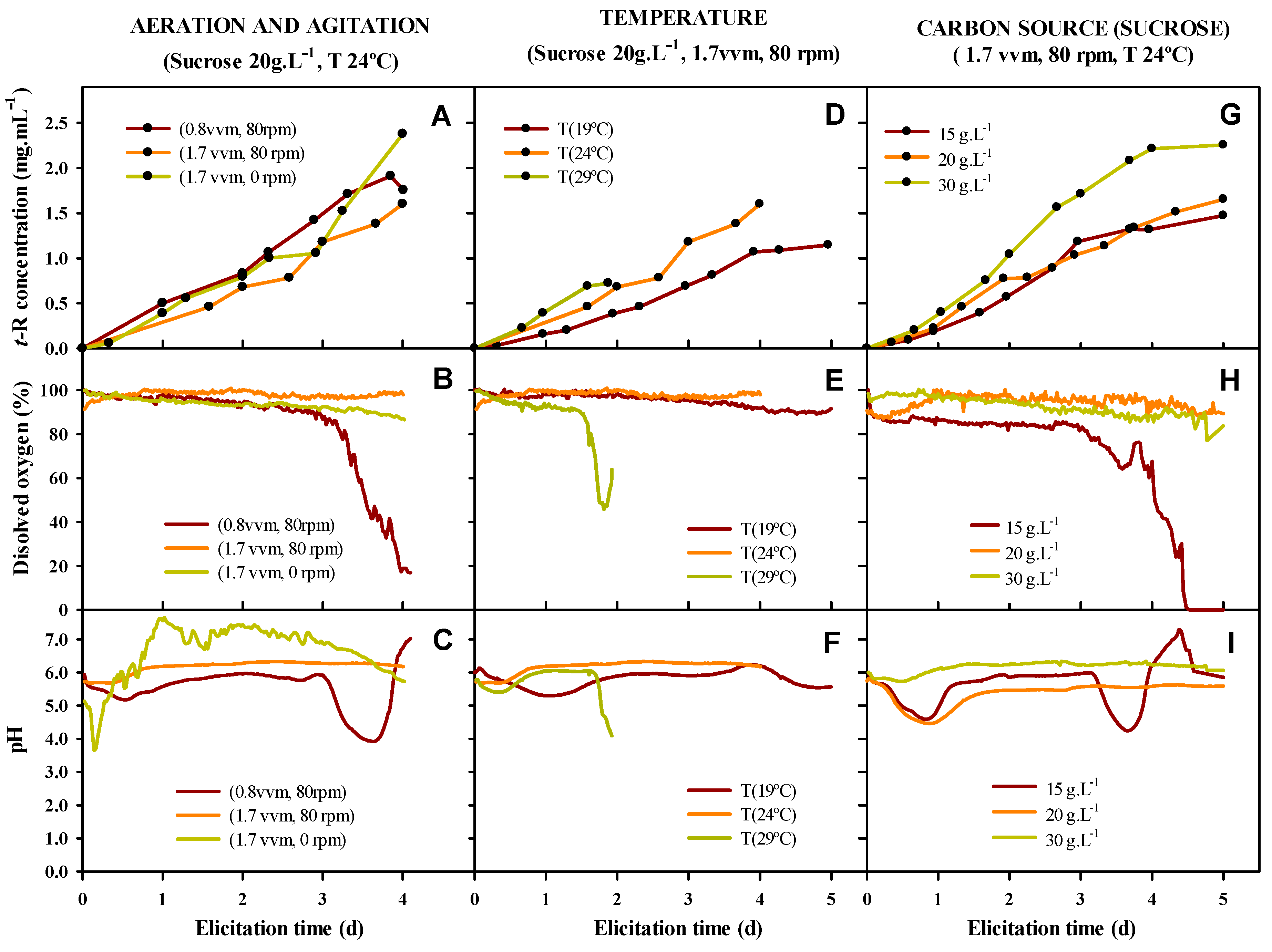
3.6. Factors Affecting t-R Bioproduction in Bioreactor
4. Discussion
4.1. Effect of Factors Related to Cell Physiology, Growth Phase, and Aging, on t-R Bioproduction
4.2. Effect of Factors Related to Elicitation Handling: Biomass Density and Elicitors Concentration, on t-R Bioproduction
4.3. Effect of the Order of Addition of Elicitors on t-R Bioproduction
4.4. Effect of Darkness on t-R Bioproduction
4.5. Scale-Up to 2 L Bioreactor for t-R Bioproduction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langcake, P.; Pryce, R.J. The Production of Resveratrol and the Viniferins by Grapevines in Response to Ultraviolet Irradiation. Phytochemistry 1977, 16, 1193–1196. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Douillet-Breuil, A.-C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Stilbene Derivatives by Plant Cells and Microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Fregoni, M.; Trevisan, M.; Mattivi, F.; Vrhovsek, U.; Falchetti, R. The Occurrence of the Stilbene Piceatannol in Grapes. Vitis J. Grapevine Res. 2002, 41, 133–136. [Google Scholar]
- Bavaresco, L.; Fregoni, C.; van Zeller de Macedo Basto Gonçalves, M.I.; Vezzulli, S. Physiology & Molecular Biology of Grapevine Stilbenes: An Update. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 341–364. ISBN 978-90-481-2305-6. [Google Scholar]
- Liswidowati; Melchior, F.; Hohmann, F.; Schwer, B.; Kindl, H. Induction of Stilbene Synthase by Botrytis Cinerea in Cultured Grapevine Cells. Planta 1991, 183, 307–314. [Google Scholar] [CrossRef]
- Tassoni, A.; Fornalè, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B.; Bagni, N. Jasmonates and Na-Orthovanadate Promote Resveratrol Production in Vitis vinifera cv. Barbera Cell Cultures. New Phytol. 2005, 166, 895–905. [Google Scholar] [CrossRef]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.-F.; Mérillon, J.-M. Methyl Jasmonate Induces Defense Responses in Grapevine and Triggers Protection against Erysiphe Necator. J. Agric. Food Chem. 2006, 54, 9119–9125. [Google Scholar] [CrossRef]
- Ferri, M.; Tassoni, A.; Franceschetti, M.; Righetti, L.; Naldrett, M.J.; Bagni, N. Chitosan Treatment Induces Changes of Protein Expression Profile and Stilbene Distribution in Vitis vinifera Cell Suspensions. Proteomics 2009, 9, 610–624. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, W.; Deng, M. Hyper-Production of 13C-Labeled Trans-Resveratrol in Vitis vinifera Suspension Cell Culture by Elicitation and In Situ Adsorption. Biochem. Eng. J. 2011, 53, 292–296. [Google Scholar] [CrossRef]
- Morales, M.; Bru, R.; García-Carmona, F.; Ros Barceló, A.; Pedreño, M.A. Effect of Dimethyl-β-Cyclodextrins on Resveratrol Metabolism in Gamay Grapevine Cell Cultures before and after Inoculation with Shape Xylophilus Ampelinus. Plant Cell Tissue Organ Cult. 1998, 53, 179–187. [Google Scholar] [CrossRef]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified Cyclodextrins Are Chemically Defined Glucan Inducers of Defense Responses in Grapevine Cell Cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, A.; Vrhovsek, U.; Kassemeyer, H.-H.; Mattivi, F.; Velasco, R. Elicitor-Induced Resveratrol Production in Cell Cultures of Different Grape Genotypes (Vitis spp.). Vitis-Geilweilerhof 2006, 45, 63–68. [Google Scholar]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedrẽo, M.A. Synergistic Effect of Methyljasmonate and Cyclodextrin on Stilbene Biosynthesis Pathway Gene Expression and Resveratrol Production in Monastrell Grapevine Cell Cultures. BMC Res. Notes 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Martinez-Esteso, M.J.; Sellés-Marchart, S.; Vera-Urbina, J.C.; Pedreño, M.A.; Bru-Martinez, R. Changes of Defense Proteins in the Extracellular Proteome of Grapevine (Vitis vinifera cv. Gamay) Cell Cultures in Response to Elicitors. J. Proteom. 2009, 73, 331–341. [Google Scholar] [CrossRef]
- Martinez-Esteso, M.J.; Sellés-Marchart, S.; Vera-Urbina, J.C.; Pedreño, M.A.; Bru-Martinez, R. DIGE Analysis of Proteome Changes Accompanying Large Resveratrol Production by Grapevine (Vitis vinifera cv. Gamay) Cell Cultures in Response to Methyl-β-Cyclodextrin and Methyl Jasmonate Elicitors. J. Proteom. 2011, 74, 1421–1436. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced Extracellular Production of Trans-Resveratrol in Vitis vinifera Suspension Cultured Cells by Using Cyclodextrins and Methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Early Signaling Events in Grapevine Cells Elicited with Cyclodextrins and Methyl Jasmonate. Plant Physiol. Biochem. 2013, 62, 107–110. [Google Scholar] [CrossRef]
- Almagro, L.; Carbonell-Bejerano, P.; Belchí-Navarro, S.; Bru, R.; Martínez-Zapater, J.M.; Lijavetzky, D.; Pedreño, M.A. Dissecting the Transcriptional Response to Elicitors in Vitis vinifera Cells. PLoS ONE 2014, 9, e109777. [Google Scholar] [CrossRef]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Márquez, A.; Bru, R.; Pedreño, M.A. Enhanced Extracellular Production of Trans-Resveratrol in Vitis vinifera Suspension Cultured Cells by Using Cyclodextrins and Coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef]
- Mueller, M.J. Enzymes Involved in Jasmonic Acid Biosynthesis. Physiol. Plant 1997, 100, 653–663. [Google Scholar] [CrossRef]
- Gundlach, H.; Müller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic Acid Is a Signal Transducer in Elicitor-Induced Plant Cell Cultures. Proc. Natl. Acad. Sci. USA 1992, 15, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- Menke, F.L.; Champion, A.; Kijne, J.W.; Memelink, J.A. Novel Jasmonate- and Elicitor-Responsive Element in the Periwinkle Secondary Metabolite Biosynthetic Gene Str Interacts with a Jasmonate- and Elicitor-Inducible AP2-Domain Transcription Factor, ORCA2. EMBO J. 1999, 18, 4455–4463. [Google Scholar] [CrossRef] [PubMed]
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl Jasmonate Is a Potent Elicitor of Multiple Defense Responses in Grapevine Leaves and Cell-Suspension Cultures. Biol. Plant 2004, 48, 273–283. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.-M. Effect of Methyl Jasmonate in Combination with Carbohydrates on Gene Expression of PR Proteins, Stilbene and Anthocyanin Accumulation in Grapevine Cell Cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef]
- Krisa, S.; Larronde, F.; Budzinski, H.; Decendit, A.; Deffieux, G.; Mérillon, J.-M. Stilbene Production by Vitis vinifera Cell Suspension Cultures: Methyl Jasmonate Induction and 13C Biolabeling. J. Nat. Prod. 1999, 62, 1688–1690. [Google Scholar] [CrossRef]
- Cardillo, A.B.; Perassolo, M.; Giulietti, A.M.; Rodriguez, J. Cyclodextrins: A tool in plant cell and organ culture bioprocesses for the production of secondary metabolites. Plant Cell Tissue Organ Cult. 2021, 146, 1–19. [Google Scholar] [CrossRef]
- Ferri, M.; Dipalo, S.C.F.; Bagni, N.; Tassoni, A. Chitosan Elicits Mono-Glucosylated Stilbene Production and Release in Fed-Batch Bioreactor Cultures of Grape Cells. Food Chem. 2011, 124, 1473–1479. [Google Scholar] [CrossRef]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Viniferins by an Elicited Grapevine Cell Culture in a 2L Stirred Bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Vera Urbina, J.C. Producción de Resveratrol Mediante Cultivos de Células Vegetales de Vid (Vitis vinifera L.Cv. Gamay): Escalado y Diseño de Biorreactores; Universidad de Alicante: Alicante, Spain, 2012. [Google Scholar]
- Vera-Urbina, J.C.; Sellés-Marchart, S.; Martínez-Esteso, M.J.; Pedreño, M.; Bru, R. Production of Grapevine Cell Biomass (Vitis vinifera L. Gamay) and Resveratrol in Custom Commercial Bioreactors Using Cyclodextrins Methyl-Jasmonate Elicitors. In Resveratrol: Sources, Production and Health Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 19–39. [Google Scholar]
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor. Plants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Morel, G. Le Problème de La Transformation Tumorale Chez Les Végétaux. Physiol. VÉG. 1970, 8, 189–191. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; pp. 478–479. [Google Scholar]
- Pan, Z.-W.; Wang, H.-Q.; Zhong, J.-J. Scale-up Study on Suspension Cultures of Taxus Chinensis Cells for Production of Taxane Diterpene. Enzym. Microb. Technol. 2000, 27, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Paiva, N.L. Plant Cell Culture. In Manual of Industrial Microbiology and Biotechnology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 196–211. ISBN 9781683671282. [Google Scholar]
- Lu, M.; Wong, H.; Teng, W. Effects of Elicitation on the Production of Saponin in Cell Culture of Panax Ginseng. Plant Cell Rep. 2001, 20, 674–677. [Google Scholar] [CrossRef]
- Lamboursain, L.; Jolicoeur, M. Critical Influence of Eschscholzia Californica Cells Nutritional State on Secondary Metabolite Production. Biotechnol. Bioeng. 2005, 91, 827–837. [Google Scholar] [CrossRef]
- Smith, B.A.; Reider, M.L.; Fletcher, J.S. Relationship between Vital Staining and Subculture Growth during the Senescence of Plant Tissue Cultures. Plant Physiol. 1982, 70, 1228–1230. [Google Scholar] [CrossRef]
- Fu, C.; Li, L.; Wu, W.; Li, M.; Yu, X.; Yu, L. Assessment of Genetic and Epigenetic Variation during Long-Term Taxus Cell Culture. Plant Cell Rep. 2012, 31, 1321–1331. [Google Scholar] [CrossRef]
- Kiselev, K.; Dubrovina, A.; Shumakova, O. DNA Mutagenesis in 2- and 20-Yr-Old Panax Ginseng Cell Cultures. Vitr. Cell. Dev. Biol.-Plant 2012, 49, 175–182. [Google Scholar] [CrossRef]
- Wei, L.-R.; Qin, W.-Y.; Li, Y.-C. Effects of Demethylating Reagent 5-Aza-2′-Deoxycytidine on the Growth and Cephalotaxine Production in Cephalotaxus Mannii Suspension Cells. Plant Cell Tissue Organ Cult. PCTOC 2019, 139, 359–368. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, Y.; Nie, L.; Lu, M.; Fu, C.; Yu, L. High-Throughput Sequencing Reveals MiRNA Effects on the Primary and Secondary Production Properties in Long-Term Subcultured Taxus Cells. Front. Plant Sci. 2015, 6, 604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Wang, L. An Evolutionary View of Plant Tissue Culture: Somaclonal Variation and Selection. Plant Cell Rep. 2012, 31, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V.; Tyunin, A.P.; Manyakhin, A.Y.; Zhuravlev, Y.N. Resveratrol Content and Expression Patterns of Stilbene Synthase Genes in Vitis amurensis Cells Treated with 5-Azacytidine. Plant Cell Tissue Organ Cult. PCTOC 2011, 105, 65–72. [Google Scholar] [CrossRef]
- Perassolo, M.; Smith, M.E.; Giulietti, A.M.; Rodriguez Talou, J. Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tissue Organ Cult. 2016, 124, 319–330. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, Y.-J.; Devi, B.S.R.; Oh, J.Y.; Kim, S.-Y.; Kwon, W.-S.; Yang, D.-C. Sodium Nitroprusside Enhances the Elicitation Power of Methyl Jasmonate for Ginsenoside Production in Panax Ginseng Roots. Res. Chem. Intermed. 2016, 42, 2937–2951. [Google Scholar] [CrossRef]
- Sukito, A.; Tachibana, S. Effect of Methyl Jasmonate and Salycilic Acid Synergism on Enhancement of Bilobalide and Ginkgolide Production by Immobilized Cell Cultures of Ginkgo Biloba. Bioresour. Bioprocess. 2016, 3, 24. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazón, J.; Pedreño, M.A.; Cusidó, R.M. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping Methyl Jasmonate-Mediated Transcriptional Reprogramming of Metabolism and Cell Cycle Progression in Cultured Arabidopsis Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–666. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009, 21, 2220–2236. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Fischer, S.; Merz, P.; Bogs, J.; Riemann, M.; Nick, P. An ancestral allele of grapevine transcription factor MYB14 promotes plant defence. J. Exp. Bot. 2016, 67, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Höll, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell. 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, A.; Wong, D.C.J.; Höll, J.; Hmmam, I.; Matus, J.T.; Bogs, J.; Ziegler, T.; Dry, I.; Barcaccia, G.; Lucchin, M. Combinatorial Regulation of Stilbene Synthase Genes by WRKY and MYB Transcription Factors in Grapevine (Vitis vinifera L.). Plant Cell Physiol. 2018, 59, 1043–1059. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Mérillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Hurtado-Gaitán, E.; Sellés-Marchart, S.; Hartwell, J.; Martínez-Esteso, M.J.; Bru-Martínez, R. Down-Regulation of Phosphoenolpyruvate Carboxylase Kinase in Grapevine Cell Cultures and Leaves Is Linked to Enhanced Resveratrol Biosynthesis. Biomolecules 2021, 11, 1641. [Google Scholar] [CrossRef]
- Jiang, J.; Xi, H.; Dai, Z.; Lecourieux, F.; Yuan, L.; Liu, X.; Patra, B.; Wei, Y.; Li, S.; Wang, L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J. Exp. Bot. 2019, 70, 715–729. [Google Scholar] [CrossRef]
- Mu, H.; Li, Y.; Yuan, L.; Jiang, J.; Wei, Y.; Duan, W.; Fan, P.; Li, S.; Liang, Z.; Wang, L. MYB30 and MYB14 form a repressor-activator module with WRKY8 that controls stilbene biosynthesis in grapevine. Plant Cell 2023, 35, 552–573. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Martínez-Esteso, M.J.; Vilella-Antón, M.T.; Sellés-Marchart, S.; Morante-Carriel, J.A.; Hurtado, E.; Palazon, J.; Bru-Martínez, R. A Tau Class Glutathione-S-Transferase is Involved in Trans-Resveratrol Transport out of Grapevine Cells. Front. Plant Sci. 2017, 8, 1457. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Mahadevappa, P.; Giridhar, P. Influence of Photoperiod on Growth, Bioactive Compounds and Antioxidant Activity in Callus Cultures of Basella rubra L. J. Photochem. Photobiol. B 2020, 209, 111937. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.M.; Rushing, J.W.; Rajapakse, N.C.; Thomas, R.L.; Riley, M.B. Potential Implications of Medicinal Plant Production in Controlled Environments: The Case of Feverfew (Tanacetum Parthenium). HortScience 2006, 41, 531–535. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Simic, S.G. Phenolic Profile of Dark-Grown and Photoperiod-Exposed Hypericum perforatum L. Hairy Root Cultures. Sci. World J. 2013, 2013, 602752. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (Cell) Factories for Advanced Production of Plant Secondary Metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef] [PubMed]




| Age I | Age II | |||||||
|---|---|---|---|---|---|---|---|---|
| Cycle | Concentration of t-R (mg·mL−1) | Specific Production of t-R (mg·g biomass−1) | Concentration of t-R (mg·mL−1) | Specific Production of t-R (mg·g biomass−1) | ||||
| Photoperiod | Darkness | Photoperiod | Darkness | Photoperiod | Darkness | Photoperiod | Darkness | |
| 1 | 3.05 ± 0.09 | 3.16 ± 0.16 | 9.75 ± 0.10 | 10.67 ± 0.32 | 3.02 ± 0.05 | 4.24 ± 0.02 | 9.28 ± 0.09 | 12.08 ± 0.05 |
| 2 | 3.38 ± 0.03 | 4.45 ± 0.12 | 9.70 ± 0.04 | 13.33 ± 0.14 | 1.66 ± 0.02 | 2.90 ± 0.03 | 5.57 ± 0.04 | 9.32 ± 0.04 |
| 3 | 3.72 ± 0.08 | 4.72 ± 0.17 | 10.99 ± 0.21 | 14.75 ± 0.18 | 2.05 ± 0.03 | 2.67 ± 0.10 | 6.63 ± 0.08 | 8.48 ± 0.31 |
| 4 | 3.23 ± 0.03 | 4.81 ± 0.11 | 9.69 ± 0.10 | 14.34 ± 0.12 | 2.86 ± 0.03 | 3.65 ± 0.05 | 8.53 ± 0.04 | 10.61 ± 0.12 |
| 5 | 4.54 ± 0.14 | 4.54 ± 0.07 | 13.16 ± 0.41 | 14.17 ± 0.08 | 3.16 ± 0.01 | 3.46 ± 0.09 | 9.22 ± 0.03 | 10.16 ± 0.26 |
| 6 | 3.50 ± 0.09 | 3.16 ± 0.05 | 10.12 ± 0.11 | 9.80 ± 0.05 | 2.76 ± 0.06 | 2.95 ± 0.02 | 8.36 ± 0.14 | 8.98 ± 0.02 |
| 7 | 4.52 ± 0.03 | 5.22 ± 0.04 | 13.10 ± 0.02 | 15.59 ± 0.08 | 2.98 ± 0.06 | 3.12 ± 0.02 | 8.84 ± 0.06 | 9.19 ± 0.02 |
| 8 | 3.27 ± 0.03 | 4.64 ± 0.07 | 8.94 ± 0.08 | 13.86 ± 0.07 | 1.87 ± 0.01 | 2.76 ± 0.02 | 5.44 ± 0.01 | 7.94 ± 0.05 |
| Avg of 8 cycles | 3.65 ± 0.58 | 4.34 ± 0.76 | 10.68 ± 1.61 | 13.32 ± 2.02 | 2.54 ± 0.59 | 3.22 ± 0.53 | 7.73 ± 1.60 | 9.60 ± 1.32 |
| Trans-Resveratrol | Aeration (vvm)/ Agitation Speed (rpm) | Temperature (°C) | Sucrose (g·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (0.8/80) | (1.7/80) | (1.7/0) | 19 | 24 | 29 | 15 | 20 | 30 | |
| Concentration (mg·mL−1) | 1.90 | 1.60 | 2.42 | 1.15 | 1.60 | 0.70 | 1.31 | 1.65 | 2.26 |
| Specific production (mg·g biomass−1) | 5.7 | 5.6 | 7.1 | 3.7 | 5.6 | 2.2 | 4.9 | 5.3 | 7.3 |
| Total production (mg) | 1425.0 | 1408.0 | 1780.9 | 916.0 | 1408.0 | 553.0 | 1218.3 | 1336.5 | 1827.4 |
| Specific productivity a (mg·g biomass−1·day−1) | 1.43 | 1.41 | 1.78 | 0.9 | 1.41 | 1.1 | 0.97 | 1.07 | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Urbina, J.C.; Sellés-Marchart, S.; Martínez-Márquez, A.; Martínez-Esteso, M.J.; Pedreño, M.A.; Morante-Carriel, J.; Bru-Martínez, R. Factors Affecting the Bioproduction of Resveratrol by Grapevine Cell Cultures under Elicitation. Biomolecules 2023, 13, 1529. https://doi.org/10.3390/biom13101529
Vera-Urbina JC, Sellés-Marchart S, Martínez-Márquez A, Martínez-Esteso MJ, Pedreño MA, Morante-Carriel J, Bru-Martínez R. Factors Affecting the Bioproduction of Resveratrol by Grapevine Cell Cultures under Elicitation. Biomolecules. 2023; 13(10):1529. https://doi.org/10.3390/biom13101529
Chicago/Turabian StyleVera-Urbina, Juan Carlos, Susana Sellés-Marchart, Ascensión Martínez-Márquez, María José Martínez-Esteso, María Angeles Pedreño, Jaime Morante-Carriel, and Roque Bru-Martínez. 2023. "Factors Affecting the Bioproduction of Resveratrol by Grapevine Cell Cultures under Elicitation" Biomolecules 13, no. 10: 1529. https://doi.org/10.3390/biom13101529
APA StyleVera-Urbina, J. C., Sellés-Marchart, S., Martínez-Márquez, A., Martínez-Esteso, M. J., Pedreño, M. A., Morante-Carriel, J., & Bru-Martínez, R. (2023). Factors Affecting the Bioproduction of Resveratrol by Grapevine Cell Cultures under Elicitation. Biomolecules, 13(10), 1529. https://doi.org/10.3390/biom13101529








