Protein Concentration Affects the Food Allergen γ-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of γ-Conglutin Hydrolysate
2.2. Generation of Murine Dendritic Cells
2.3. Stimulation of DCs with Proteins, Bacteria, LPS, and Cytokine Production
2.4. Preparation of Labeled Proteins and Bacteria
2.5. Protein and Bacteria Uptake by DCs
2.6. Assessment of Protein Aggregation by Light Scattering
2.7. Measurement of ROS Formation
2.8. Statistical Analysis
3. Results
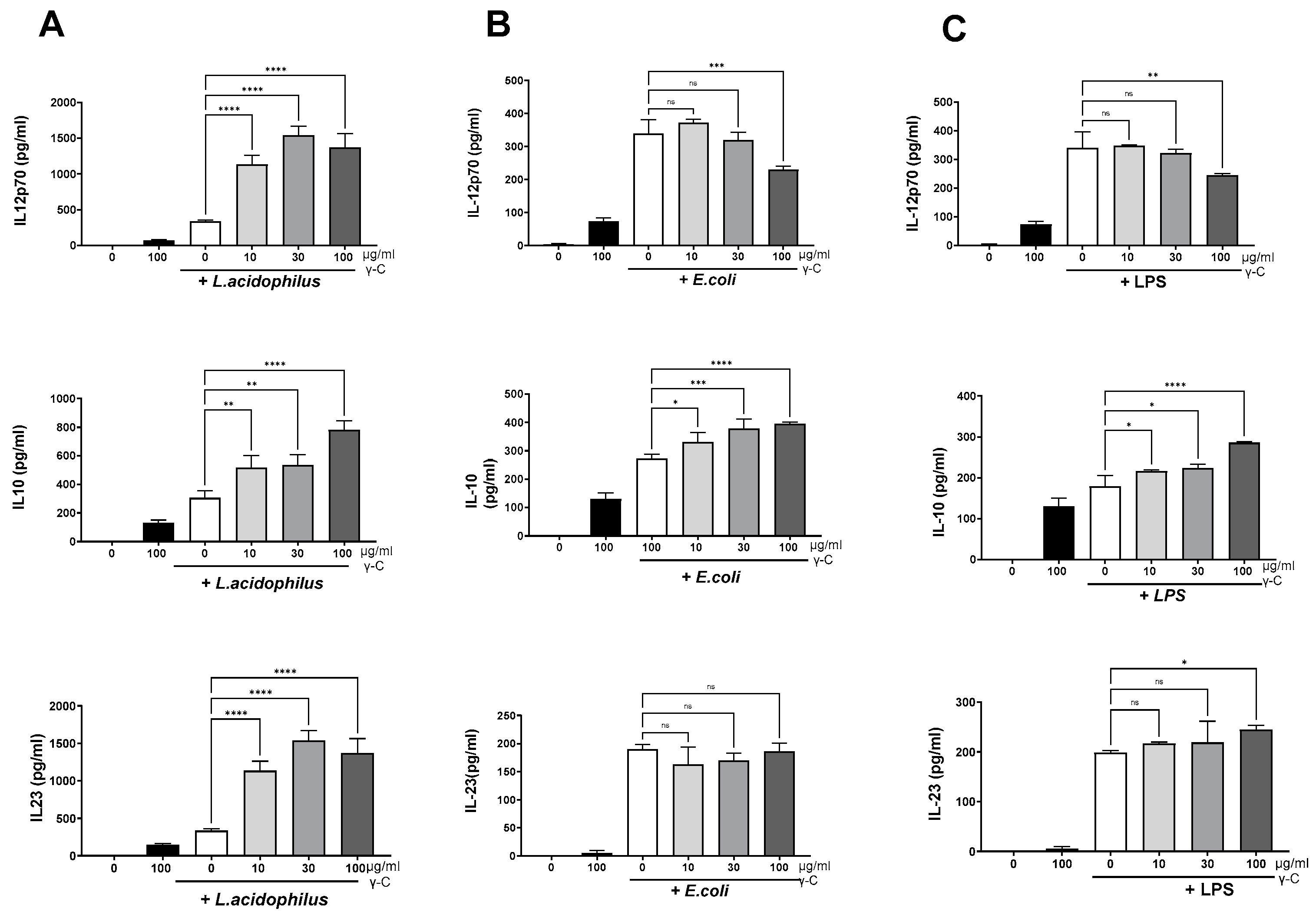
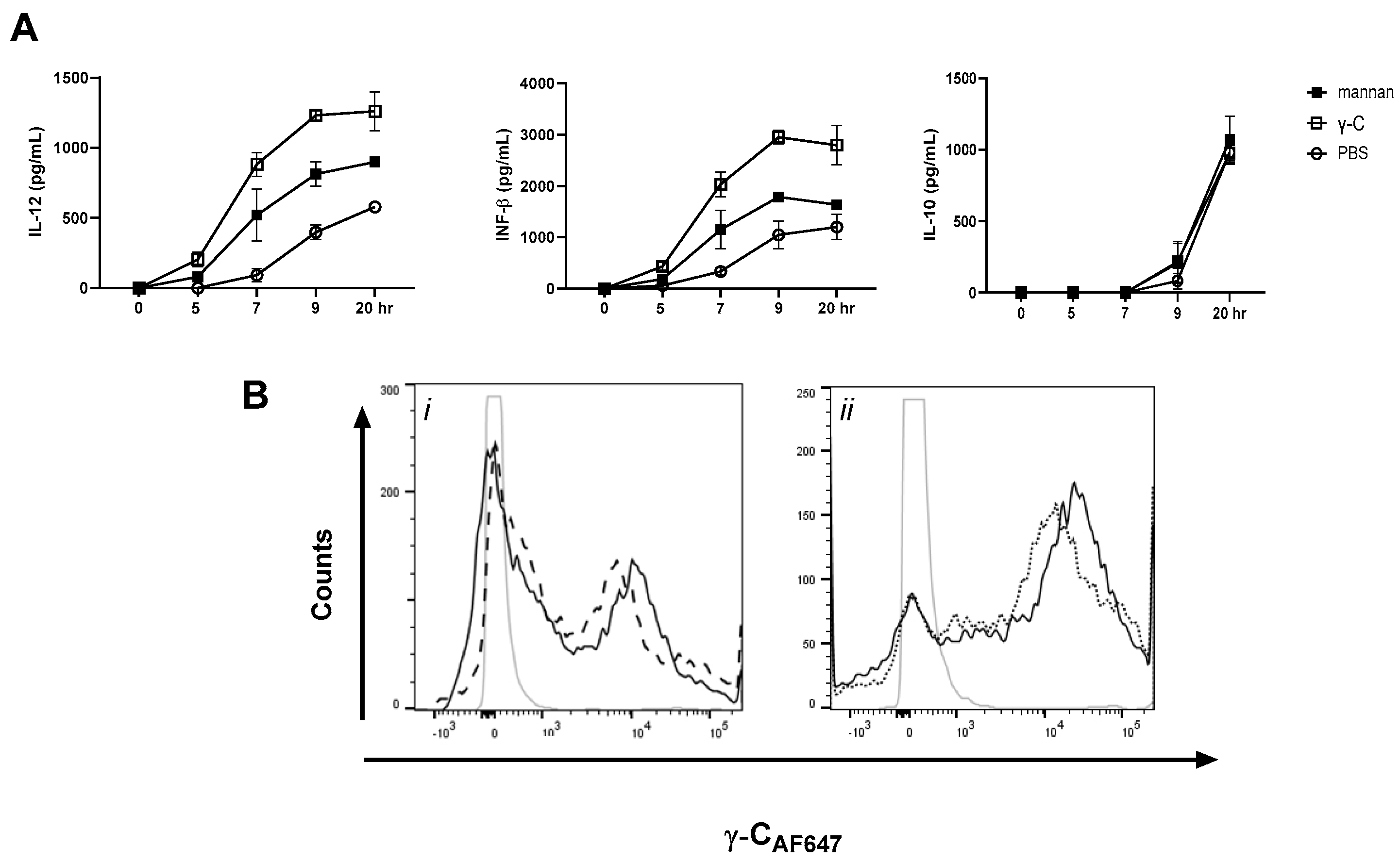
3.1. DCs Stimulated with E. coli Nissle 1917 or L. acidophilus NCFM Are Modulated Differently by γ-C
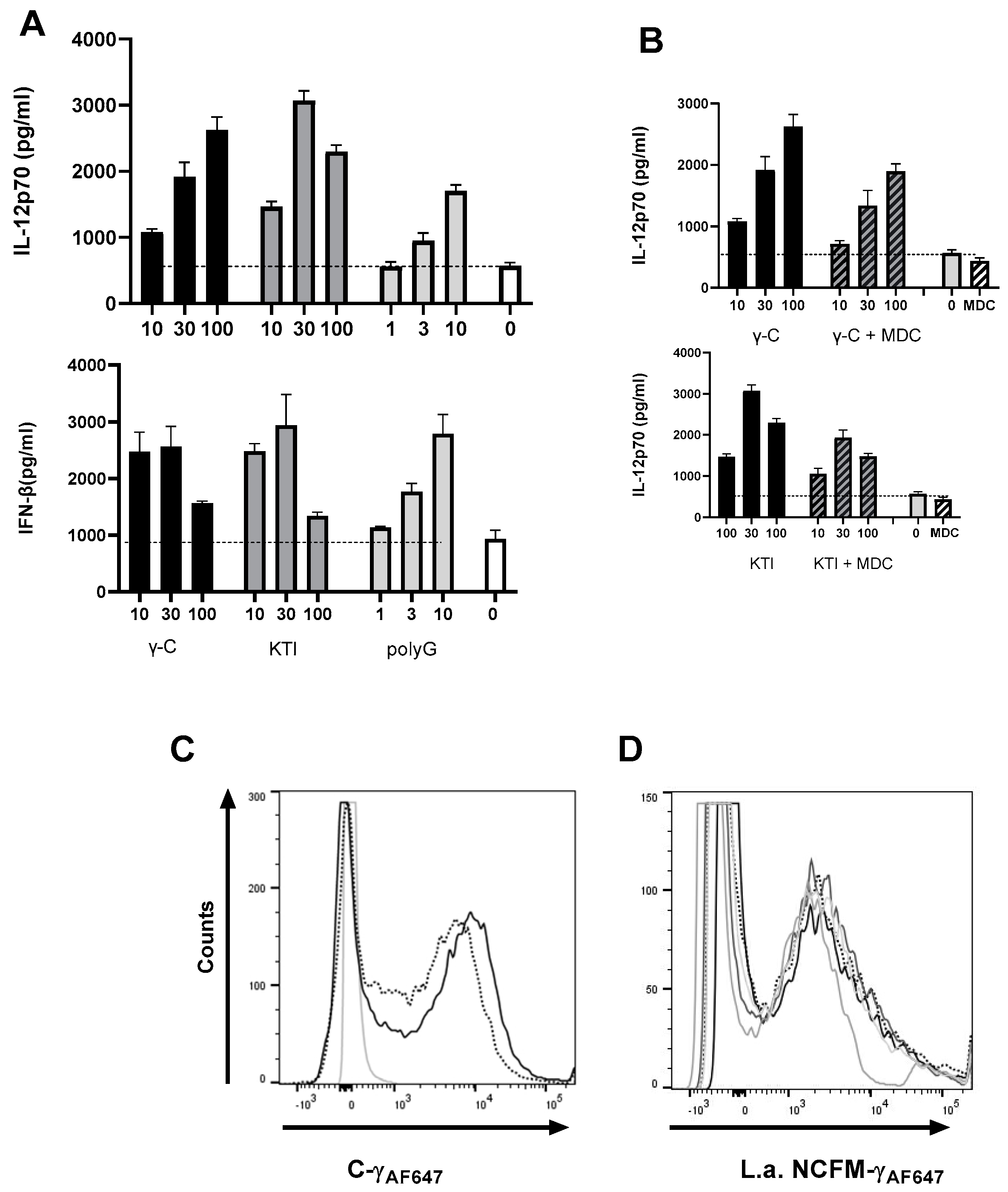
3.2. Both Intact and Degraded Protein Holds IL-12 Enhancing Capacity in L. acidophilus NCFM Stimulated DC
3.3. A Non-Glycosylated Protein Is as Potent as γ-C and Mannan in Enhancing L. acidophilus NCFM-Induced IL-12 and IFN-β
3.4. Addition of γ-C or KTI Induces ROS Production
3.5. In High Concentration, γ-C Is Endocytosed in Higher Concentrations Due to Aggregation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The Future of Lupin as a Protein Crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.A.B.M.; Nordlee, J.A.; Penninks, A.H.; Chen, L.; Goodman, R.E.; Bruijnzeel-Koomen, C.A.F.M.; Hefle, S.L.; Taylor, S.L.; Knulst, A.C. Lupine Allergy: Not Simply Cross-Reactivity with Peanut or Soy. J. Allergy Clin. Immunol. 2007, 120, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.A.B.M.; Koppelman, S.J.; Penninks, A.H.; Lebens, A.; Bruijnzeel-Koomen, C.A.F.M.; Hefle, S.L.; Taylor, S.L.; van Hoffen, E.; Knulst, A.C. Clinical Relevance of Sensitization to Lupine in Peanut-Sensitized Adults. Allergy 2009, 64, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Roberts, G.; Grimshaw, K.; White, S.; Hourihane, J. Short Communication: Lupin Allergy in Peanut-Allergic Children and Teenagers: Lupin Allergy in Peanut-Allergic Children. Allergy 2007, 63, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Dooper, M.; Plassen, C.; Holden, L.; Lindvik, H. Immunoglobulin E Cross-Reactivity Between Lupine Conglutins and Peanut Allergens in Serum of Lupine-Allergic Individuals. J. Investig. Allergol. Clin. Immunol. 2009, 19, 283–291. [Google Scholar]
- Hefle, S.L.; Lemanske, R.F.; Bush, R.K. Adverse Reaction to Lupine-Fortified Pasta. J. Allergy Clin. Immunol. 1994, 94, 167–172. [Google Scholar] [CrossRef]
- Matheu, V.; Manuel de Barrio, V.; Sierra, Z.; Gracia-Bara, M.T.; Tornero, P.; Baeza, M.L. Lupine-Induced Anaphylaxis. Ann. Allergy Asthma Immunol. 1999, 83, 406–408. [Google Scholar] [CrossRef]
- Radcliffe, M.; Scadding, G.; Brown, H.M. Lupin Flour Anaphylaxis. Lancet 2005, 365, 1360. [Google Scholar] [CrossRef]
- Soller, L.; La Vieille, S.; Chan, E.S. First Reported Case in Canada of Anaphylaxis to Lupine in a Child with Peanut Allergy. Allergy Asthma Clin. Immunol. 2018, 14, 64. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Mafra, I. Lupine Allergens: Clinical Relevance, Molecular Characterization, Cross-reactivity, and Detection Strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar] [CrossRef]
- Fiocchi, A.; Sarratud, P.; Terracciano, L.; Vacca, E.; Bernardini, R.; Fuggetta, D.; Ballabio, C.; Duranti, M.; Magni, C.; Restani, P. Assessment of the Tolerance to Lupine-Enriched Pasta in Peanut-Allergic Children. Clin. Exp. Allergy 2009, 39, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Magni, C.; Restani, P.; Mottini, M.; Fiocchi, A.; Tedeschi, G.; Duranti, M. IgE-Mediated Cross-Reactivity among Leguminous Seed Proteins in Peanut Allergic Children. Plant Foods Hum. Nutr. 2010, 65, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Peñas, E.; Uberti, F.; Fiocchi, A.; Duranti, M.; Magni, C.; Restani, P. Characterization of the Sensitization Profile to Lupin in Peanut-Allergic Children and Assessment of Cross-Reactivity Risk. Pediatr. Allergy Immunol. 2013, 24, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The Health Benefits of Sweet Lupin Seed Flours and Isolated Proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Gomez Galan, A.M.; Brohée, M.; Scaravelli, E.; van Hengel, A.J.; Chassaigne, H. Development of Real-Time PCR Assays for the Detection of Lupin Residues in Food Products. Eur. Food Res. Technol. 2010, 230, 597–608. [Google Scholar] [CrossRef]
- Islam, S.; Yan, G.; Appels, R.; Ma, W. Comparative Proteome Analysis of Seed Storage and Allergenic Proteins among Four Narrow-Leafed Lupin Cultivars. Food Chem. 2012, 135, 1230–1238. [Google Scholar] [CrossRef]
- Czubinski, J.; Montowska, M.; Pospiech, E.; Lampart-Szczapa, E. Proteomic Analysis of Lupinus angustifolius (Var. Zeus and Bojar) and Lupinus luteus (Var. Lord and Parys) Seed Proteins and Their Hydrolysates: Lupin Seed Proteins and Their Hydrolysates. J. Sci. Food Agric. 2017, 97, 5423–5430. [Google Scholar] [CrossRef]
- Li, X.-M.; Serebrisky, D.; Lee, S.-Y.; Huang, C.-K.; Bardina, L.; Schofield, B.H.; Stanley, J.S.; Burks, A.W.; Bannon, G.A.; Sampson, H.A. A Murine Model of Peanut Anaphylaxis: T- and B-Cell Responses to a Major Peanut Allergen Mimic Human Responses. J. Allergy Clin. Immunol. 2000, 106, 150–158. [Google Scholar] [CrossRef]
- Foss, N.; Duranti, M.; Magni, C.; Frøkiær, H. Assessment of Lupin Allergenicity in the Cholera Toxin Model: Induction of IgE Response Depends on the Intrinsic Properties of the Conglutins and Matrix Effects. Int. Arch. Allergy Immunol. 2006, 141, 141–150. [Google Scholar] [CrossRef]
- Duranti, M.; Restani, P.; Poniatowska, M.; Cerletti, P. The Seed Globulins of Lupinus albus. Phytochemistry 1981, 20, 2071–2075. [Google Scholar] [CrossRef]
- Capraro, J.; Spotti, P.; Magni, C.; Scarafoni, A.; Duranti, M. Spectroscopic Studies on the PH-Dependent Structural Dynamics of γ-Conglutin, the Blood Glucose-Lowering Protein of Lupin Seeds. Int. J. Biol. Macromol. 2010, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The Immunology of the Allergy Epidemic and the Hygiene Hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.; Ghaemmaghami, A.M. Allergen Recognition by Innate Immune Cells: Critical Role of Dendritic and Epithelial Cells. Front. Immunol. 2013, 4, 356. [Google Scholar] [CrossRef]
- Royer, P.-J.; Emara, M.; Yang, C.; Al-Ghouleh, A.; Tighe, P.; Jones, N.; Sewell, H.F.; Shakib, F.; Martinez-Pomares, L.; Ghaemmaghami, A.M. The Mannose Receptor Mediates the Uptake of Diverse Native Allergens by Dendritic Cells and Determines Allergen-Induced T Cell Polarization through Modulation of IDO Activity. J. Immunol. 2010, 185, 1522–1531. [Google Scholar] [CrossRef]
- Emara, M.; Royer, P.-J.; Abbas, Z.; Sewell, H.F.; Mohamed, G.G.; Singh, S.; Peel, S.; Fox, J.; Shakib, F.; Martinez-Pomares, L.; et al. Recognition of the Major Cat Allergen Fel d 1 through the Cysteine-Rich Domain of the Mannose Receptor Determines Its Allergenicity. J. Biol. Chem. 2011, 286, 13033–13040. [Google Scholar] [CrossRef]
- Salazar, F.; Hall, L.; Negm, O.H.; Awuah, D.; Tighe, P.J.; Shakib, F.; Ghaemmaghami, A.M. The Mannose Receptor Negatively Modulates the Toll-like Receptor 4–Aryl Hydrocarbon Receptor–Indoleamine 2,3-Dioxygenase Axis in Dendritic Cells Affecting T Helper Cell Polarization. J. Allergy Clin. Immunol. 2016, 137, 1841–1851.e2. [Google Scholar] [CrossRef]
- Mathiesen, R.; Eld, H.M.S.; Sørensen, J.; Fuglsang, E.; Lund, L.D.; Taverniti, V.; Frøkiær, H. Mannan Enhances IL-12 Production by Increasing Bacterial Uptake and Endosomal Degradation in L. acidophilus and S. Aureus Stimul. Dendritic Cells. Front. Immunol. 2019, 10, 2646. [Google Scholar] [CrossRef]
- Heinzl, G.C.; Tretola, M.; De Benedetti, S.; Silacci, P.; Scarafoni, A. Lupinus albus γ-Conglutin: New Findings about Its Action at the Intestinal Barrier and a Critical Analysis of the State of the Art on Its Postprandial Glycaemic Regulating Activity. Nutrients 2022, 14, 3666. [Google Scholar] [CrossRef]
- Schiarea, S.; Arnoldi, L.; Fanelli, R.; De Combarieu, E.; Chiabrando, C. In-Depth Glycoproteomic Characterization of γ-Conglutin by High-Resolution Accurate Mass Spectrometry. PLoS ONE 2013, 8, e73906. [Google Scholar] [CrossRef]
- Eld, H.M.S.; Johnsen, P.R.; Nielsen, E.M.; Jørgensen, F.Z.; Lindstrøm-Svendsen, M.; Baldry, M.; Ingmer, H.; Frøkiær, H. Soluble C-Type Lectin-Receptor Ligands Stimulate ROS Production in Dendritic Cells and Potentiate Killing of MRSA as Well as the MRSA Induced IL-12 Production. Front. Immunol. 2022, 13, 845881. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frøkiaer, H. Lactobacillus acidophilus Induces Virus Immune Defence Genes in Murine Dendritic Cells by a Toll-like Receptor-2-Dependent Mechanism: Induction of Virus Defence in Dendritic Cells by Lactobacillus acidophilus. Immunology 2010, 131, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Maaetoft-Udsen, K.; Stifter, S.A.; Hertzog, P.; Goriely, S.; Thomsen, A.R.; Paludan, S.R.; Frøkiær, H. MyD88 Drives the IFN-β Response to Lactobacillus acidophilus in Dendritic Cells through a Mechanism Involving IRF1, IRF3, and IRF7. J. Immunol. 2012, 189, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic Cells Use Macropinocytosis and the Mannose Receptor to Concentrate Macromolecules in the Major Histocompatibility Complex Class II Compartment: Downregulation by Cytokines and Bacterial Products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef]
- Schweizer, A.; Stahl, P.D.; Rohrer, J. A Di-Aromatic Motif in the Cytosolic Tail of the Mannose Receptor Mediates Endosomal Sorting. J. Biol. Chem. 2000, 275, 29694–29700. [Google Scholar] [CrossRef]
- Heinsbroek, S.E.M.; Taylor, P.R.; Martinez, F.O.; Martinez-Pomares, L.; Brown, G.D.; Gordon, S. Stage-Specific Sampling by Pattern Recognition Receptors during Candida Albicans Phagocytosis. PLoS Pathog. 2008, 4, e1000218. [Google Scholar] [CrossRef]
- Cho, S.; Park, E.-M.; Febbraio, M.; Anrather, J.; Park, L.; Racchumi, G.; Silverstein, R.L.; Iadecola, C. The Class B Scavenger Receptor CD36 Mediates Free Radical Production and Tissue Injury in Cerebral Ischemia. J. Neurosci. 2005, 25, 2504–2512. [Google Scholar] [CrossRef]
- Cominacini, L.; Rigoni, A.; Pasini, A.F.; Garbin, U.; Davoli, A.; Campagnola, M.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. The Binding of Oxidized Low Density Lipoprotein (Ox-LDL) to Ox-LDL Receptor-1 Reduces the Intracellular Concentration of Nitric Oxide in Endothelial Cells through an Increased Production of Superoxide. J. Biol. Chem. 2001, 276, 13750–13755. [Google Scholar] [CrossRef]
- Wang, W.; He, B.; Shi, W.; Liang, X.; Ma, J.; Shan, Z.; Hu, Z.; Danesh, F.R. Deletion of Scavenger Receptor A Protects Mice from Progressive Nephropathy Independent of Lipid Control during Diet-Induced Hyperlipidemia. Kidney Int. 2012, 81, 1002–1014. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Ben, J.; Yue, S.; Bai, H.; Guan, X.; Bai, X.; Jiang, L.; Ji, Y.; Fan, L.; et al. The Di-Leucine Motif Contributes to Class A Scavenger Receptor-Mediated Internalization of Acetylated Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1317–1322. [Google Scholar] [CrossRef]
- Brix, S.; Kjær, T.M.R.; Barkholt, V.; Frøkiær, H. Lipopolysaccharide Contamination of β-Lactoglobulin Affects the Immune Response against Intraperitoneally and Orally Administered Antigen. Int. Arch. Allergy Immunol. 2004, 135, 216–220. [Google Scholar] [CrossRef]
- Suri, R.M.; Austyn, J.M. Bacterial Lipopolysaccharide Contamination of Commercial Collagen Preparations May Mediate Dendritic Cell Maturation in Culture. J. Immunol. Methods 1998, 214, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Wen, M.-H. Lipopolysaccharide-mediated Reactive Oxygen Species and Signal Transduction in the Regulation of Interleukin-1 Gene Expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Vissers, Y.M.; Snel, J.; Zuurendonk, P.F.; Smit, B.A.; Wichers, H.J.; Savelkoul, H.F.J. Differential Effects of Lactobacillus acidophilus and Lactobacillus plantarum Strains on Cytokine Induction in Human Peripheral Blood Mononuclear Cells. FEMS Immunol. Med. Microbiol. 2010, 59, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. The Mannose Receptor Ligands and the Macrophage Glycome. Curr. Opin. Struct. Biol. 2022, 75, 102394. [Google Scholar] [CrossRef]
- Czubinski, J.; Lattová, E.; Zdráhal, Z.; Strasser, R. Characteristics of N -Glycosylation and Its Impact on the Molecular Behavior of Lupinus Angustifolius γ-Conglutin. J. Agric. Food Chem. 2023, 71, 7359–7369. [Google Scholar] [CrossRef]
- Autenrieth, S.E.; Autenrieth, I.B. Variable Antigen Uptake Due to Different Expression of the Macrophage Mannose Receptor by Dendritic Cells in Various Inbred Mouse Strains. Immunology 2009, 127, 523–529. [Google Scholar] [CrossRef]
- Brown, M.S.; Basu, S.K.; Falck, J.R.; Ho, Y.K.; Goldstein, J.L. The Scavenger Cell Pathway for Lipoprotein Degradation: Specificity of the Binding Site That Mediates the Uptake of Negatively-Charged LDL by Macrophages. J. Supramol. Struct. 1980, 13, 67–81. [Google Scholar] [CrossRef]
- Harshyne, L.A.; Zimmer, M.I.; Watkins, S.C.; Barratt-Boyes, S.M. A Role for Class A Scavenger Receptor in Dendritic Cell Nibbling from Live Cells. J. Immunol. 2003, 170, 2302–2309. [Google Scholar] [CrossRef]
- Siegemund, S.; Schütze, N.; Freudenberg, M.A.; Lutz, M.B.; Straubinger, R.K.; Alber, G. Production of IL-12, IL-23 and IL-27p28 by Bone Marrow-Derived Conventional Dendritic Cells Rather than Macrophages after LPS/TLR4-Dependent Induction by Salmonella Enteritidis. Immunobiology 2008, 212, 739–750. [Google Scholar] [CrossRef]
- Swanson, J.A.; Baer, S.C. Phagocytosis by Zippers and Triggers. Trends Cell Biol. 1995, 5, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Bavaro, S.L.; Benedé, S.; Diaz-Perales, A.; Bueno-Diaz, C.; Gelencser, E.; Klueber, J.; Larré, C.; Lozano-Ojalvo, D.; Lupi, R.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens? Clinic. Rev. Allerg. Immunol. 2022, 62, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.R.; Bruun, S.W.; Frøkiær, H. Antigenic Specificity of Serum Antibodies in Mice Fed Soy Protein. Int. Arch. Allergy Immunol. 2003, 132, 58–67. [Google Scholar] [CrossRef] [PubMed]




| Preparation | Endotoxin (EU/mg) |
|---|---|
| Conglutin-γ | 20 |
| Kunitz trypsin inhibitor | 0.5 |
| PolyG | nd |
| Pep-pan conglutin-γ | 30 |
| Trp Conglutin-γ | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinzl, G.C.; Eriksen, D.B.; Johnsen, P.R.; Scarafoni, A.; Frøkiær, H. Protein Concentration Affects the Food Allergen γ-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells. Biomolecules 2023, 13, 1531. https://doi.org/10.3390/biom13101531
Heinzl GC, Eriksen DB, Johnsen PR, Scarafoni A, Frøkiær H. Protein Concentration Affects the Food Allergen γ-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells. Biomolecules. 2023; 13(10):1531. https://doi.org/10.3390/biom13101531
Chicago/Turabian StyleHeinzl, Giuditta C., Danny Blichfeldt Eriksen, Peter Riber Johnsen, Alessio Scarafoni, and Hanne Frøkiær. 2023. "Protein Concentration Affects the Food Allergen γ-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells" Biomolecules 13, no. 10: 1531. https://doi.org/10.3390/biom13101531
APA StyleHeinzl, G. C., Eriksen, D. B., Johnsen, P. R., Scarafoni, A., & Frøkiær, H. (2023). Protein Concentration Affects the Food Allergen γ-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells. Biomolecules, 13(10), 1531. https://doi.org/10.3390/biom13101531








