Altered Osteogenic Differentiation in Mesenchymal Stem Cells Isolated from Compact Bone of Chicken Treated with Varying Doses of Lipopolysaccharides
Abstract
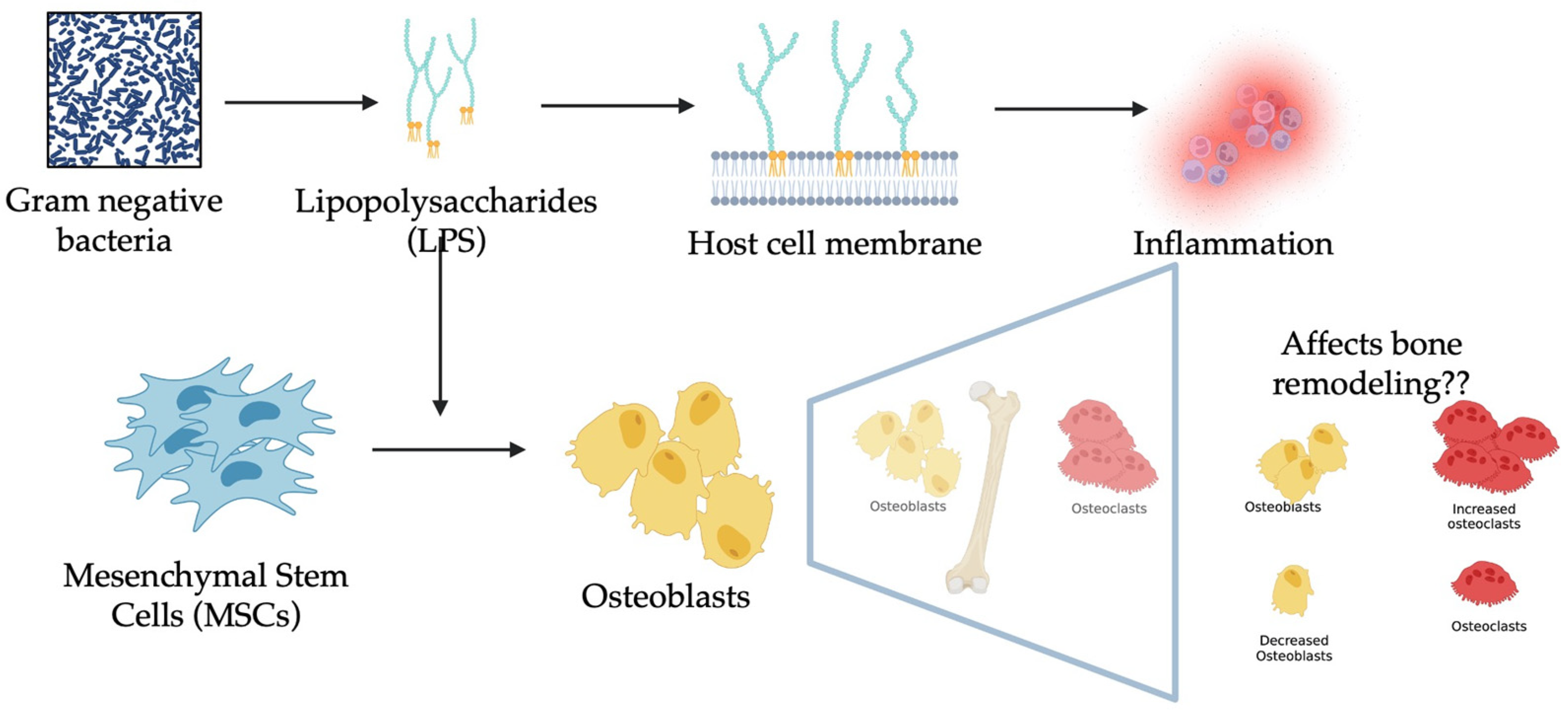
:1. Introduction
2. Materials and Methods
2.1. Animal Use and Ethics Statement
2.2. Isolation of MSCs
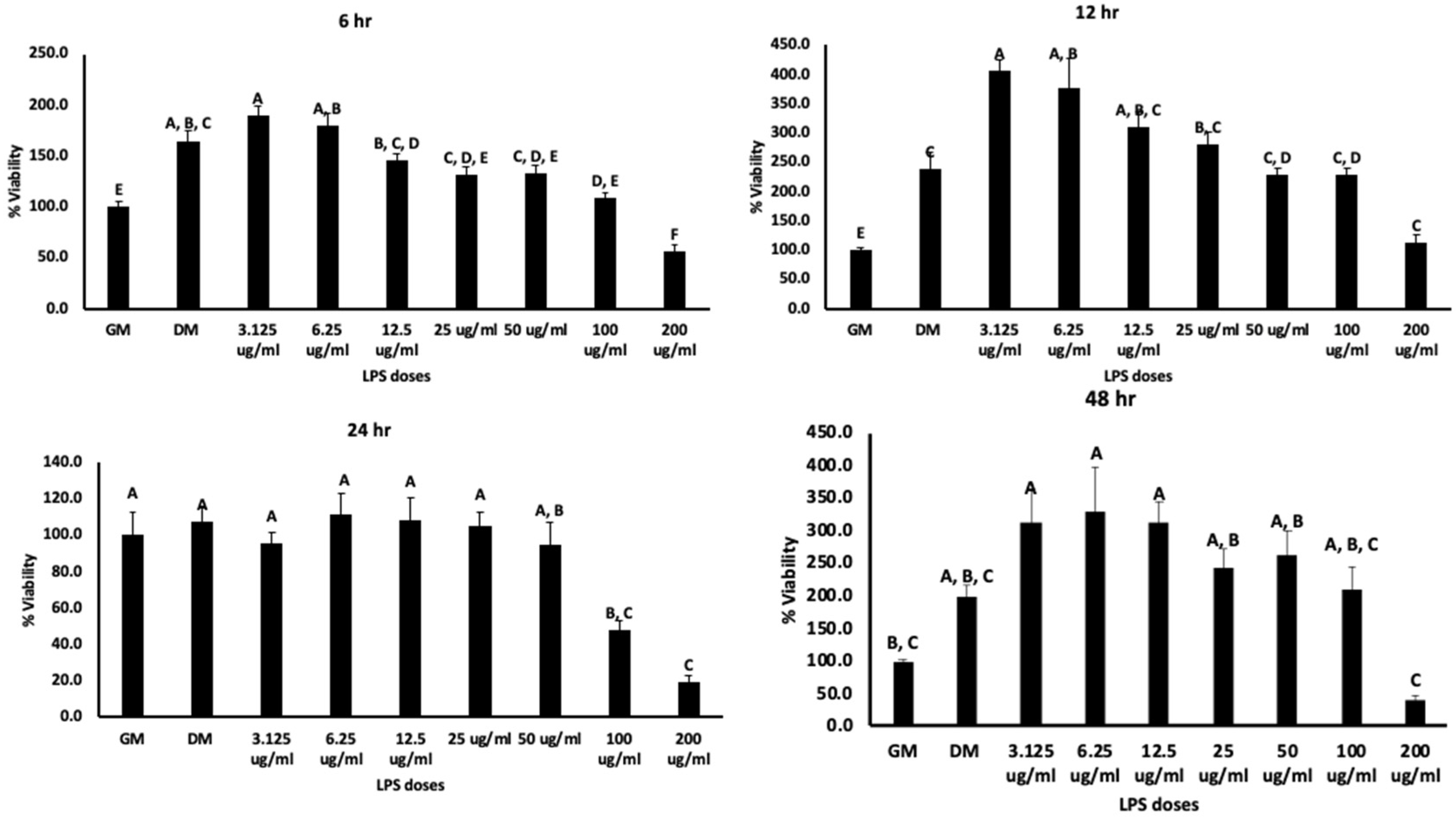
2.3. Cell Viability Assay
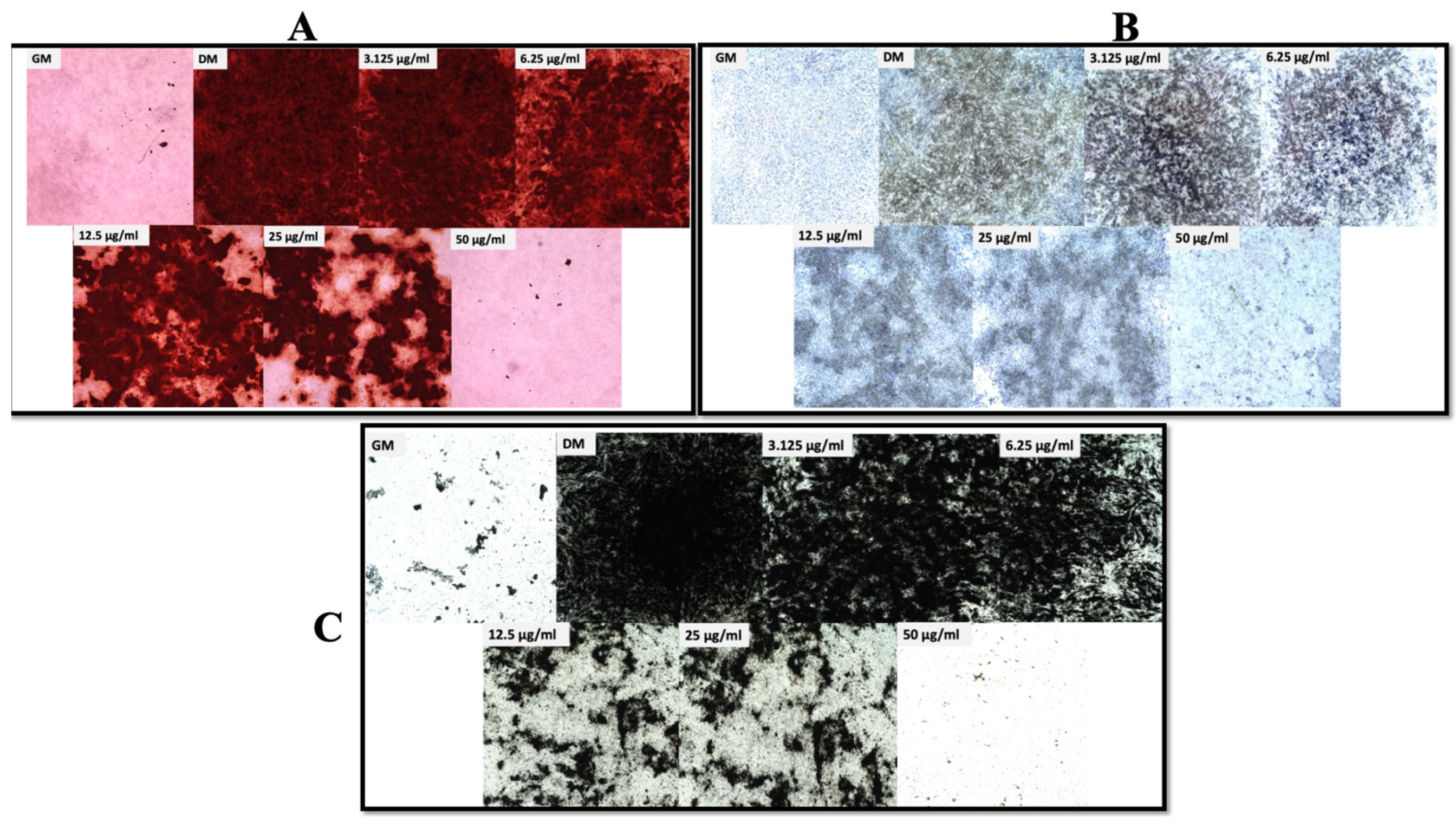
2.4. Osteogenic Differentiation
2.5. Alizarin Red S Staining (AZ) and Mineral Deposit Quantification
2.6. Alkaline Phosphatase Expression Assay
2.7. Von Kossa Staining
2.8. Cellular ROS Assay
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
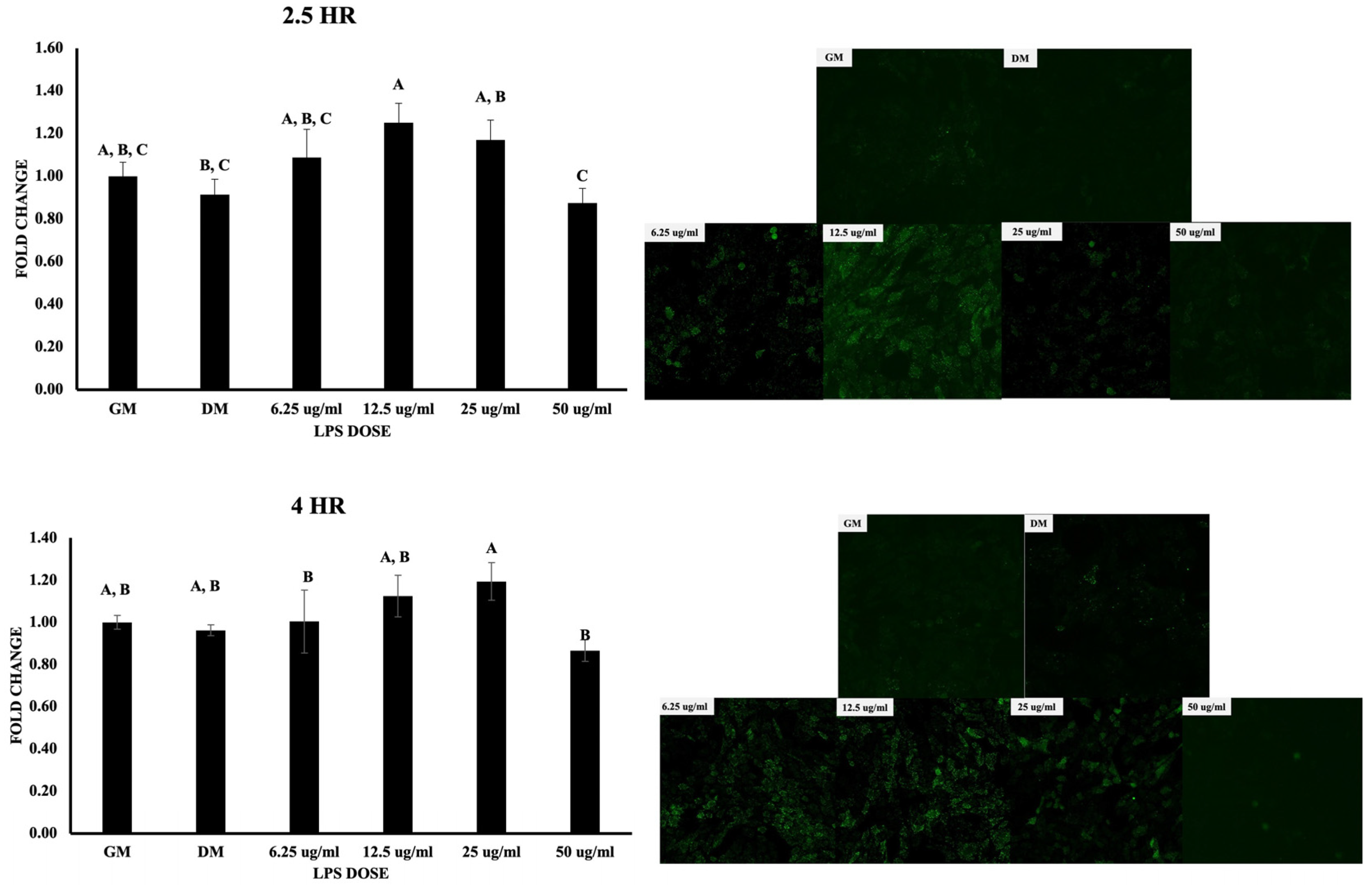
3.1. Cell Viability, Osteogenic Differentiation, and ROS Production
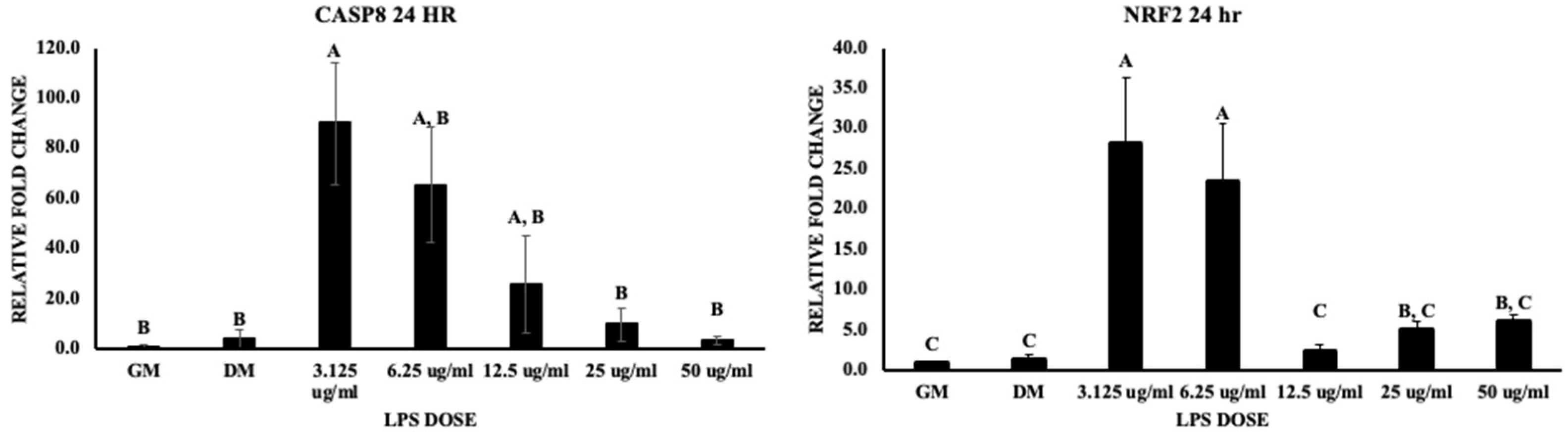
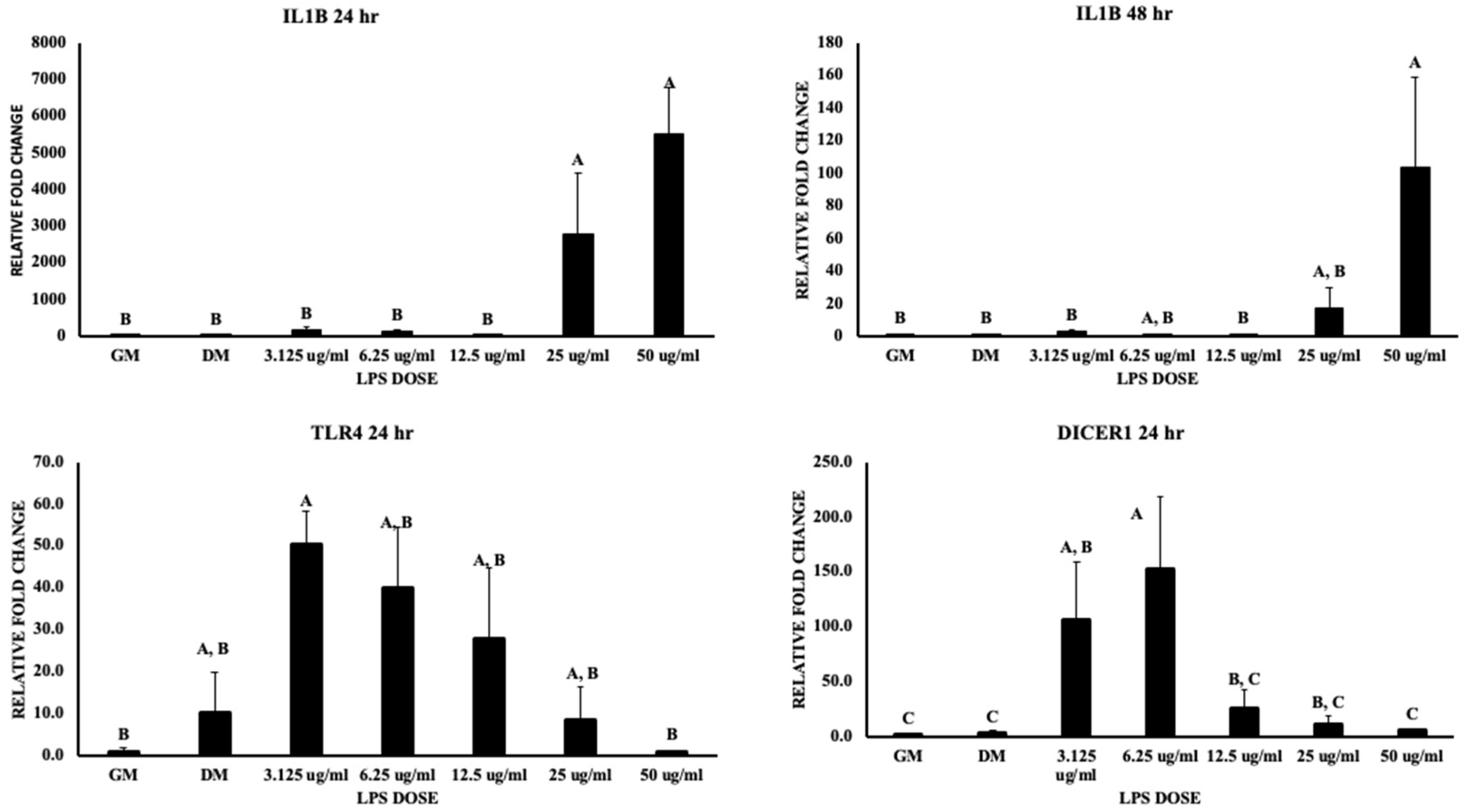
3.2. Inflammatory Pathways
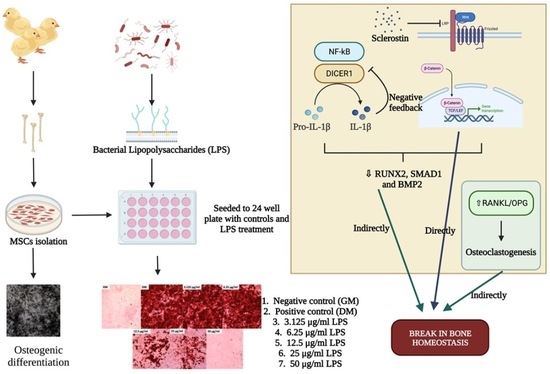
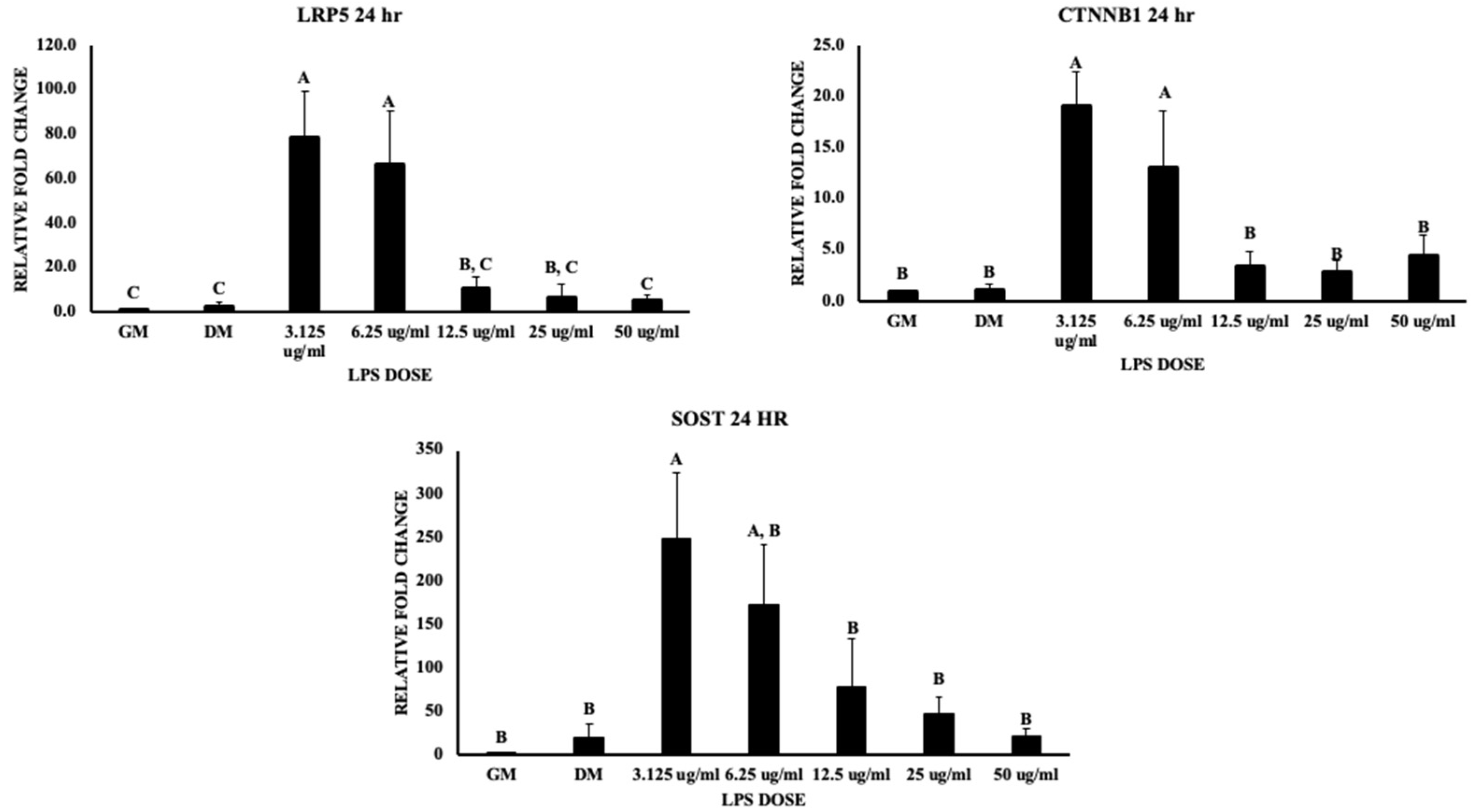
3.3. Wnt Signaling and Bone Homeostasis
4. Discussion
4.1. Inflammatory Pathways
4.2. The Wnt Signaling Pathway
4.3. Bone Formation and Resorption
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weimer, S.L.; Wideman, R.F.; Scanes, C.G.; Mauromoustakos, A.; Christensen, K.D.; Vizzier-Thaxton, Y. The utility of infrared thermography for evaluating lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult. Sci. 2019, 98, 1575–1588. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Fornari, M.B.; Zanella, R.; Ibelli, A.M.G.; Fernandes, L.T.; Cantão, M.E.; Thomaz-Soccol, V.; Ledur, M.C.; Peixoto, J.O. Unraveling the associations of osteoprotegerin gene with production traits in a paternal broiler line. Springerplus 2014, 3, 682. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, Y.; Liu, G.; Marshall, B.; Sharma, M.K.; Kim, W.K. Effect of Hydrogen Oxide-Induced Oxidative Stress on Bone Formation in the Early Embryonic Development Stage of Chicken. Biomolecules 2023, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.R.; Bressan, R.B.; Forner, S.; Martini, A.C.; Rode, M.; Delben, P.B.; Rae, G.A.; Figueiredo, C.P.; Trentin, A.G. Transplantation of human skin-derived mesenchymal stromal cells improves locomotor recovery after spinal cord injury in rats. Cell. Mol. Neurobiol. 2017, 37, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Verfaillie, C.M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013, 2013, 130763. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in food, food supplements, and probiotics: Should we be worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]
- Leimer, N.; Rachmühl, C.; Palheiros Marques, M.; Bahlmann, A.S.; Furrer, A.; Eichenseher, F.; Seidl, K.; Matt, U.; Loessner, M.J.; Schuepbach, R.A. Nonstable Staphylococcus aureus small-colony variants are induced by low pH and sensitized to antimicrobial therapy by phagolysosomal alkalinization. J. Infect. Dis. 2016, 213, 305–313. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Herzmann, N.; Salamon, A.; Fiedler, T.; Peters, K. Lipopolysaccharide induces proliferation and osteogenic differentiation of adipose-derived mesenchymal stromal cells in vitro via TLR4 activation. Exp. Cell Res. 2017, 350, 115–122. [Google Scholar] [CrossRef]
- Croes, M.; Oner, F.C.; Kruyt, M.C.; Blokhuis, T.J.; Bastian, O.; Dhert, W.J.A.; Alblas, J. Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PLoS ONE 2015, 10, e0132781. [Google Scholar] [CrossRef] [PubMed]
- Bandow, K.; Maeda, A.; Kakimoto, K.; Kusuyama, J.; Shamoto, M.; Ohnishi, T.; Matsuguchi, T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 2010, 402, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, B.; Dong, Z.; Gao, L.; He, X.; Liao, L.; Hu, C.; Wang, Q.; Jin, Y. Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor κB pathway. Stem Cell Res. Ther. 2014, 5, 67. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C. Wnt pathway in bone repair and regeneration–what do we know so far. Front. Cell Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Shen, Y.-S.; He, M.-C.; Yang, F.; Yang, P.; Pang, F.-X.; He, W.; Cao, Y.; Wei, Q.-S. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2019, 112, 108746. [Google Scholar] [CrossRef]
- Greene, E.; Flees, J.; Dhamad, A.; Alrubaye, A.; Hennigan, S.; Pleimann, J.; Smeltzer, M.; Murray, S.; Kugel, J.; Goodrich, J.; et al. Double-Stranded RNA Is a Novel Molecular Target in Osteomyelitis Pathogenesis: A Translational Avian Model for Human Bacterial Chondronecrosis with Osteomyelitis. Am. J. Pathol. 2019, 189, 2077–2089. [Google Scholar] [CrossRef]
- Adhikari, R.; Chen, C.; Waters, E.; West, F.D.; Kim, W.K. Isolation and differentiation of mesenchymal stem cells from broiler chicken compact bones. Front. Physiol. 2019, 9, 1892. [Google Scholar] [CrossRef]
- Chen, C.; Adhikari, R.; White, D.L.; Kim, W.K. Role of 1,25-Dihydroxyvitamin D(3) on Osteogenic Differentiation and Mineralization of Chicken Mesenchymal Stem Cells. Front. Physiol. 2021, 12, 479596. [Google Scholar] [CrossRef]
- Sharma, M.K.; Liu, G.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Effects of mixed Eimeria challenge on performance, body composition, intestinal health, and expression of nutrient transporter genes of Hy-Line W-36 pullets (0–6 wks of age). Poult. Sci. 2022, 101, 102083. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Y.; Chen, Y.; Yang, L.; Song, J.; Tang, Y.; Deng, F.; Zheng, L. Dicer-dependent pathway contribute to the osteogenesis mediated by regulation of Runx2. Am. J. Transl. Res. 2016, 8, 5354. [Google Scholar]
- Zheng, L.; Tu, Q.; Meng, S.; Zhang, L.; Yu, L.; Song, J.; Hu, Y.; Sui, L.; Zhang, J.; Dard, M. Runx2/DICER/miRNA pathway in regulating osteogenesis. J. Cell. Physiol. 2017, 232, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Choppa, V.S.R.; Kim, W.K. A Review on Pathophysiology, and Molecular Mechanisms of Bacterial Chondronecrosis and Osteomyelitis in Commercial Broilers. Biomolecules 2023, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Murchison, E.P.; Tang, R.; Callis, T.E.; Tatsuguchi, M.; Deng, Z.; Rojas, M.; Hammond, S.M.; Schneider, M.D.; Selzman, C.H. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2008, 105, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Al-Aalim, A.M.; Al-Iedani, A.A.; Hamad, M.A. Study of the effects of Escherichia coli lipopolysaccharide on innate immunity: The expression profile of TLR4 and CD14 genes in rat liver. Open Vet. J. 2021, 11, 771–779. [Google Scholar] [CrossRef]
- Lang, C.H.; Silvis, C.; Deshpande, N.; Nystrom, G.; Frost, R.A. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1β,-6, and high-mobility-group protein-1 in skeletal muscle. Shock 2003, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Z.; Zhao, J.; Miao, W.; Ye, T.; Chen, A. Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2045. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Smeekens, S.P.; Joosten, L.A.B.; Kullberg, B.J.; Dinarello, C.A.; van der Meer, J.W.M.; Netea, M.G. Reactive oxygen species–independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 2010, 107, 3030–3033. [Google Scholar] [CrossRef]
- Smeekens, S.P.; van de Veerdonk, F.L.; Joosten, L.A.B.; Kullberg, B.J.; Dinarello, C.A.; van der Meer, J.W.M.; Netea, M.G. Reactive oxygen species-independent activation of the interleukin-1β inflammasome in cells from patients with chronic granulomatous disease. In Immunological and Genetic Components of the Anti-Candida Host Immune Response; Off Page: Amsterdam, The Netherlands, 2014; p. 157. [Google Scholar]
- Fan, H.; Zhao, G.; Liu, L.; Liu, F.; Gong, W.; Liu, X.; Yang, L.; Wang, J.; Hou, Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell. Mol. Immunol. 2012, 9, 473–481. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Ranera, B.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Inflammation affects the viability and plasticity of equine mesenchymal stem cells: Possible implications in intra-articular treatments. J. Vet. Sci. 2017, 18, 39–49. [Google Scholar] [CrossRef]
- Shi, J.; Chi, S.; Xue, J.; Yang, J.; Li, F.; Liu, X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J. Immunol. Res. 2016, 2016, 9392132. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zylstra-Diegel, C.R.; Schumacher, C.A.; Baker, J.J.; Carpenter, A.C.; Rao, S.; Yao, W.; Guan, M.; Helms, J.A.; Lane, N.E. Wntless functions in mature osteoblasts to regulate bone mass. Proc. Natl. Acad. Sci. USA 2012, 109, E2197–E2204. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, E.; Zhang, H.; Wang, J.; Wu, N.; Chen, X.; Wang, N.; Wen, S.; Nan, G.; Deng, F. Crosstalk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS ONE 2013, 8, e82436. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Vijakumaran, U.; Shanmuganantha, L.; Law, J.-X.; Alias, E.; Ng, M.-H. The Role and Mechanism of MicroRNA 21 in Osteogenesis: An Update. Int. J. Mol. Sci. 2023, 24, 11330. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Chen, H.; Ge, G.; Wang, M.; Gao, A.; Tong, L.; Xu, Y.; Yang, H.; Pan, G. Biomimetic osteogenic peptide with mussel adhesion and osteoimmunomodulatory functions to ameliorate interfacial osseointegration under chronic inflammation. Biomaterials 2020, 255, 120197. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Larose, J.; Kent, O.A.; Lim, M.; Changoor, A.; Zhang, L.; Storozhuk, Y.; Mao, X.; Grynpas, M.D.; Cong, F. RANKL coordinates multiple osteoclastogenic pathways by regulating expression of ubiquitin ligase RNF146. J. Clin. Investig. 2017, 127, 1303–1315. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef]



| Gene Name | Accession Number | F Primer Sequence | R Primer Sequence |
|---|---|---|---|
| BMP 2 | NM_001398170.1 | CCCCTACATGTTGGACCTC | CCCACTTGTTTCTGGCAGT |
| RUNX2 | NM_204128.2 | GTGGCCAGATTCAATGACCT | CCATCCACCGTCACCTTTAT |
| CTSK (Cathepsin K) | NM_204971.3 | AGTCTGCCCTCCTTCCAGTT | CTTGATGATCCAGTGCTTGG |
| TNFSF11 (RANKL) | NM_001083361.2 | GTCCAGCGTATTCTGGGAAA | GCAAAAGGTTGCTTCTCTGG |
| LRP5 | NM_001012897.2 | GGTGCCCCCTTATATGACAG | GATCAGTAGCTGGGGATGGA |
| TLR4 | NM_001030693.2 | ACTCTTGGGGTGCTGCTG | TGTCCTGTGCATCTGAAAG |
| b-catenin (CTNNB1) | NM_205081.3 | AGGGTGCTGAAGGTGTTGTC | GCTGGCTTGGATCTGTAAGG |
| Dicer1 (ribonuclease 3) | NM_001040465.2 | GACCTGACCAATCTCAACCAG | TTTGCCTTCCTCTTCTCAGC |
| NFKB2 | NM_204413.2 | CCACGTCACCAAGAAGAACA | GGTCCATCACCTTCTTCAGC |
| NFE2L2 (NRF-2) | NM_205117.2 | ATGCAGCTCTTGGCAGAAG | CTGGGTGGCTGAGTTTGATT |
| OPG | NM_001033641.1 | ACGCTTGTGCTCTTGGACAT | CAGCGTAGTACTGGTCTGGG |
| IL-1B | XM_015297469.2 | TGCCTGCAGAAGAAGCCTCG | GACGGGCTCAAAAACCTCCT |
| SOST | XM_004948551 | ATCCCACCTCCTGCCCAACTCCATC | GGTTCGGTTTGCTGCTCCTGGCTC |
| SMAD1 | XM_040698719.1 | GTTTTGTAAAGGGTTGGGGAGC | AATGCAGGAGCTTGGGACCTTA |
| Beta-actin | NM_205518.2 | CAACACAGTGCTGTCTGGTGGTA | ATCGTACTCCTGCTTGCTGATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choppa, V.S.R.; Liu, G.; Tompkins, Y.H.; Kim, W.K. Altered Osteogenic Differentiation in Mesenchymal Stem Cells Isolated from Compact Bone of Chicken Treated with Varying Doses of Lipopolysaccharides. Biomolecules 2023, 13, 1626. https://doi.org/10.3390/biom13111626
Choppa VSR, Liu G, Tompkins YH, Kim WK. Altered Osteogenic Differentiation in Mesenchymal Stem Cells Isolated from Compact Bone of Chicken Treated with Varying Doses of Lipopolysaccharides. Biomolecules. 2023; 13(11):1626. https://doi.org/10.3390/biom13111626
Chicago/Turabian StyleChoppa, Venkata Sesha Reddy, Guanchen Liu, Yuguo Hou Tompkins, and Woo Kyun Kim. 2023. "Altered Osteogenic Differentiation in Mesenchymal Stem Cells Isolated from Compact Bone of Chicken Treated with Varying Doses of Lipopolysaccharides" Biomolecules 13, no. 11: 1626. https://doi.org/10.3390/biom13111626
APA StyleChoppa, V. S. R., Liu, G., Tompkins, Y. H., & Kim, W. K. (2023). Altered Osteogenic Differentiation in Mesenchymal Stem Cells Isolated from Compact Bone of Chicken Treated with Varying Doses of Lipopolysaccharides. Biomolecules, 13(11), 1626. https://doi.org/10.3390/biom13111626