Solid-State Preparation and Characterization of 2-Hydroxypropylcyclodextrins-Iodine Complexes as Stable Iodophors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid-State Preparation of Iodine–Cyclodextrin Complexes
- (a)
- Liquid-assisted grinding (LAG): In a representative procedure, HP-β-CD (2.50 g, 1.62 mmol) and iodine (412 mg, 1.62 mmol) were mixed in a mortar and EtOH (3 mL) was added. The resultant mixture was kneaded thoroughly with a pestle to obtain homogeneous slurry, and the mixing continued until the solvent was completely removed. The sample was then kept overnight at 25 °C in a ventilated hood, and the resultant dark red solid was finely pulverized.
- (b)
- Co-evaporation (COE): Typically, a solution of HP-β-CD (1.07 g, 0.70 mmol) and iodine (177 mg, 0.70 mmol) in EtOH (3 mL) was taken to dryness under vacuum at 30 °C in a Buchi rotavapor. The obtained dark red solid was then transferred to a petri dish and left overnight at 25 °C in a ventilated hood before the analysis.
- (c)
- Sealed-heating (SH): In a representative example, a physical mixture of HP-β-CD (2.00 g, 1.30 mmol) and iodine (330 mg, 1.30 mmol) was put in a vial crimped with a Teflon cap and maintained at 60 °C in a laboratory oven for 6 h. Then, the vial was allowed to cool to room temperature, and the dark red solid was transferred to a petri dish, left overnight at 25 °C in a ventilated hood, and then analyzed.
2.3. Solid-State Characterization of Iodine–Cyclodextrin Complexes
2.4. Stability Studies
- (a)
- Accelerated stability test: Samples of solid complexes (about 1 g each, analyses in triplicate) were spread in an open glass Petri dish (40 mm diameter) and stored at 40 °C in oven for up to 28 days. Aliquots of each sample (25 mg) were withdrawn at set intervals and analyzed for the iodine content by UV.
- (b)
- Real-time stability: The solid complexes obtained by the suitable procedure (1 g each) were stored in a transparent low-density (60 μm thickness) heat-sealed polyethylene bag and kept in the dark at 25 ± 2 °C (65 ± 5% RH). At regular intervals over three months, aliquots (25 mg) of the samples were monitored (analyses in triplicate) for the iodine content by UV.
- (c)
- Stability in solution: Solutions of the solid complexes were prepared in water at a 2.5 mg/mL concentration and sterilized by filtration (CA, 0.2 µm). The solutions were then dispensed in white opaque polyethylene bottles (5 mL) in sterile conditions and kept at room temperature (25 °C ± 2 °C; 65 ± 5% RH) for up to three months. At regular intervals, aliquots of the solution (200 mg) were diluted with 4.7 g of 1% potassium iodide solution and analyzed in triplicate for the iodine content.
2.5. Time-Kill Assay
3. Results and Discussion
3.1. Solid-State Preparation of the Iodine–CD Complexes
3.2. Solid-State Characterization of Iodine–CD Complexes
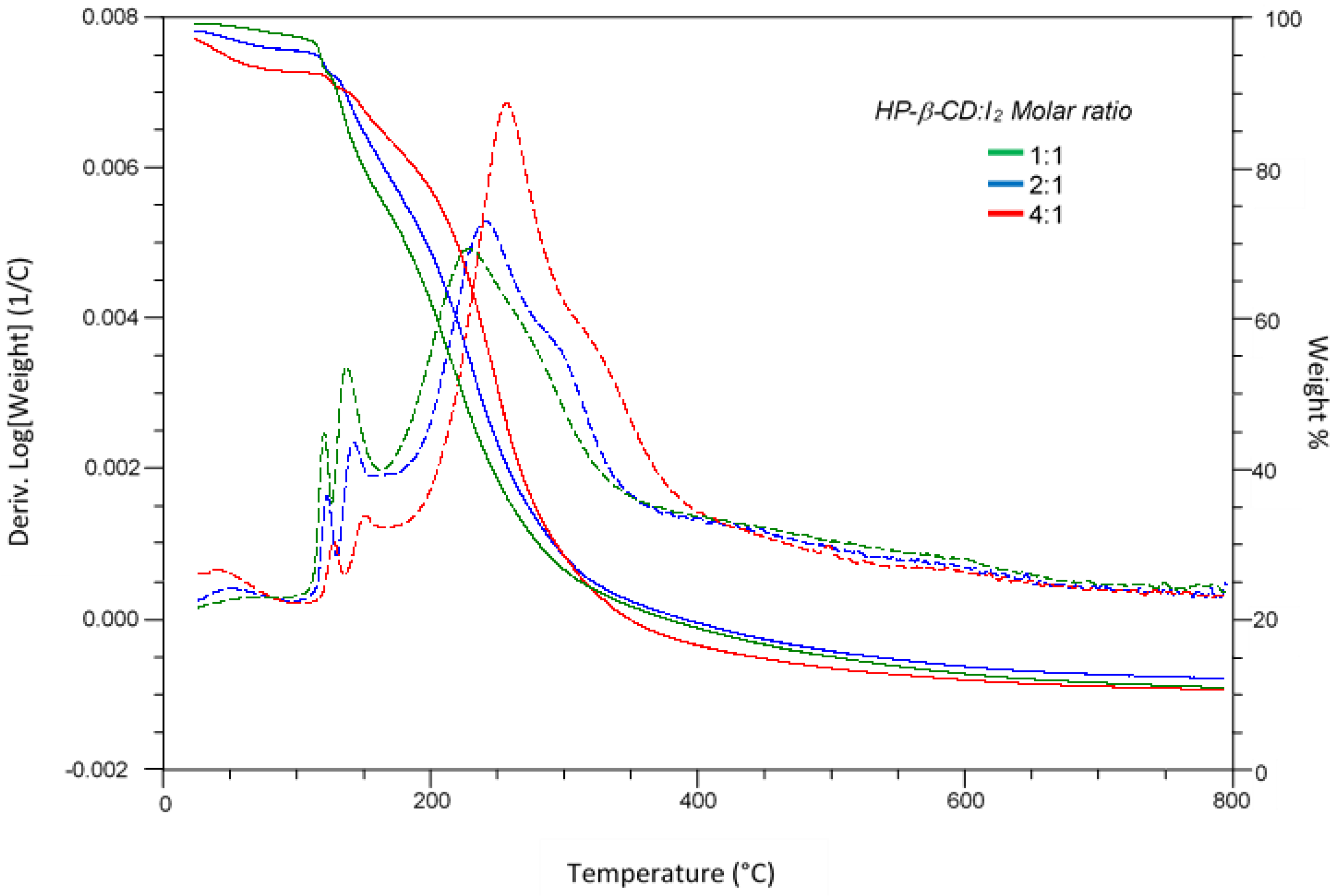
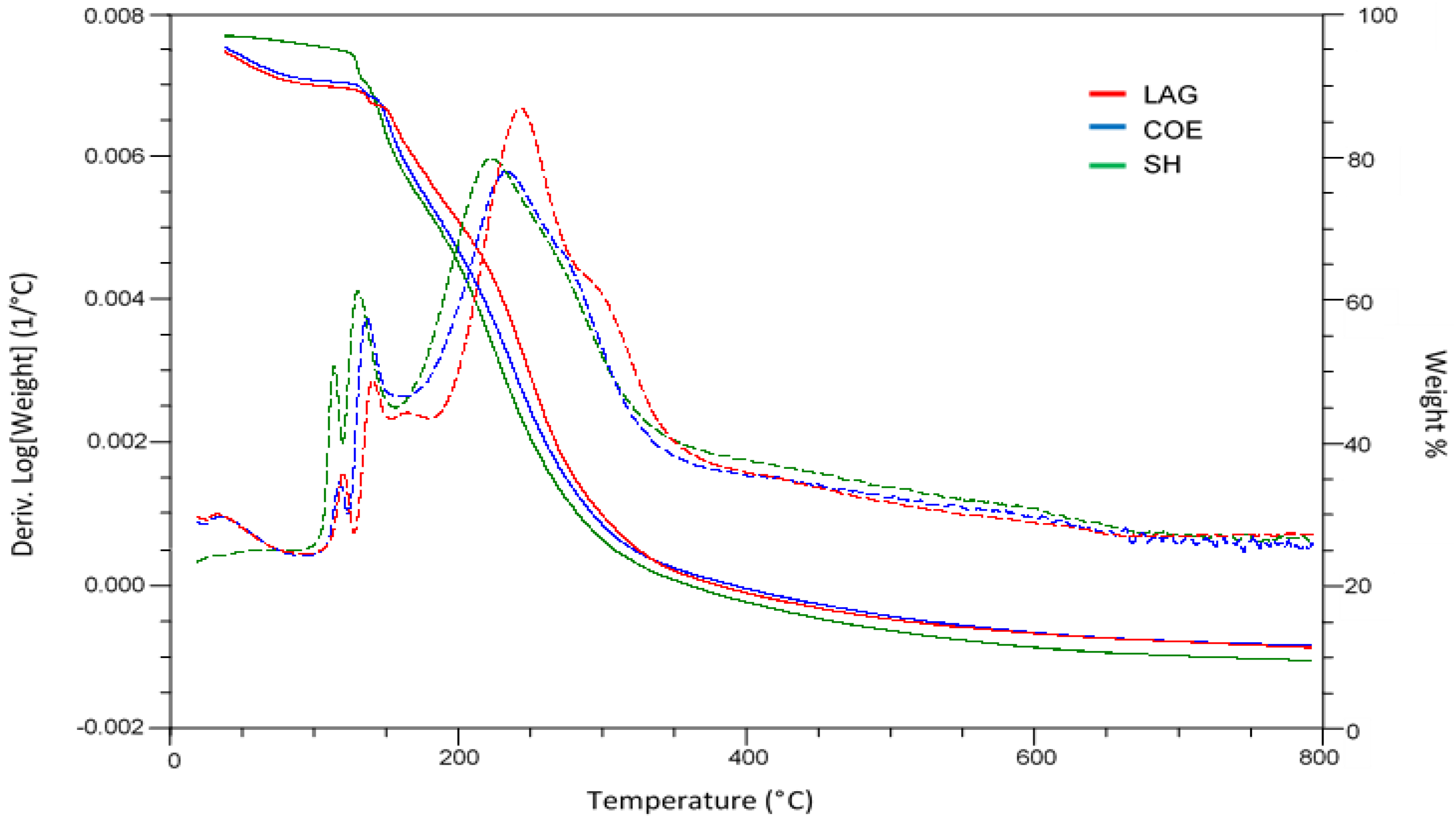
3.2.1. Thermogravimetric Analysis
3.2.2. Scanning Electron Microscopy/Energy-Dispersive X-ray Analysis (SEM/EDX)
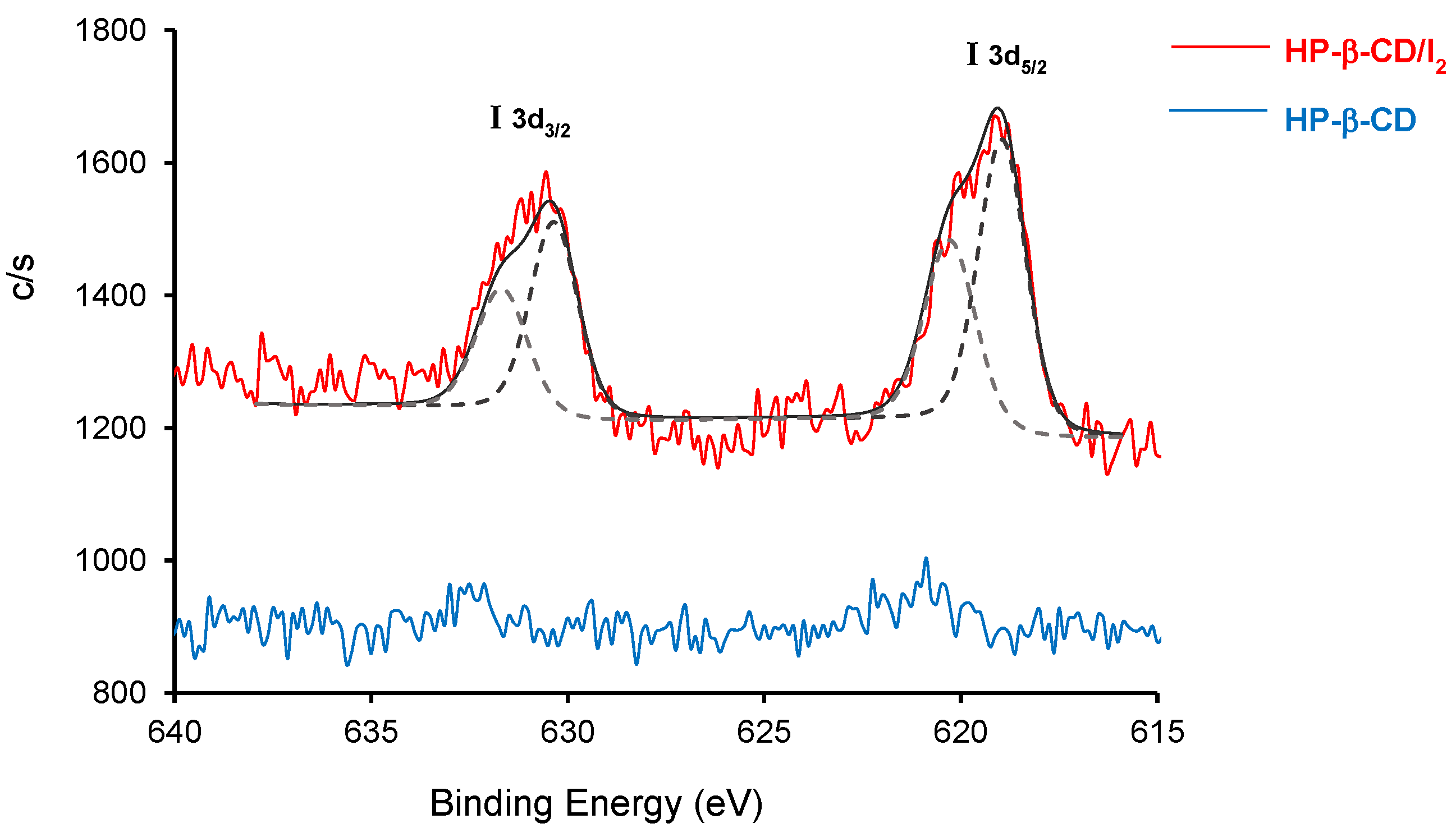
3.2.3. X-ray Photoelectron Spectroscopy (XPS)
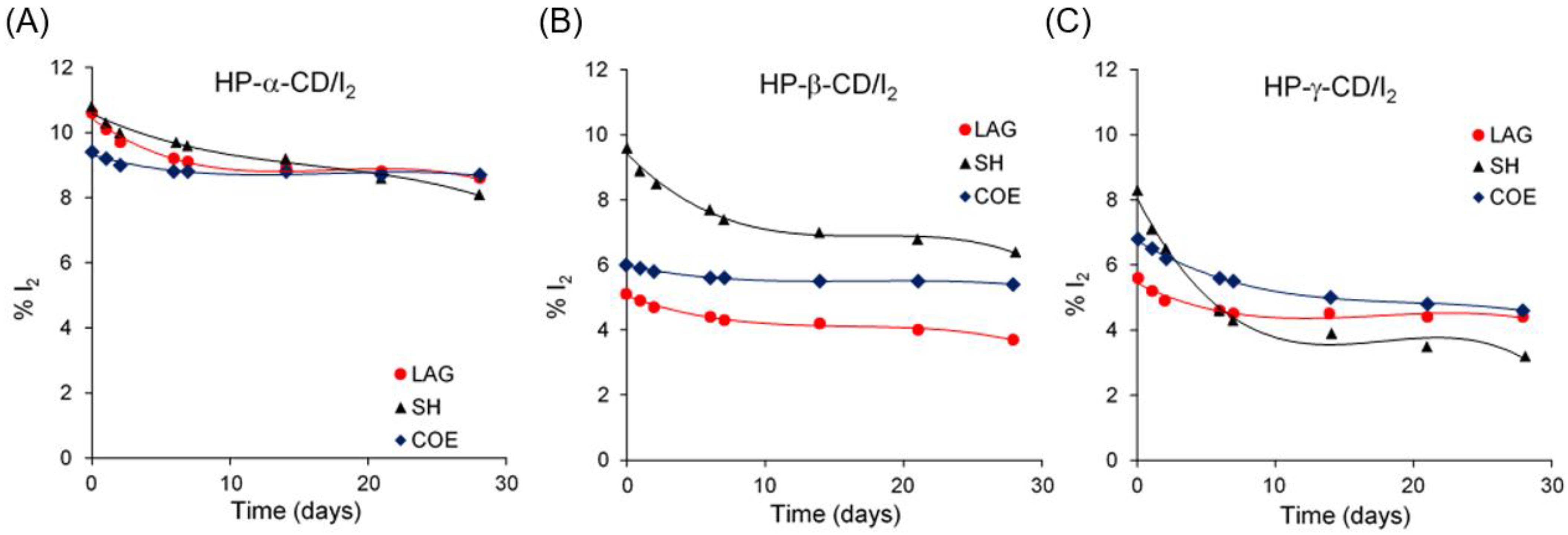
3.3. Stability Studies
3.4. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermeulen, H.; Westerbos, S.J.; Ubbink, D.T. Benefit and harm of iodine in wound care: A systematic review. J. Hosp. Infect. 2010, 76, 191–199. [Google Scholar] [CrossRef]
- Lachapelle, J.-M.; Castel, O.; Fueyo Casado, A.; Leroy, B.; Micali, G.; Tennstedt, D.; Lambert, J. Antiseptics in the era of bacterial resistance: A focus on povidone iodine. Clin. Pract. 2013, 10, 579–592. [Google Scholar] [CrossRef]
- Capriotti, K.; Pelletier, J.; Barone, S.; Capriotti, J. Efficacy of dilute povidone-iodine against multi- drug resistant bacterial biofilms, fungal biofilms and fungal spores. Clin. Res. Dermatol. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Anderson, D.E.; Sivalingam, V.; Eng, A.; Kang, Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef]
- Schreier, H.; Erdos, G.; Reimer, K.; König, B.; König, W.; Fleischer, W. Molecular effects of povidone-iodine on relevant microorganisms: An electron-microscopic and biochemical study. Dermatology 1997, 195, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Sriwilaijaroen, N.; Wilairat, P.; Hiramatsu, H.; Takahashi, T.; Suzuki, T.; Ito, M.; Ito, Y.; Tashiro, M.; Suzuki, Y. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: Its effects on hemagglutination and sialidase activities. Virol. J. 2009, 6, 124. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simone, A. Povidone iodine: Properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization. Antimicrob. Agents Chemother. 2020, 64, e00682–e00720. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Abdul, S.; Alsagoff, L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Tse, C.; Wa, C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Atemnkeng, M.A.; Plaizier-Vercammen, J.; Schuermans, A. Comparison of free and bound iodine and iodide species as a function of the dilution of three commercial povidone–iodine formulations and their microbicidal activity. Int. J. Pharm. 2006, 317, 161–166. [Google Scholar] [CrossRef]
- Van Ketel, W.G.; van den Berg, W.H. Sensitization to Povidone-Iodine. Dermatol. Clin. 1980, 8, 107–110. [Google Scholar] [CrossRef]
- Lachapelle, J.M. Allergic contact dermatitis from povidone-iodine: A re-evaluation study. Contact Dermat. 2005, 52, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Balin, A.K.; Pratt, L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol. Surg. 2002, 28, 210–214. [Google Scholar] [PubMed]
- Kramer, S.A. Effect of povidone-iodine on wound healing: A review. J. Vasc. Nurs. 1999, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, R.G.; Leaper, D.J.; Queen, D. Iodine made easy. Wounds Int. 2011, 2, 1–6. [Google Scholar]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Polymeric iodophors: Preparation, properties, and biomedical applications. Rev. J. Chem. 2020, 10, 40–57. [Google Scholar] [CrossRef]
- Mallick, S.; Sharma, S.; Banerjee, M.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Iodine-stabilized Cu nanoparticle chitosan composite for antibacterial applications. ACS Appl. Mater. Interfaces 2012, 4, 1313–1323. [Google Scholar] [CrossRef]
- Gao, T.; Fan, H.; Wang, X.; Gao, Y.; Liu, W.; Chen, W.; Dong, A.; Wang, Y.-J. Povidone–iodine-based polymeric nanoparticles for antibacterial applications. ACS Appl. Mater. Interfaces 2017, 9, 25738–25746. [Google Scholar] [CrossRef]
- Tang, C.; York, A.W.; Mikitsh, J.L.; Wright, A.C.; Chacko, A.-M.; Elias, D.R.; Xu, Y.; Lim, H.-K.; Prud’homme, R.K. Preparation of PEGylated iodine-loaded nanoparticles via polymer-directed self-assembly. Macromol. Chem. Phys. 2018, 219, 1700592. [Google Scholar] [CrossRef]
- Au-Duong, A.-N.; Lee, C.-K. Iodine-loaded metal-organic framework as growth-triggered antimicrobial agent. Mater. Sci. Eng. C 2017, 76, 477–482. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Nakhaei, M.; Akhbari, K.; Davoodi, A. Biocompatible MOF-808 as an iodophor antimicrobial agent with controlled and sustained release of iodine. CrystEngComm 2021, 23, 8538–8545. [Google Scholar] [CrossRef]
- Muhire, C.; Reda, A.T.; Zhang, D.; Xu, X.; Cui, C. An overview on metal Oxide-based materials for iodine capture and storage. Chem. Eng. J. 2022, 431, 133816. [Google Scholar] [CrossRef]
- Mishra, A.; Chaudhary, N. Study of povidone iodine loaded hydrogels as wound dressing material. Trends Biomater. Artif. Organs 2010, 23, 122–128. [Google Scholar]
- Gull, N.; Khan, S.M.; Khalid, S.; Zia, S.; Islam, A.; Sabir, A.; Sultan, M.; Hussain, F.; Khan, R.U.; Butt, M.T.Z. Designing of biocompatible and biodegradable chitosan based crosslinked hydrogel for in vitro release of encapsulated povidone-iodine: A clinical translation. Int. J. Biol. Macromol. 2020, 164, 4370–4380. [Google Scholar] [CrossRef]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Root-Bernstein, R.S.; Dillon, P.F. Small molecule complementarity as a source of novel pharmaceutical agents and combination therapies. Curr. Pharm. Des. 2008, 4, 55–62. [Google Scholar] [CrossRef]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Patel, J.K. Cyclodextrin inclusion complex to enhance solubility of poorly water soluble drugs: A review. Int. J. Pharm. Sci. Res. 2013, 4, 68–76. [Google Scholar]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Devel. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Sanemasa, I.; Kobayashi, T.; Deguchi, T. Formation constants of cyclodextrin inclusion complexes with iodine in aqueous solutions. Bull. Chem. Soc. Jpn. 1985, 58, 1033–1036. [Google Scholar] [CrossRef]
- Tomono, K.; Goto, H.; Suzuki, T.; Ueda, H.; Nagai, T.; Watanabe, J. Interaction of iodine with 2-hydroxypropyl-α-cyclodextrin and its bactericidal activity. Drug Devel. Ind. Pharm. 2002, 28, 1303–1309. [Google Scholar] [CrossRef]
- Neoh, T.-L.; Noda, Y.; Yoshii, H.; Furuta, T. Release characteristics of iodine encapsulated in cyclodextrins. J. Inc. Phenom. Macrocycl. Chem. 2006, 56, 117–123. [Google Scholar] [CrossRef]
- Asim, M.H.; Jalil, A.; Shahzadi, I.; Khan, M.; Matuszczak, B.; Bernkop-Schnürch, A. Mucoadhesive S-protected thiolated cyclodextrin-iodine complexes: A promising strategy to prolong mucosal residence time of iodine. Future Microbiol. 2019, 14, 411–424. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, É.; Szejtli, J. Entrapment of iodine with cyclodextrins: Potential application of cyclodextrins in nuclear waste management. Environ. Sci. Technol. 1999, 33, 4495–4498. [Google Scholar] [CrossRef]
- Hirota, M.; Higaki, S.; Ito, S.; Ishida, Y.; Terao, K. Effects of 2-hydroxypropyl α-cyclodextrin on the radioactive iodine sorption on activated carbon. J. Radioanal. Nucl. Chem. 2021, 328, 659–667. [Google Scholar] [CrossRef]
- Lechat, F.L.; Wouessidjewe, D.; Herrenknecht, C.; Duchěne, D. Preparation and stability of iodine/α-cyclodextrin inclusion complex. Drug Devel. Ind. Pharm. 1992, 18, 1853–1863. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Feng, Y.; Guo, Q. Preparation, quantitative analysis and bacteriostasis of solid state iodine inclusion complex with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 255–262. [Google Scholar] [CrossRef]
- Polumbryk, M.; Pasichnyi, V.; Omelchenko, C.; Vyshnevskiy, O. Determination of structure and morphology of the cyclodextrins-iodine complexes. Ukr. Food J. 2017, 6, 117–124. [Google Scholar] [CrossRef]
- Guiming, L.; Song, L.T.; Zhao, M.; Yang, Z.; Feng, S.; Huang, M.; Fu, Z.; Zhang, J.; Li, W.; Qin, Y.; et al. High-Stability Cydiodine Powder as Well as Preparation Method and Application Method Thereof. Chinese Patent CN107517963A, 29 December 2017. Available online: https://patents.google.com/patent/CN107517963A/en (accessed on 5 September 2022).
- Nakai, Y.; Yamamoto, K.; Terada, K.; Watanabe, D. New methods for preparing cyclodextrin inclusion compounds. I. Heating in a sealed container. Chem. Pharm. Bull. 1987, 35, 4609–4615. [Google Scholar] [CrossRef]
- Kreaz, R.M.A.; Abu-Eida, E.Y.; Erős, I.; Kata, M. Freeze-dried complexes of furosemide with β-cyclodextrin derivatives. J. Incl. Phen. Macroc. Chem. 1999, 34, 39–48. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Nazzal, S. Curcumin complexation with cyclodextrins by the autoclave process: Method development and characterization of complex formation. Int. J. Pharm. 2017, 520, 173–180. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P.A. Grinding as solvent-free green chemistry approach for cyclodextrin inclusion complex preparation in the solid state. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef]
- Pursell, J.L.; Pursell, C.J. Host–guest inclusion complexation of α-cyclodextrin and triiodide examined using UV–vis spectrophotometry. J. Phys. Chem. A 2016, 120, 2144–2149. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Budai, Z. Acid hydrolysis of β-cyclodextrin. Acta Chim. Hung. 1976, 91, 73–80. [Google Scholar]
- Lee, Y.R.; Do, X.H.; Cho, K.Y.; Jeong, K.; Baek, K.-Y. Amine-functionalized zeolitic imidazolate framework-8 (ZIF-8) nanocrystals for adsorption of radioactive iodine. ACS Appl. Nano Mater. 2020, 3, 9852–9861. [Google Scholar] [CrossRef]
- Berkelman, R.L.; Holland, B.W.; Anderson, R.L. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J. Clin. Microbiol. 1982, 15, 635–639. [Google Scholar] [CrossRef]
- Bhagwat, D.; Iny, O.; Pedi, F. Stabilizing Packaged Iodophor and Minimizing Leaching of Iodine through Packaging. U.S. Patent US4996048, 26 February 1991. Available online: https://patents.google.com/patent/US4996048A/en (accessed on 5 September 2022).
- Musumeci, R.; Bandello, F.; Martinelli, M.; Calaresu, E.; Cocuzza, C.E. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur. J. Ophthalmol. 2019, 29, 673–677. [Google Scholar] [CrossRef] [PubMed]
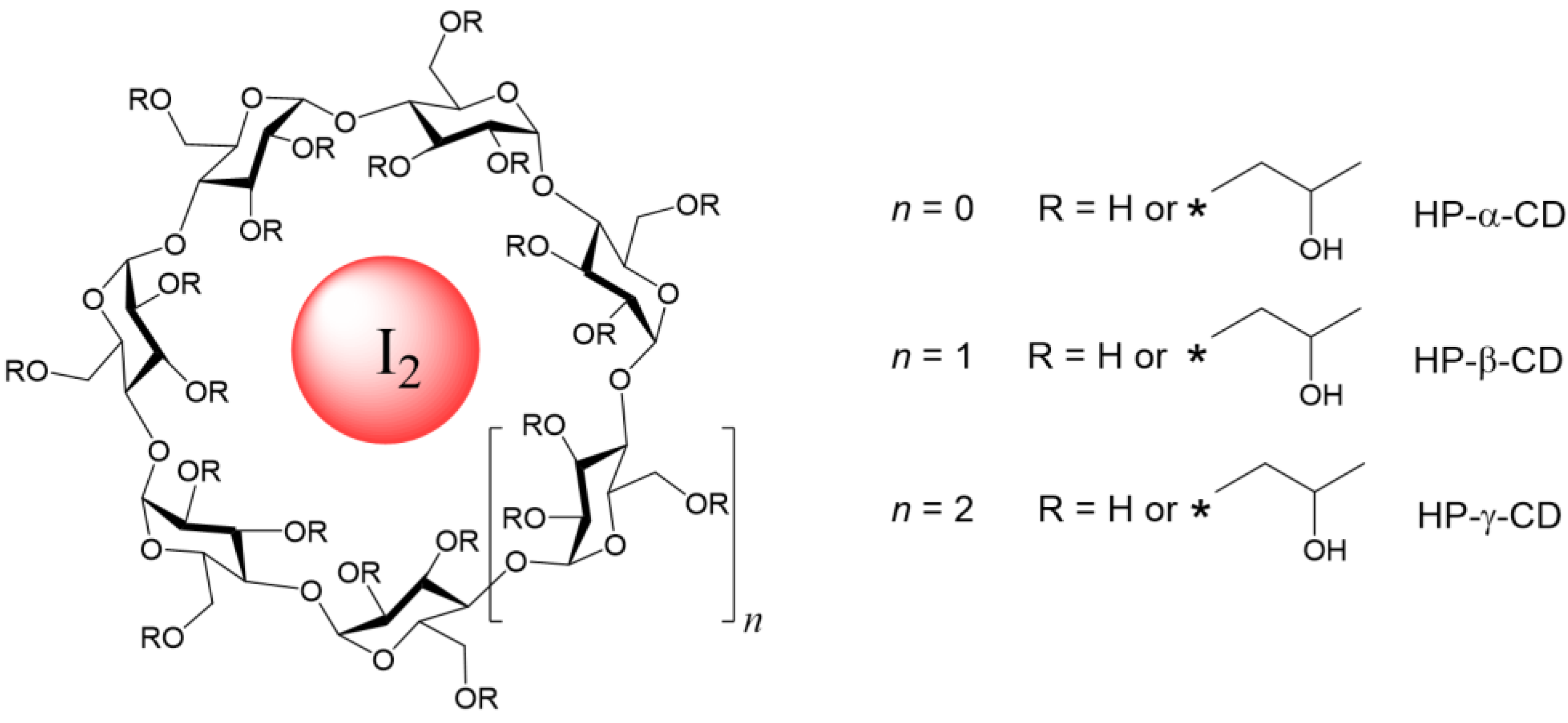


| Entry | Cyclodextrin | Preparation Method a | Temperature (°C) | % Iodine (w/w) b | % Loading c |
|---|---|---|---|---|---|
| 1 | HP-β-CD | LAG | 25 | 5.14 ± 0.03 | 36.2 |
| 2 | HP-β-CD | COE | 30 | 6.01 ± 0.04 | 42.2 |
| 3 | HP-β-CD | SH | 60 | 9.64 ± 0.03 | 67.9 |
| 4 | HP-β-CD | SH d | 60 | 9.78 ± 0.05 | 68.9 |
| 5 | HP-β-CD | SH d | 40 | 4.40 ± 0.04 | 31.0 |
| 6 | HP-β-CD | SH e | 60 | 4.71 ± 0.02 | 61.8 |
| 7 | HP-β-CD | SH f | 60 | 2.40 ± 0.06 | 60.6 |
| 8 | HP-α-CD | LAG | 25 | 10.58 ± 0.02 | 62.7 |
| 9 | HP-α-CD | COE | 30 | 9.45 ± 0.03 | 55.9 |
| 10 | HP-α-CD | SH | 60 | 10.81 ± 0.01 | 63.9 |
| 11 | HP-γ-CD | LAG | 25 | 5.56 ± 0.05 | 40.0 |
| 12 | HP-γ-CD | COE | 30 | 6.77 ± 0.05 | 48.7 |
| 13 | HP-γ-CD | SH | 60 | 8.32 ± 0.03 | 59.9 |
| Entry | Cyclodextrin | Prep. Method | Td1 (°C) b | Td2 (°C) b | Td3 (°C) b | Td4 (°C) b | TDm=50% (°C) c | % R d |
|---|---|---|---|---|---|---|---|---|
| 1 | HP-α-CD | PM | - | - | 363 | - | 341 | 2.3 |
| 2 | HP-β-CD | PM | - | - | 364 | - | 342 | 1.7 |
| 3 | HP-γ-CD | PM | - | - | 342 | - | 330 | 2.6 |
| 4 | HP-β-CD | LAG | 136 | 153 | 258 | 303 | 253 | 12.9 |
| 5 | HP-β-CD | COE | 136 | 155 | 249 | - | 243 | 13.2 |
| 6 | HP-β-CD | SH | 128 | 148 | 240 | - | 234 | 11.1 |
| 7 | HP-β-CD | SH e | 134 | 153 | 250 | 299 | 248 | 12.3 |
| 8 | HP-β-CD | SH f | 139 | 161 | 266 | 320 | 261 | 10.8 |
| 9 | HP-α-CD | LAG | 134 | 151 | 226 | - | 236 | 19.9 |
| 10 | HP-α-CD | COE | 133 | 148 | 230 | - | 233 | 15.3 |
| 11 | HP-α-CD | SH | 130 | 148 | 241 | - | 232 | 13.6 |
| 12 | HP-γ-CD | LAG | 145 | 160 | 255 | 295 | 253 | 12.7 |
| 13 | HP-γ-CD | COE | 141 | 157 | 250 | 288 | 248 | 13.6 |
| 14 | HP-γ-CD | SH | 139 | 163 | 256 | 300 | 251 | 12.5 |
| Complex | I% | I% (UV) a |
|---|---|---|
| HP-α-CD/I2 | 7.7 ± 0.3 | 10.26 ± 0.02 |
| HP-β-CD/I2 | 7.2 ± 0.2 | 8.75 ± 0.06 |
| HP-γ-CD/I2 | 2.9 ± 0.2 | 7.62 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattilo, S.; Spitaleri, F.; Aleo, D.; Saita, M.G.; Patti, A. Solid-State Preparation and Characterization of 2-Hydroxypropylcyclodextrins-Iodine Complexes as Stable Iodophors. Biomolecules 2023, 13, 474. https://doi.org/10.3390/biom13030474
Dattilo S, Spitaleri F, Aleo D, Saita MG, Patti A. Solid-State Preparation and Characterization of 2-Hydroxypropylcyclodextrins-Iodine Complexes as Stable Iodophors. Biomolecules. 2023; 13(3):474. https://doi.org/10.3390/biom13030474
Chicago/Turabian StyleDattilo, Sandro, Fabiola Spitaleri, Danilo Aleo, Maria Grazia Saita, and Angela Patti. 2023. "Solid-State Preparation and Characterization of 2-Hydroxypropylcyclodextrins-Iodine Complexes as Stable Iodophors" Biomolecules 13, no. 3: 474. https://doi.org/10.3390/biom13030474
APA StyleDattilo, S., Spitaleri, F., Aleo, D., Saita, M. G., & Patti, A. (2023). Solid-State Preparation and Characterization of 2-Hydroxypropylcyclodextrins-Iodine Complexes as Stable Iodophors. Biomolecules, 13(3), 474. https://doi.org/10.3390/biom13030474









