Abstract
Circular RNA (circRNA) is a newly discovered noncoding RNA that regulates gene transcription, binds to RNA-related proteins, and encodes protein microRNAs (miRNAs). The development of molecular biomarkers such as circRNAs holds great promise in the diagnosis and prognosis of clinical disorders. Importantly, circRNA-mediated maternal-fetus risk factors including environmental (high altitude), maternal (preeclampsia, smoking, and chorioamnionitis), placental, and fetal (preterm birth and low birth weight) factors are the early origins and likely to contribute to the occurrence and progression of developmental and pediatric cardiopulmonary disorders. Although studies of circRNAs in normal cardiopulmonary development and developmental diseases have just begun, some studies have revealed their expression patterns. Here, we provide an overview of circRNAs’ biogenesis and biological functions. Furthermore, this review aims to emphasize the importance of circRNAs in maternal-fetus risk factors. Likewise, the potential biomarker and therapeutic target of circRNAs in developmental and pediatric lung diseases are explored.
1. Introduction
Circular RNA (circRNA) was first described in RNA viruses/viroids as an error of endogenous RNA junctions [1]. Initially, the function of circRNA was thought to be restricted only to the region of Y chromosome involved in sex determination [2]. In recent years, however, sequencing techniques and computational analyses have revealed that circRNAs have diverse functions [3]. Despite these advances, many questions remain unanswered, such as how microRNAs or RNA binding proteins interact with circRNA, and how circRNAs act as a scaffold to modulate protein complex formation.
The lack of a 3′ and 5′ end in circRNA prevents it from being digested by ribonucleases such as RNase R. Hence, circRNA has a half-life ten times that of linear RNA. CircRNA sequences are highly conserved [4]. CircRNAs are widely found in animal and human cells and play a crucial role in gene transcription and post-transcriptional gene expression. CircRNA can function as an RNA transporter, protein binder, transcription factor, regulatory factor, and miRNA sponge [5,6,7,8]. Circular RNAs contain miRNA response elements, which bind to miRNAs and compete for miRNA-binding sites. Consequently, circRNAs function as intracellular competitive endogenous RNA (ceRNA) by antagonizing miRNA function, which plays a key role in lung development and disease.
Currently over 20,000 circRNAs have been identified. In general, circRNAs are named based on their parental genes or specific functions. For instance, cerebellar degeneration-related protein antisense RNA (CDR1as) is also called ciRS-7 (circRNA sponge for miR-7) [9,10]. In mammals, circRNAs play an essential role in many tissues, including brain, blood, heart, liver, kidney, placenta, and lung [11]. Clinically, the contribution of aberrant circRNA expression to the pathogenesis of cardiopulmonary injuries, as well as the potential of targeting circRNA-miRNA associations is being pursued. (Clinical trials: NCT04864457, NCT03170830, NCT03766204). In addition to adult tissues, circular RNA has been discovered in developing tissues [11,12,13]. In recent years, circRNAs have emerged as important regulatory molecules for cardiovascular and pulmonary development, as well as diseases of aberrant development [11,12,14,15,16,17,18,19]. Recent research has proven that genetic factors play an integral role in the progression of developmental lung diseases such as bronchopulmonary dysplasia (BPD) [7,20] and neonatal acute respiratory distress syndrome (ARDS). Preterm birth can profoundly affect and delay cardiopulmonary development and transition contributing to BPD and neonatal ARDS [21,22].
Remarkable advances have been achieved in circRNA biology over the past few decades, but the effect of regulatory networks on circRNA function and regulation remain largely unexplored in health and disease. Here, we briefly overview circRNA biogenesis and their mechanisms of action in general and important analytic approaches of circRNA profiling. Additionally, we will discuss the latest data regarding their role in mesendoderm differentiation, pulmonary bud formation, lung branching morphogenesis, and vascularization relevant to pulmonary development and related diseases. Finally, circRNA analysis in diagnostic and therapeutic contexts are discussed.
2. Biogenesis and Biological Functions of circRNAs
mRNA precursers (pre-mRNA) typically include one to five exons and introns. Together, the combined sequence of both exons and introns can be as much as three times longer than linear processed mRNA alone [23]. CircRNA appears to originate from pre-mRNA, forming a single-stranded RNA circle with covalently joined 5′ and 3′ ends [3,23]. Three types of circRNAs can be classified according to their sequences: intronic circRNA (CiRNA), extron-intron circRNA (ElcRNA), and exonic circRNA (EcRNA) [24,25]. CircRNAs with exonic sequences are exported to the cytoplasm, whereas circRNAs with introns are anchored in the nucleus. To date, the majority of discovered circRNAs display a predominantly exonic structure and are primarily located in the cytoplasm [26].
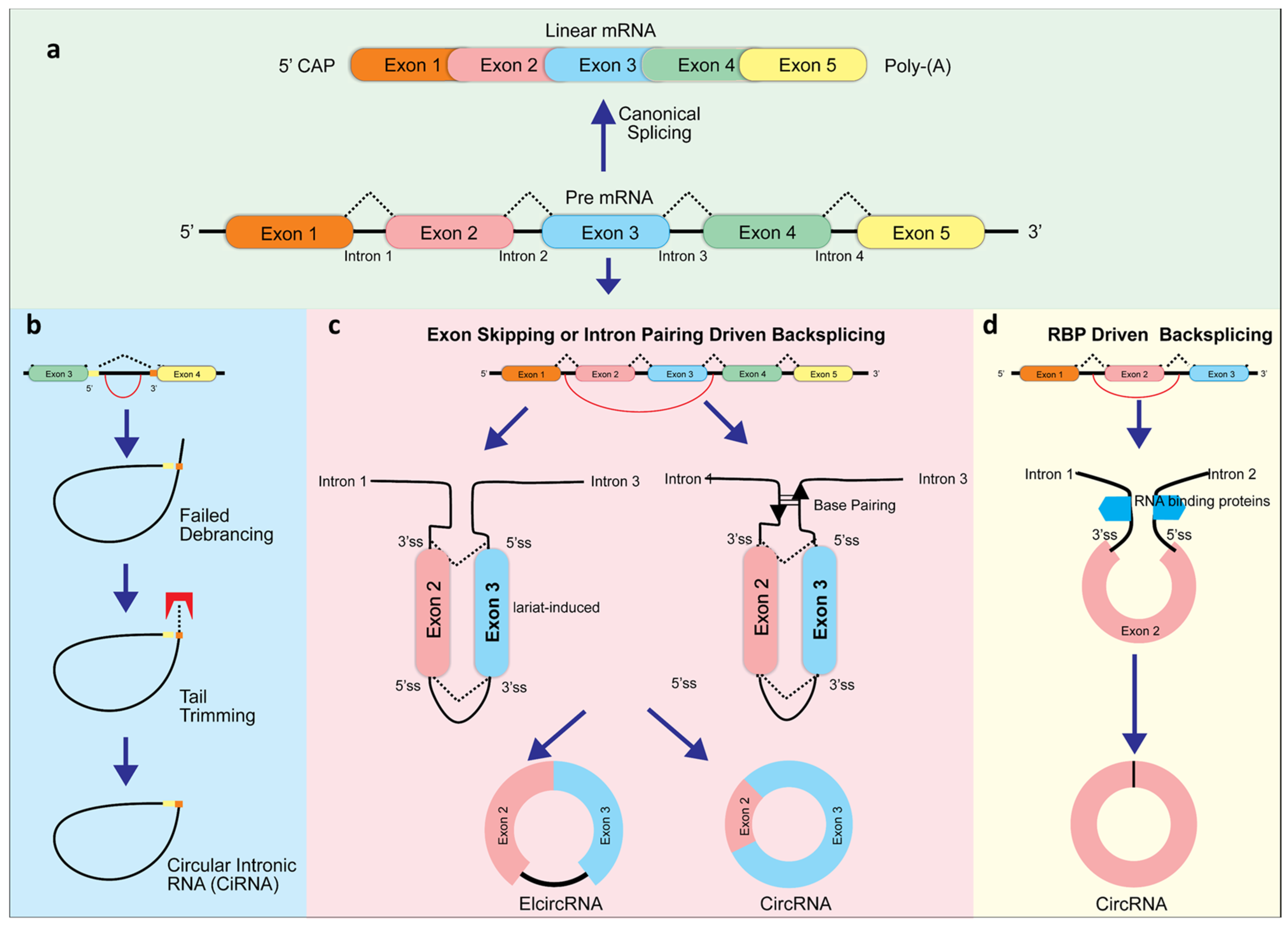
CircRNAs usually are composed of exons 2 and 3 of the gene locus. Most circular RNAs are generated at the expense of their linear counterparts since they are derived from constitutive exons. A large portion of circRNAs contain the canonical splice site motif, suggesting that the canonical spliceosome is involved. When transcription termination factors (for example, SF3b or SF3a) or core spliceosome components (for example, SF3b) are inhibited, circRNAs can become the dominant RNA transcript. Transcripts that are read through can then be backspliced into downstream genes. CircRNAs can also be produced by exon skipping, through either the lariat-guided or intron-guided methods [27]. Currently, there are three models for exon splicing during circRNA formation: exon skipping backsplicing, intron paring backsplicing, and RNA binding proteins (RBP)-driven backsplicing [2,28,29] (Figure 1). The primary working hypothesis for formation of circRNA is that looping the intron sequences along the upstream junction site brings these two sites close together allowing a phosphodiester bond junction to occur where the 3′ and 5′ sites are joined [30]. This complex can be mediated by specific motifs at the 5′ and 3′ sides of introns (exon skipping backsplicing), base pairing between inverted repeating elements (intron paring driven backsplicing), or by dimerization with RNA binding proteins. When exons are skipped, they form a giant lariat, which undergoes internal cleavage, removing the intron and generating ElcRNA and EcRNA [31]. Both intron-pairing circularization or lariat intron driven ciRNA generation involve classic spliceosomes to generate circRNA [29]. Additionally, it has been found that when lariat introns do not detach from their parent mRNA at the level of the branch point site, ciRNAs are formed after trimming the lariat tail [32] (Figure 1).
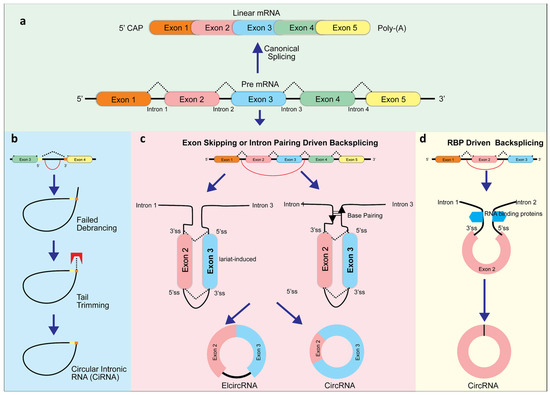
Figure 1.
CircRNA biogenesis. The classic splicing typically generates linear RNA. However, this splicing can also produce circular intronic RNAs (ciRNAs) from intronic lariat precursors (a) when avoiding debranching via the presence of consensus RNA sequences (yellow) (b). In addition, long flanking introns, inverted repeat elements, and RNA binding proteins (RBP) boost backsplicing, including exon skipping (c), intron pairing driven (c), and RBP-driven backsplicing (d).
As research continues, more will be learned regarding the regulation of splicing and circularization to generate circRNAs. CircRNA biogenesis can contain the 5′ end of the pre-mRNA upstream exon coupled to the downstream exon at the 3′ end. CircRNAs have covalently closed loops with no 3′ cap and no 5′ end. Therefore, their resistance to fluid absorption and ribonuclease (RNase) degradation leads to the relative stability of circRNA in the body compared to linear RNA [33,34]. The back-splicing of circRNA is dependent on DNA sequences (complementary intronic (cis)-elements sequences) and RNA-binding proteins (trans-factors) [33,35]. Circular RNAs are almost exclusively exonic and lack intron segregation. In fact, it was found that it is not exon sequences but circRNA’s complementary introns that regulate circRNA synthesis [28,36]. Moreover, complementary side sequences are enriched with circRNA introns in various species such as Caenorhabditis elegans, rodent, pig, and human [37,38,39,40].
Several mechanisms have been discovered for regulating circRNA biogenesis. Inhibiting spliceosomes by depleting U2 small nuclear ribonucleoprotein components, can increase circular to linear RNA ratios [41]. Alternative pathways can direct the newly formed RNA to a pathway that boosts backsplicing when pre-mRNA processing slows down. RNA binding proteins (RBPs) such as heterogeneous nuclear ribonucleoprotein (hnRNP), serine-arginine (SR), and FUS protein [42,43], as well as splicing regulators such as NF110 and NF90, Muscleblind, and NOVA2 can bind to intronic sequences flanking circularized exons and stabilize CIS pairs enhancing the production of circRNA [44,45,46]. Conversely, circRNA formation is inhibited by some RBPs. As an example, by binding to reverse complementary ALU elements, DEAH-box helicase 9 (DHX9) influences the uncoiling of Alu (Arthrobacter luteus) elements and inhibits the formation of circRNA [47]. Alu elements, transposable segments of DNA that are recognized by the Alu endonuclease restriction enzyme, are found throughout the genome. Originally thought to be parasitic DNA, evolutionary and functional roles are being discovered for this large family of retrotransposons. Among the newly discovered roles, complementary Alu segments in introns facilitate a more accessible junction leading to more circRNA generation [48]. In addition, when other factors such as the pre-mRNA 3′ end processing endonuclease Cpsf73 are depleted, each one may cumulatively disrupt circRNA formation [41]. ATP-dependent RNA helicase A and endogenous double-stranded RNA are required for the biogenesis of circRNAs that rely on base pairing between reverse repeats [44].
2.1. Transcriptional Regulation
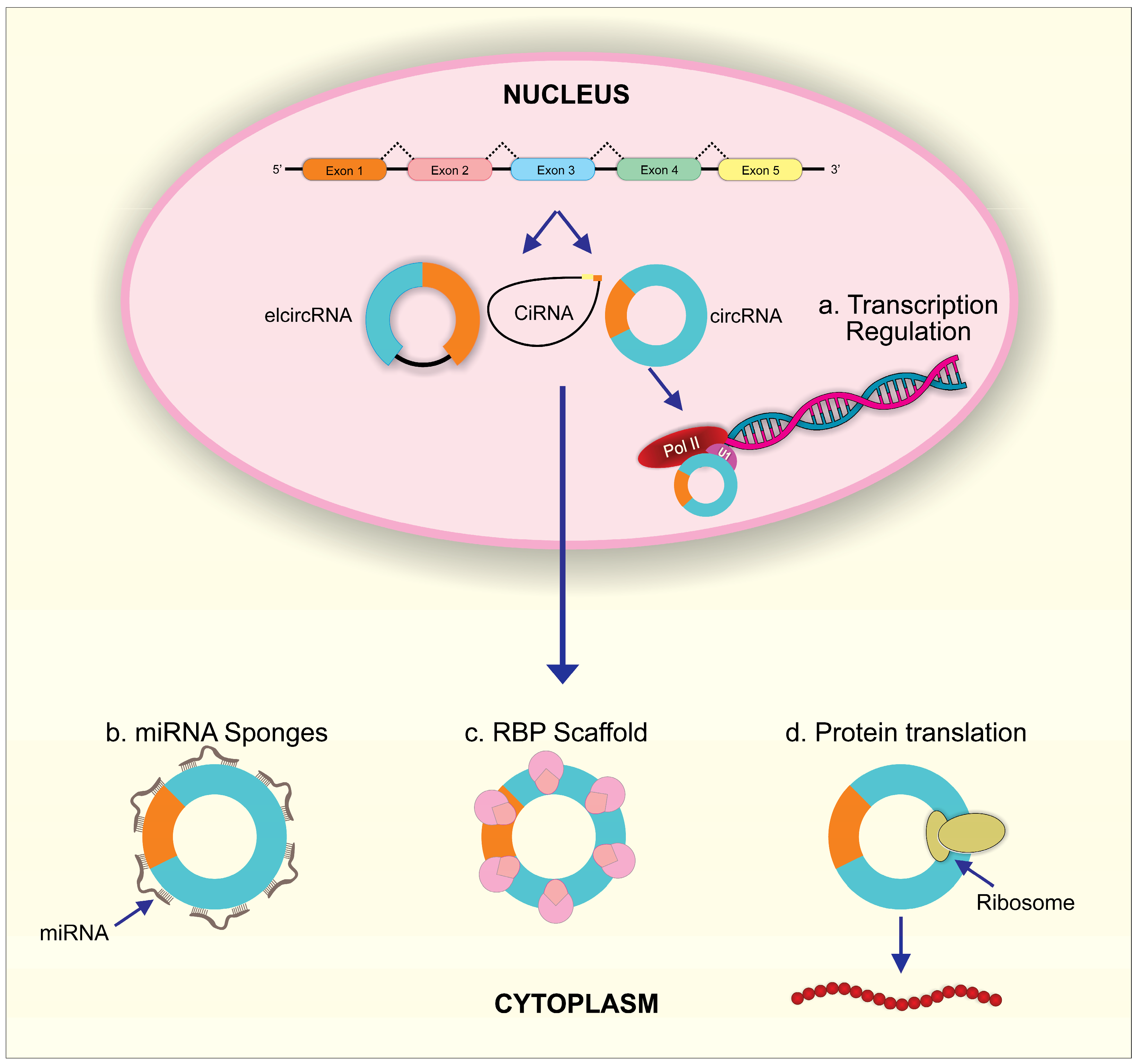
In the nucleus, circRNAs such as EIciRNAs can directly bind to elongated RNA Pol II binding sites or interact with the Pol II transcription complex after forming EIciRNA–U1 snRNP complexes through RNA–RNA interactions. Both interactions regulate the transcription of circRNA parent genes (Figure 2). In human cells, ci-ankrd52 activates RNA polymerase II and enhances transcription of the parent gene ANKRD52 [49]. Binding of CircEIF3J and circPAIP2 to U1 snRNA through the U1-binding site in EIciRNA was demonstrated to be required for the transcription-enhancing effect of these two EIciRNAs [50]. In addition, some circRNAs such as circITGA7, circ-HuR, circ-STAT3, and circ-DAB1 regulate the parent genes’ transcription via their interaction and modulation of transcriptional factors [51,52,53,54]. CircRNAs, such as FECR1 and TAH-circRNAs, induced DNA methylation and thus contributed to circRNA-mediated transcriptional regulation of parent genes [55,56].
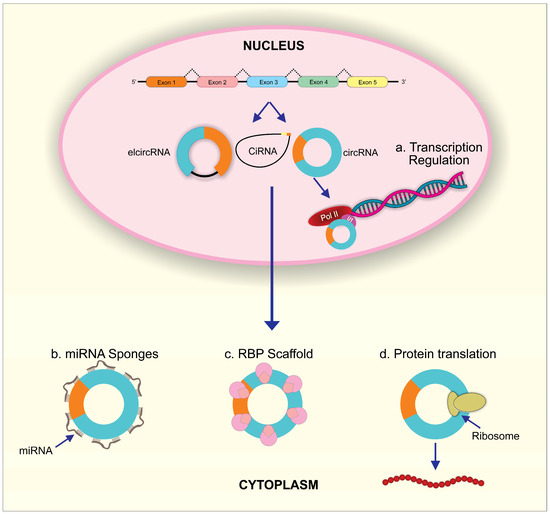
Figure 2.
The biological functions of circRNAs. (a) In the nucleus, circRNAs can regulate parent RNA transcription through combining with U1 snRNP and then interacting with Pol II. In the cytoplasm, (b) circRNAs can act as miRNA sponges to inhibit miRNA activity; (c) circRNAs can scaffold RBPs to regulate post-transcriptional process; (d) circRNAs can initiate protein translation by recruiting ribosome.
2.2. CircRNA as Super Sponge for miRNA
miRNA is noncoding RNA approximately 18 to 25 nucleotides long and an essential epigenetic regulator in eukaryotes. Mature miRNA can directly bind to target mRNAs, resulting in degradation of target mRNAs or suppression of target mRNA’s translation in lung development and diseases [57,58,59]. Remarkably, it has been verified that circRNAs contain miRNA response elements (MER), which can competitively bind to miRNAs [32] (Figure 2). Therefore, circRNAs act as intracellular competitive endogenous RNA (ceRNA) to antagonize miRNA function. The powerful miRNA sponge function of circRNAs was verified for the first time in 2013 [10]. Circular RNA, ciRS-7 (also termed CDR1as), was identified and co-expressed with miR-7 in the mouse brain. Significantly, the combination of ciRS-7 and miR-7 contributed to increased miR-7 target gene expression through suppressed miR-7 activity. This supports ciRS-7 as a miR-7 inhibitor, attributable to the more than 70 conventional binding sites for miR-7 on ciRS-7 [10]. At the same time, the testis-specific circRNA, sex-determining region Y (Sry)9, was found to have 16 putative target sites for miR-138 and was demonstrated to interact with miR-138, leading to decreased knockdown potential for miR-138 on its target genes [10]. Increasing evidence has accumulated for miRNA sponge effects by circRNA as a general phenomenon in the normal development and progression of various disease.
2.3. CircRNA as RNA Binding Protein Sponge
RNA binding proteins (RBPs) can target RNA through RNA-binding domains to regulate gene expression in different cellular processes such as cell morphology and differentiation, cell proliferation, response to oxidative stress, aging, and apoptosis [60]. CircRNAs have been found to play an essential role in forming this stable RNA protein complex that influences host gene expression [35] (Figure 2). In particular, circ-Mbl, circ-PAIP2, and circ-EIF3J interact with RNA polymerase II to enhance parent gene expression [41,50,61]. CircE2F2 or circPABPN1 interacts with HuR to regulate the stability and translation of target E2F2 or PABPN1mRNA [62]. CircCUX1, through its interaction with EWS RNA binding protein 1, facilitates MAZ-mediated CUX1 transcription [63]. In addition, circRNAs can sequester RBPs in order to affect the translocation of these proteins, which then affects the RBP-target gene regulation [64,65]. To be noted, circRNAs’ dynamic tertiary structure may be affected by different cell types, tissues, and developmental stages, which can affect their ability to bind to various proteins. Circular RNAs can display a variety of functions as a result of different circRNA–RBP interactions [66].
2.4. CircRNA as mRNA for Protein Coding
Several aspects of circRNA sequence have raised the possibility of translation. Several in vitro and in vivo studies have shown that circular RNA have an intrinsic entry site (IRES) or a modification of N6-methyladenosine (m6A) that can facilitate translation into peptides (Figure 2) [67,68,69]. Approximately 13% of all discovered circRNA sequences contain m6A consensus motifs. The YTHDF3 reader can recruit translation initiation factors to begin protein translation at a single m6A site and initiate circRNA translation. m6A modifications are commonly distributed in circRNAs and can strongly influence the efficiency of circRNA translation [70]. Many circRNAs such as circZNF609, circPINTexon2, circFBXW7, circSHPRH, circ-AKT3 and circβ-catenin contain the initial codon of ribosome-associated mRNAs [71,72,73,74,75,76]. Peptides can be translated from the small open reading frames (sORFs) of these circRNAs using coupling-dependent and non-constrained mechanisms, but not cap-dependent mechanisms since circRNAs lack a 5′cap [67].
Pamudurti et al. in their study presented evidence supporting circRNA translation: 1. circRNA was associated explicitly with translation ribosome; 2. proteins were generated from circRNA minigenes; 3. ribosome footprint readings were supported by the stop codon in circMbl; 4. sequences were identified that promoted cap-independent translation on several circRNAs; 5. a novel isoform of a peptide was detected coinciding with circMbl. However, circRNAs associated with ribosomes were detected in lower abundance than free circRNAs and some minigenes did not lead to protein production, suggesting that a specific process promotes translation of a subset of circRNAs [77].
2.5. Post-Translation Regulation
Recent studies revealed that circRNA could compete with enzymes and influence the post-translational modifications of full-length proteins coded by parent genes. Circβ-catenin-coding β-catenin-370aa competitively interacted with GSK3β and inhibited its binding to β-catenin, leading to the antagonization of GSK3β-induced β-catenin degradation [76]. AKT3-174aa encoded by circ-AKT3 competed with this AKT isotype for binding to pPDK1 and reduced AKT-Thr308 phosphorylation, indicating that AKT3-174aa plays a negative regulatory role in regulating phosphatidylinositol 3-kinase (PI3K)/AKT signaling activity [75]. Alternately, circFBXW7 was observed to interact with the deubiquitinating enzyme USP28, preventing USP28 from binding to FBXW7 and thus upregulating the expression of FBXW7 [78].
3. CircRNA and Risk Factors of Developmental and Pediatric Lung Diseases
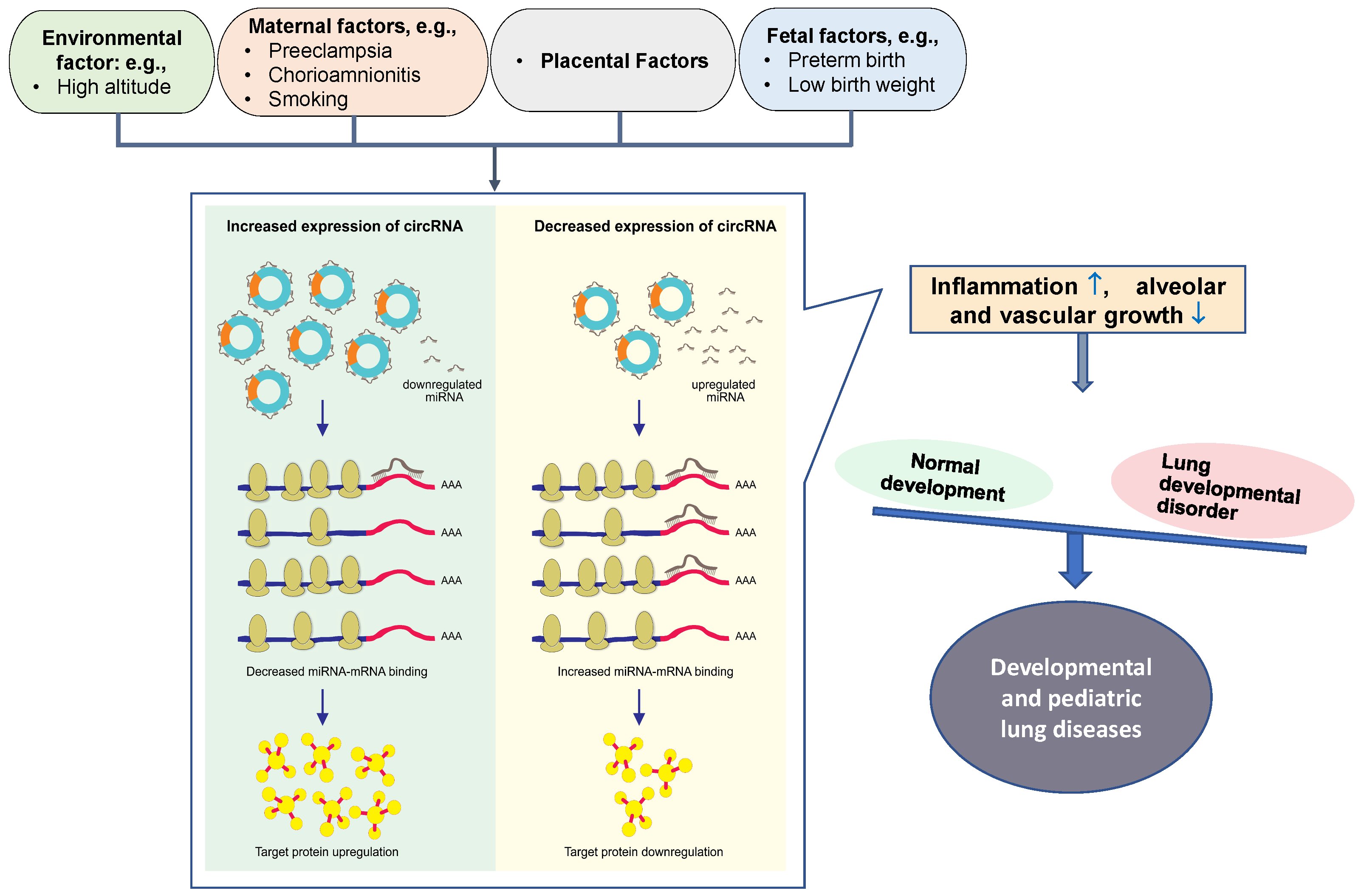
Developmental and pediatric lung diseases are disorders of lung development affecting neonates, infants, children, and adolescents. The risk factors include environmental (high altitude), maternal (preeclampsia, smoking, and chorioamnionitis), placental, and fetal (preterm birth and low birth weight) factors. These risk factors are interrelated and interact during fetal development. They impact perinatal “plasticity”, lung development, as well as lung injury and repair processes. There is an increasing body of evidence that abnormal circRNA expression is associated with developmental diseases in humans and is associated with gene dysregulation [13,79].
In one animal model used to study the effects of high altitude (hypoxic) environmental stress and adaptation, circRNAs and mRNAs (SKIV2L2, PRKCSH, NewGene.10854.1, POR and LOC102286089) were found to be downregulated in yak lungs exposed to high altitude [80]. This study revealed the role of circRNAs in transcriptional changes in response to high altitude adaptation. In a human subject study, Qian et al. examined circRNA expression patterns in the placental tissues of pregnant women with preeclampsia and pregnant women who delivered healthily but prematurely [81]. They discovered significant differential expression of circRNAs in the placental tissues of women with preeclampsia: 143 circRNAs were upregulated, and 158 were downregulated [81]. In another study, 300 circRNAs were identified as differentially expressed between preeclampsia and normal placental tissues [82]. Among them, hg38_circ_0014736 and hsa_circ_0015382 were validated as significantly upregulated and hsa_circ_0007121 was significantly downregulated [82]. Remarkedly, the GEO databases GSE102897 and GSE96985 show different expression profiles of circRNAs in human preeclampsia and normal placentas. It has been shown that increased circRNAs such as circ_0001687, circLRRK1, circ_0008726, circ_0085296, circ_0011460, circ_0026552 and decreased circRNAs such as circ_0001513 play a role in trophoblast proliferation, migration, and angiogenesis during preeclampsia development [83,84,85,86,87,88].
Preeclampsia is one of the leading causes of premature birth. As a result of premature birth, there are a number of complications that result in significant morbidity and mortality, including bronchopulmonary dysplasia (BPD), hyaline membrane disease, and pulmonary interstitial emphysema [89]. A recent study has documented a circRNA expression profile associated with preterm birth, and preliminarily analyzed its regulatory mechanism and predictive value for preterm birth [90]. About 211 abnormally expressed circRNAs existed in the peripheral blood of preterm women. Among them, the top 20 circRNAs, including hsa-SCARF1_0001, hsa-GCN1_0003, hsa-RAD54L2_002, hsa-CREBBP_0001, hsa-FAM13B_0019, hsa-NUSAP1_0010, hsa-YY1AP1_0001, hsa-MORC3_0001, and hsa-RANBP9_0002, are related to immune/inflammatory pathways mediating the process of preterm birth [90]. Although there is not yet a clear and direct relationship between maternal-fetus risk factors and these disorders, these risk factors can affect prenatal lung development and can have a direct impact on neonatal health. Therefore, circRNA-mediated maternal-fetus risk factors are likely to contribute to the occurrence and progression of developmental and pediatric lung disorders (Figure 3).
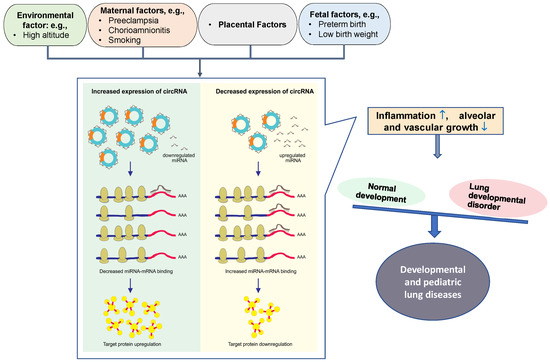
Figure 3.
Schematic diagram of circRNA-miRNA interaction on controlling developmental and pediatric lung diseases. CircRNAs contain miRNA-binding sites. Maternal-fetus risk factors cause the alteration of circRNAs in placenta or/and circulation. Increased circRNAs can absorb more miRNAs, leading to miRNA downregulation; this reduces miRNA-mRNA binding, thereby upregulating miRNA target gene expression and protein translation. Conversely, decreased circRNAs and circRNA-miRNA interaction will downregulate miRNA target gene expression and protein translation. Eventually, the dysregulated proteins involved in the abnormal lung development can induce inflammation ( ) and suppress the growth of alveoli and blood vessels (
) and suppress the growth of alveoli and blood vessels ( ), potentially leading to developmental and pediatric lung diseases.
), potentially leading to developmental and pediatric lung diseases.
 ) and suppress the growth of alveoli and blood vessels (
) and suppress the growth of alveoli and blood vessels ( ), potentially leading to developmental and pediatric lung diseases.
), potentially leading to developmental and pediatric lung diseases.
Current research into the role of circRNAs in developmental and pediatric lung disorders is restricted to BPD. The current definition of BPD describes a developmental and pediatric lung disease with less heterogeneity, simplified alveolar surface, and reduced and dysmorphic vascular bed [91]. In preterm infants, placental, environmental, and genetic insults may result in abnormal alveolarization and vascularization of the lungs, increasing the possibility of developing BPD. There is clear evidence that pulmonary vascular and alveolar development are interdependent processes, and a negative correlation between alveolar simplification and distal lung angiogenesis has been observed [92]. Although BPD treatment and diagnosis are progressing, many patients still suffer from lung damage, leading to long-term lung dysfunction [93,94]. Much is still unknown regarding the multi-faceted and complex pathological process of BPD [95]. Further clarification of the molecular mechanisms leading to BPD can suggest novel molecular targets for diagnosis and treatment.
4. Co-Expression Networks of CircRNA-miRNA in the Progression of Developmental and Pediatric Lung Diseases
Although non-coding RNAs (ncRNAs) were once considered a waste product, they are now recognized as molecules that regulate many lung disorders. Since circRNAs act as sponges in the human body for miRNAs and can alter the function of those miRNAs, presumably circRNA can influence the occurrence and progression of diseases through regulation of miRNAs [13] (Figure 3).
Recent discoveries revealing the profile of circRNA expression in preterm infants with BPD is beginning to elucidate the role of circRNA in BPD and its dysregulated biological processes. In the peripheral venous blood of neonates with BPD, 491 circRNAs were markedly altered [20]. Significantly increased were circ_FANCL, circ_0009256, circ_0003037, circ_0009983, circ_0003357, and circ_0003122, while significantly decreased were circ_0014932, circ_0015109, circ_0017811, circ_0020588, and circ_0015066. These altered circRNAs likely contribute to the complex signaling pathways and biological processes in BPD [20]. Individuals with moderate BPD displayed a significant increase in circ_FANCL in correlation with oxygen treatment [20]. In another study, circABCC-4 levels in peripheral blood from 31 preterm infants with BPD were significantly increased, and the significance of the increase was positively correlated with poor long-term outcomes [96]. Further, both in vitro and in vivo, circABCC-4 promotes apoptosis and inhibits cell proliferation, important interrupted processes in BPD development [96]. Therefore, there is a clear indication that an interconnected circRNA expression profile in peripheral blood in neonates has important implications in both the diagnosis and pathogenesis of BPD.
In recent years, there has been considerable interest in exploring ncRNAs and miRNAs in BPD. Tao et al. [97] illustrated the interaction between the gene, RNA imprinted and accumulated in nucleus (Rian), and miR-421 in BPD models. They determined that Rian was downregulated in hyperoxia-induced BPD, which induced inflammatory responses via targeting miR-421 and upregulating miR-421 expression. Using analysis of miRNA-circRNA co-expression networks in peripheral venous blood from neonates with BPD, it was determined that many miRNAs can bind to one circRNA. At the same time, one miRNA can also regulate different circRNAs functions (Table 1). For instance, upregulated circ_FANCL interacts with let-7, miR-196, miR-20a, miR-22, and miR-26a. This network can be expanded through let-7 which can regulate the TGF-β/RAS/HMGA2 pathways [20,98]. CircABCC4 has been found to be related to BPD from genetic screening; this circRNA was found to share the miRNA response element of miR-663a with PLA2G6, strongly indicating that there exists an axis between these three molecules [96]. Furthermore, a circABCC4/miR-663a/PLA2G6 network was associated with the severity of the development and clinicopathological features of BPD [96]. In vitro studies documented circABCC4 targeting and downregulation of miR-663a expression, which directly inhibited PLA2G6 expression. In rat, six circRNAs were identified and positively correlated with BPD, while seven other circRNAs were negatively correlated with BPD [99]. In addition, Wang et al. [7] identified 634 miRNAs and 1545 circRNAs in BPD mouse models. Further, they generated circRNA-miRNA co-expression networks for seven upregulated circRNAs (e.g., Chr8:11226466|11231468, Chr9:108218013|108218410, Chr8:127415570|127426753, Chr13:10386649|103897928, Chr2:160750963|160752574, Chr11:106868535|106875939 and Chr3:15411189|15542472) and three downregulated circRNAs (e.g., Chr1:85202140|85659862, Chr1:177096967|177109738 and Chr14:70256360|70267506) (Table 2). Among the miRNAs identified in this circRNA-miRNA co-expression network, some miRNAs such as the let-7 family, miR-141, miR-100, miR-181b, miR-503, miR-29a, miR-135b, and miR-17 have previously been identified in the development of BPD [7,100,101,102,103]. This suggests that circRNA-miRNA networks can influence genes in signaling pathways associated with the progression of BPD, both positively and negatively.

Table 1.
CircRNAs-miRNA involved in lung development.

Table 2.
Dysregulated circRNAs in BPD.
In a recent study, circRNA expression profiles were investigated in neonatal acute respiratory distress syndrome (ARDS) [111]. In the United States, neonatal ARDS is the most common cause of respiratory distress in premature infants. It is noteworthy that babies born with ARDS are likely to develop BPD. Prematurity and the low birth weight of the infant are the most significant risk factors for both. Additionally, maternal diabetes, hypoxia, and ischemia during pregnancy contribute to the risk [22]. The Montreaux definition is a consensus definition for neonatal ARDS that covers neonates from birth to 44 weeks postmenstrual age (4 weeks if the baby is born at term) [112]. Neonatal ARDS is characterized by inflammation of the lungs and catabolism of surfactant molecules, leading to pulmonary dysfunction in neonates [112]. Neonatal ARDS remains one of the leading causes of morbidity and mortality in preterm infants despite treatment advances, such as antenatal corticosteroids and surfactants. Physician-scientists are continuing to research the mechanisms of neonatal ARDS and search for new therapeutic targets. In the blood samples of newborns with neonatal ARDS, Zhou et al. discovered 741 circRNAs that were downregulated and 588 that were upregulated compared to those in normal newborn blood [111]. Based on bioinformatic analysis of the parental genes of differentially expressed circRNAs, these circRNAs could be involved in protein synthesis and metabolism in neonatal ARDS [111]. As an example, the hsa_circ_0005389 gene regulates the amino acid transporter SLC38A10 [111], which is involved in immune response, nascent protein synthesis, and cell survival under oxidative stress [113]. In neonatal ARDS, three upregulated circRNAs (hsa_circ_0005389, hsa_circ_0000367, hsa_circ_0059571) and two downregulated circRNAs (hsa_circ_0058495, hsa_circ_0006608) were found to interact with 25 miRNAs and 125 target genes [111]. These genes contribute to inflammation in the early stages of neonatal ARDS by synthesizing and secreting endocrine hormones, such as glucocorticoids, and affecting gene regulatory cascades, such as the stress-activated MAPK cascade [111]. As a result, circRNAs may offer new approaches to diagnose neonatal ARDS and decrease the inflammatory response in neonatal ARDS and related diseases, potentially offering a new therapeutic route.
5. Potential Biomarkers or Therapeutic Targets of circRNAs in Developmental and Pediatric Lung Diseases
There is growing evidence that exosome-noncoding RNAs likely contribute to lung disease. Research has demonstrated that exosomes isolated from tracheal aspirates of infants with severe BPD and bronchoalveolar lavage fluids from hyperoxia-treated newborn mice contain reduced miR-876 levels, a miRNA implicated in BPD development [114]. A large body of research on exosome-noncoding RNAs in lung disorders has focused on exosome-miRNAs and exosome-, long, noncoding RNA (lncRNAs), while very little research has been conducted on other noncoding RNAs species such as exosome-circRNAs. In addition to microRNAs and lncRNAs, circRNAs were identified in exosomes, thus the important role of exosome-bound circRNAs in developmental lung disorders cannot be ignored. In a recent study, circRNAs, long noncoding RNAs, and mRNAs were profiled in the umbilical cord blood of newborns with BPD, and 317 circRNAs, 104 long noncoding RNAs, and 135 mRNAs were found to be altered. Through bioinformatic analysis, several potential exosomal circRNA/lncRNA–miRNA–mRNA networks were identified with relevance to BPD pathogenesis [115]. However, our knowledge of the connection of circRNAs with exosomes remains limited compared to that of exosomal lncRNA or miRNA, especially in regards to diagnosis and treatment of developmental and pediatric lung diseases.
A substantial amount of research has been conducted in the field of cardiovascular diseases on stem cell exosome-based biomarkers, therapy strategies, and drug delivery [116]. The use of nanoparticles as a carrier to deliver RNA specifically and efficiently to target cells is a significant development towards therapeutic exosome engineering. Exosomal circRNAs have been extensively studied as therapeutic targets for cancer. For example, overexpression of exosomal circSHKBP1 may promote gastric cancer proliferation, migration, invasion, and angiogenesis, while knockdown of exosomal circSHKBP1 reduces lung metastatic tumor size and number [117]. Once the mechanism by which exosomal circRNAs or exosomal circRNA/miRNA disseminate through body fluid and their role in abnormal lung development and lung disorders has been determined, circRNA-exosome-targeted therapy may offer novel targets for therapeutic intervention for lung regeneration and prevention of the harmful effects of developmental lung diseases.
In fascinating investigations, circRNAs have been engineered to act as sequestering sponges for miRNAs associated with human diseases [118,119]. This makes circRNA a useful tool for the study of molecular biology and molecular medicine because the engineered circRNA (circmiRs) can be targeted to the nucleus as well as the cytoplasm. In recent work, Lavenniah et al. designed a circRNA sponge to target miR-132 and -212, known pro-hypertrophic miRs in the heart [119]. Artificial circRNA sponges successfully targeted the miR-212/132 family, were successfully delivered to cardiomyocytes in vivo, and successfully reduced left ventricular hypertrophy [119]. In light of this, the potential of engineered circRNAs as a future therapeutic in humans is promising. The implications of this are tremendous as it suggests that circRNA has great promise as a method of controlling developmental and pediatric lung disease progression. Nevertheless, the biological functions and mechanisms of circRNA-miRNA must be explored and verified further to be used as a clinical approach for developmental and pediatric lung diseases.
CircRNAs serve as scaffolds for proteins and miRNA sponges, and can affect translation, transcription, and degradation of specific mRNAs. There will likely be wide-ranging developments in the circulating RNA field in the coming years. CircRNA transport, localization, degradation, and biological function will be characterized in greater depth. Though newly emerging, circRNA has been identified as an essential player in lung development and developmental lung diseases. The role of circRNAs as miRNA sponges in the normal development of lung tissue and lung diseases is still being explored, and their precise mechanism of action is still unknown. It is, therefore, necessary to perform more analyses on samples collected from humans to identify circRNAs involved in cardiopulmonary development and related diseases. Further discovery of circRNA and circRNA-miRNA networks offer promising targets for therapies for developmental and pediatric cardiopulmonary disorders. As circRNAs are identified as potential biomarkers for developmental lung disease, more in-depth exploration is needed to demonstrate their relative accuracy and reliability in BPD as well as other developmental and pediatric lung diseases. Much is still unknown regarding the molecular function of circRNA in lung development and diseases. With our increased understanding of circRNA mechanisms, we expect increased opportunities for innovative treatments targeting the many circRNA-based roles in physiological and pathological processes, including those of the developing lung.
Author Contributions
Contributions: conceptualization, D.Y. and R.S.; writing—original draft preparation, Y.T. and S.Z.; writing—review and editing, S.R., D.Y. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Science and Technology Plan Project of Liaoning Province (No.2020JH2/10300128) and Natural Science Foundation of Liaoning Province (No.201602873).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cocquerelle, C.; Mascrez, B.; Hetuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell. Mol. Life Sci. 2018, 75, 1071–1098. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Tian, Y.; Gao, Y.; Dong, X.; Chen, W.; Yuan, X.; Yin, W.; Xu, J.; Chen, K.; et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 2020, 19, 128. [Google Scholar] [CrossRef]
- Wang, J.; Yin, J.; Wang, X.; Liu, H.; Hu, Y.; Yan, X.; Zhuang, B.; Yu, Z.; Han, S. Changing expression profiles of mRNA, lncRNA, circRNA, and miRNA in lung tissue reveal the pathophysiological of bronchopulmonary dysplasia (BPD) in mouse model. J. Cell. Biochem. 2019, 120, 9369–9380. [Google Scholar] [CrossRef]
- Tang, M.; Kui, L.; Lu, G.; Chen, W. Disease-Associated Circular RNAs: From Biology to Computational Identification. Biomed. Res. Int. 2020, 2020, 6798590. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA expression profiles and features in human tissues: A study using RNA-seq data. BMC Genomics 2017, 18, 680. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Huo, C.; Ding, N.; Li, J.; Xiao, J.; Lin, X.; Cai, B.; Zhang, Y.; Xu, J. Dynamic Organization of lncRNA and Circular RNA Regulators Collectively Controlled Cardiac Differentiation in Humans. EBioMedicine 2017, 24, 137–146. [Google Scholar] [CrossRef]
- Lee, E.C.S.; Elhassan, S.A.M.; Lim, G.P.L.; Kok, W.H.; Tan, S.W.; Leong, E.N.; Tan, S.H.; Chan, E.W.L.; Bhattamisra, S.K.; Rajendran, R.; et al. The roles of circular RNAs in human development and diseases. Biomed. Pharmacother. 2019, 111, 198–208. [Google Scholar] [CrossRef]
- Lim, T.B.; Lavenniah, A.; Foo, R.S. Circles in the heart and cardiovascular system. Cardiovasc. Res. 2020, 116, 269–278. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhuang, B.; Yin, J.; Chen, X.H.; Wang, J.; Li, M.M.; Xu, J.; Wang, X.Y.; Yu, Z.B.; et al. Differential Expression of CircRNAs in Embryonic Heart Tissue Associated with Ventricular Septal Defect. Int. J. Med. Sci. 2018, 15, 703–712. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Pan, J.; Chen, C.; Xia, S.; Song, Y. Circular RNAs: A rising star in respiratory diseases. Respir. Res. 2019, 20, 3. [Google Scholar] [CrossRef]
- Zhang, M.J.; Yin, J.W.; Wu, J.H.; Gu, J.; Yuan, C.Y.; Miao, H.J.; Yu, Z.B. Circular RNAs are abundant and dynamically expressed during the embryonic lung development of C57BL/6 mice. Heliyon 2020, 6, e03437. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Pan, J.J.; Sun, Z.Y.; Chen, X.Q.; Zhou, X.G.; Zhou, X.Y.; Cheng, R.; Yang, Y. Differential expression of circRNAs during rat lung development. Int. J. Mol. Med. 2019, 44, 1399–1413. [Google Scholar] [CrossRef]
- Liu, C.; Li, N.; Dai, G.; Cavdar, O.; Fang, H. A narrative review of circular RNAs as potential biomarkers and therapeutic targets for cardiovascular diseases. Ann. Transl. Med. 2021, 9, 578. [Google Scholar] [CrossRef]
- Mao, X.; Guo, Y.; Qiu, J.; Zhao, L.; Xu, J.; Yin, J.; Lu, K.; Zhang, M.; Cheng, R. Next-generation sequencing to investigate circular RNA profiles in the peripheral blood of preterm neonates with bronchopulmonary dysplasia. J. Clin. Lab. Anal. 2020, 34, e23260. [Google Scholar] [CrossRef]
- Mirza, H.; Garcia, J.A.; Crawford, E.; Pepe, J.; Zussman, M.; Wadhawan, R.; Oh, W. Natural History of Postnatal Cardiopulmonary Adaptation in Infants Born Extremely Preterm and Risk for Death or Bronchopulmonary Dysplasia. J. Pediatr. 2018, 198, 187–193 e181. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Zhang, D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: A meta-analysis. Acta Diabetol. 2019, 56, 729–740. [Google Scholar] [CrossRef]
- Szabo, L.; Salzman, J. Detecting circular RNAs: Bioinformatic and experimental challenges. Nat. Rev. Genet. 2016, 17, 679–692. [Google Scholar] [CrossRef]
- Vromman, M.; Vandesompele, J.; Volders, P.J. Closing the circle: Current state and perspectives of circular RNA databases. Brief. Bioinform. 2021, 22, 288–297. [Google Scholar] [CrossRef]
- Panda, A.C.; De, S.; Grammatikakis, I.; Munk, R.; Yang, X.; Piao, Y.; Dudekula, D.B.; Abdelmohsen, K.; Gorospe, M. High-purity circular RNA isolation method (RPAD) reveals vast collection of intronic circRNAs. Nucleic Acids Res. 2017, 45, e116. [Google Scholar] [CrossRef]
- Lei, M.; Zheng, G.; Ning, Q.; Zheng, J.; Dong, D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer 2020, 19, 30. [Google Scholar] [CrossRef]
- Gasparini, S.; Licursi, V.; Presutti, C.; Mannironi, C. The Secret Garden of Neuronal circRNAs. Cells 2020, 9, 1815. [Google Scholar] [CrossRef]
- Meganck, R.M.; Liu, J.; Hale, A.E.; Simon, K.E.; Fanous, M.M.; Vincent, H.A.; Wilusz, J.E.; Moorman, N.J.; Marzluff, W.F.; Asokan, A. Engineering highly efficient backsplicing and translation of synthetic circRNAs. Mol. Ther. Nucleic Acids 2021, 23, 821–834. [Google Scholar] [CrossRef]
- Panda, A.C.; Grammatikakis, I.; Munk, R.; Gorospe, M.; Abdelmohsen, K. Emerging roles and context of circular RNAs. Wiley Interdiscip. Rev. RNA 2017, 8. [Google Scholar] [CrossRef]
- LaRoche-Johnston, F.; Monat, C.; Verreault, E.; Cousineau, B. Molecular characterization of both transesterification reactions of the group II intron circularization pathway. Nucleic Acids Res. 2021, 49, 6996–7010. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Khanabdali, R.; Kalionis, B.; Tai, X.; Xia, S. Circular RNAs: Isolation, characterization and their potential role in diseases. RNA Biol. 2017, 14, 1715–1721. [Google Scholar] [CrossRef]
- Chen, L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell. Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Guo, X.L.; Li, X. The novel roles of circular RNAs in metabolic organs. Genes. Dis. 2018, 5, 16–23. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-protein interactions: Functions, mechanisms, and identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef]
- Patop, I.L.; Wust, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Knupp, D.; Jorgensen, B.G.; Alshareef, H.Z.; Bhat, J.M.; Grubbs, J.J.; Miura, P.; van der Linden, A.M. Loss of circRNAs from the crh-1 gene extends the mean lifespan in Caenorhabditis elegans. Aging Cell. 2022, 21, e13560. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Okholm, T.L.H.; Veno, M.T.; Kjems, J. Circular RNAs are abundantly expressed and upregulated during human epidermal stem cell differentiation. RNA Biol. 2018, 15, 280–291. [Google Scholar] [CrossRef]
- Hong, L.; Gu, T.; He, Y.; Zhou, C.; Hu, Q.; Wang, X.; Zheng, E.; Huang, S.; Xu, Z.; Yang, J.; et al. Genome-Wide Analysis of Circular RNAs Mediated ceRNA Regulation in Porcine Embryonic Muscle Development. Front. Cell. Dev. Biol. 2019, 7, 289. [Google Scholar] [CrossRef]
- Stagsted, L.V.W.; O’Leary, E.T.; Ebbesen, K.K.; Hansen, T.B. The RNA-binding protein SFPQ preserves long-intron splicing and regulates circRNA biogenesis in mammals. Elife 2021, 10. [Google Scholar] [CrossRef]
- Liang, D.; Tatomer, D.C.; Luo, Z.; Wu, H.; Yang, L.; Chen, L.L.; Cherry, S.; Wilusz, J.E. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol. Cell. 2017, 68, 940–954 e943. [Google Scholar] [CrossRef]
- Kramer, M.C.; Liang, D.; Tatomer, D.C.; Gold, B.; March, Z.M.; Cherry, S.; Wilusz, J.E. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes. Dev. 2015, 29, 2168–2182. [Google Scholar] [CrossRef] [PubMed]
- Errichelli, L.; Dini Modigliani, S.; Laneve, P.; Colantoni, A.; Legnini, I.; Capauto, D.; Rosa, A.; De Santis, R.; Scarfo, R.; Peruzzi, G.; et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017, 8, 14741. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.X.; Xue, W.; Zhang, Y.; Jiang, S.; Yin, Q.F.; Wei, J.; Yao, R.W.; Yang, L.; Chen, L.L. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol. Cell. 2017, 67, 214–227 e217. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Patop, I.L.; Krishnamoorthy, A.; Bartok, O.; Maya, R.; Lerner, N.; Ashwall-Fluss, R.; Konakondla, J.V.V.; Beatus, T.; Kadener, S. circMbl functions in cis and in trans to regulate gene expression and physiology in a tissue-specific fashion. Cell. Rep. 2022, 39, 110740. [Google Scholar] [CrossRef] [PubMed]
- Knupp, D.; Cooper, D.A.; Saito, Y.; Darnell, R.B.; Miura, P. NOVA2 regulates neural circRNA biogenesis. Nucleic Acids Res. 2021, 49, 6849–6862. [Google Scholar] [CrossRef]
- Aktas, T.; Avsar Ilik, I.; Maticzka, D.; Bhardwaj, V.; Pessoa Rodrigues, C.; Mittler, G.; Manke, T.; Backofen, R.; Akhtar, A. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature 2017, 544, 115–119. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Ju, C.J.; Hao, J.; Chen, M.; Wang, W. JEDI: Circular RNA prediction based on junction encoders and deep interaction among splice sites. Bioinformatics 2021, 37, i289–i298. [Google Scholar] [CrossRef]
- Verduci, L.; Strano, S.; Yarden, Y.; Blandino, G. The circRNA-microRNA code: Emerging implications for cancer diagnosis and treatment. Mol. Oncol. 2019, 13, 669–680. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, C.; Lin, C.; Zhang, J.; Zhang, W.; Zhang, W.; Lu, Y.; Zheng, L.; Li, X. Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene ITGA7. J. Pathol. 2018, 246, 166–179. [Google Scholar] [CrossRef]
- Yang, F.; Hu, A.; Li, D.; Wang, J.; Guo, Y.; Liu, Y.; Li, H.; Chen, Y.; Wang, X.; Huang, K.; et al. Circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol. Cancer 2019, 18, 158. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.; Liu, Y.; Zhou, Z.; Wang, X. Transcription activation of circ-STAT3 induced by Gli2 promotes the progression of hepatoblastoma via acting as a sponge for miR-29a/b/c-3p to upregulate STAT3/Gli2. J. Exp. Clin. Cancer Res. 2020, 39, 101. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.; Liu, J.; Huang, Y.G.; Zhang, C. A circular RNA derived from DAB1 promotes cell proliferation and osteogenic differentiation of BMSCs via RBPJ/DAB1 axis. Cell. Death Dis. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, G.; Yan, X.; Lv, Z.; Yin, H.; Zhang, S.; Song, W.; Li, X.; Li, L.; Du, Z.; et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Tang, Z.D.; Peng, L.; Li, Y.Y.; Qian, F.C.; Zhao, J.M.; Ding, L.W.; Du, X.J.; Li, M.; Zhang, J.; et al. Integrative Epigenomic Analysis of Transcriptional Regulation of Human CircRNAs. Front. Genet. 2020, 11, 590672. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Q.; Yan, Q.; Chen, Z.G.; Jia, C.H.; Li, X.H.; Liang, Z.Y.; Gu, J.; Wei, H.L.; Lian, C.Y.; Zheng, J.; et al. Umbilical Cord Blood-Derived Exosomes From Very Preterm Infants With Bronchopulmonary Dysplasia Impaired Endothelial Angiogenesis: Roles of Exosomal MicroRNAs. Front. Cell. Dev. Biol. 2021, 9, 637248. [Google Scholar] [CrossRef]
- Garberg, H.T.; Huun, M.U.; Baumbusch, L.O.; Asegg-Atneosen, M.; Solberg, R.; Saugstad, O.D. Temporal Profile of Circulating microRNAs after Global Hypoxia-Ischemia in Newborn Piglets. Neonatology 2017, 111, 133–139. [Google Scholar] [CrossRef]
- Syed, M.; Das, P.; Pawar, A.; Aghai, Z.H.; Kaskinen, A.; Zhuang, Z.W.; Ambalavanan, N.; Pryhuber, G.; Andersson, S.; Bhandari, V. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat. Commun. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Gebauer, F.; Schwarzl, T.; Valcarcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet. 2021, 22, 185–198. [Google Scholar] [CrossRef]
- Czubak, K.; Taylor, K.; Piasecka, A.; Sobczak, K.; Kozlowska, K.; Philips, A.; Sedehizadeh, S.; Brook, J.D.; Wojciechowska, M.; Kozlowski, P. Global Increase in Circular RNA Levels in Myotonic Dystrophy. Front. Genet. 2019, 10, 649. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Zhang, Y.; Li, B.; Lou, G. Circular RNA circE2F2 promotes malignant progression of ovarian cancer cells by upregulating the expression of E2F2 protein via binding to HuR protein. Cell. Signal. 2021, 84, 110014. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell. Metab. 2019, 30, 157–173 e157. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, G.; Wang, X.; Liu, J.; Lu, Z.; Wang, Q.; Xu, B.; Liu, Z.; Li, J. CircBACH1 (hsa_circ_0061395) promotes hepatocellular carcinoma growth by regulating p27 repression via HuR. J. Cell. Physiol. 2020, 235, 6929–6941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, F.; Fang, E.; Xiao, W.; Mei, H.; Li, H.; Li, D.; Song, H.; Wang, J.; Hong, M.; et al. Circular RNA circAGO2 drives cancer progression through facilitating HuR-repressed functions of AGO2-miRNA complexes. Cell. Death Differ. 2019, 26, 1346–1364. [Google Scholar] [CrossRef]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183–4191. [Google Scholar] [CrossRef]
- Stagsted, L.V.; Nielsen, K.M.; Daugaard, I.; Hansen, T.B. Noncoding AUG circRNAs constitute an abundant and conserved subclass of circles. Life Sci. Alliance 2019, 2, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Zhao, J.; Li, Q.; Chen, S.; Guo, T.; Li, Y.; Lai, H.; Chen, Z.; Meng, Z.; et al. HNRNPL Circularizes ARHGAP35 to Produce an Oncogenic Protein. Adv. Sci. (Weinh) 2021, 8, 2001701. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Jia, Y.; Zhang, Y.; Shi, L.; Li, Q.; Zang, A.; Wang, H. Circular RNA: Biogenesis, degradation, functions and potential roles in mediating resistance to anticarcinogens. Epigenomics 2020, 12, 267–283. [Google Scholar] [CrossRef]
- Rossi, F.; Beltran, M.; Damizia, M.; Grelloni, C.; Colantoni, A.; Setti, A.; Di Timoteo, G.; Dattilo, D.; Centron-Broco, A.; Nicoletti, C.; et al. Circular RNA ZNF609/CKAP5 mRNA interaction regulates microtubule dynamics and tumorigenicity. Mol. Cell. 2022, 82, 75–89 e79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, K.; Xu, X.; Yang, Y.; Yan, S.; Wei, P.; Liu, H.; Xu, J.; Xiao, F.; Zhou, H.; et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat. Commun. 2018, 9, 4475. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, N.; Yang, X.; Luo, J.; Yan, S.; Xiao, F.; Chen, W.; Gao, X.; Zhao, K.; Zhou, H.; et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene 2018, 37, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, X.; Li, F.; Wu, X.; Zhang, M.; Zhou, H.; Huang, N.; Yang, X.; Xiao, F.; Liu, D.; et al. Correction to: A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol. Cancer 2019, 18, 149. [Google Scholar] [CrossRef]
- Liang, W.C.; Wong, C.W.; Liang, P.P.; Shi, M.; Cao, Y.; Rao, S.T.; Tsui, S.K.; Waye, M.M.; Zhang, Q.; Fu, W.M.; et al. Translation of the circular RNA circbeta-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019, 20, 84. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell. 2017, 66, 9–21 e27. [Google Scholar] [CrossRef]
- Ye, F.; Gao, G.; Zou, Y.; Zheng, S.; Zhang, L.; Ou, X.; Xie, X.; Tang, H. circFBXW7 Inhibits Malignant Progression by Sponging miR-197-3p and Encoding a 185-aa Protein in Triple-Negative Breast Cancer. Mol. Ther. Nucleic Acids 2019, 18, 88–98. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Tan, C.; Liu, X. Circular RNAs: A new frontier in the study of human diseases. J. Med. Genet. 2016, 53, 359–365. [Google Scholar] [CrossRef]
- Ge, Q.; Guo, Y.; Zheng, W.; Zhao, S.; Cai, Y.; Qi, X. Molecular mechanisms detected in yak lung tissue via transcriptome-wide analysis provide insights into adaptation to high altitudes. Sci. Rep. 2021, 11, 7786. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, Y.; Rui, C.; Qian, Y.; Cai, M.; Jia, R. Potential Significance of Circular RNA in Human Placental Tissue for Patients with Preeclampsia. Cell. Physiol. Biochem. 2016, 39, 1380–1390. [Google Scholar] [CrossRef]
- Bai, Y.; Rao, H.; Chen, W.; Luo, X.; Tong, C.; Qi, H. Profiles of circular RNAs in human placenta and their potential roles related to preeclampsia. Biol. Reprod. 2018, 98, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gong, Y.; Zhong, L.; Ding, X.; Yang, Z.; Su, X.; Chen, M.; Zhang, F.; Yang, L. Circular RNA expression profile and competing endogenous RNA regulatory network in preeclampsia. Placenta 2022, 119, 32–38. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, Z.; Han, W. CircLRRK1 targets miR-223-3p to inhibit the proliferation, migration and invasion of trophoblast cells by regulating the PI3K/AKT signaling pathway. Placenta 2021, 104, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Xu, P.; Han, J.; Han, S.; He, J. Upregulation of circRNA hsa_circ_0008726 in Pre-eclampsia Inhibits Trophoblast Migration, Invasion, and EMT by Regulating miR-345-3p/RYBP Axis. Reprod. Sci. 2022, 29, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Niu, X.; Li, Q.; Zhao, Y.; Chen, X.; Sun, H. Circ_0085296 suppresses trophoblast cell proliferation, invasion, and migration via modulating miR-144/E-cadherin axis. Placenta 2020, 97, 18–25. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Q.; Deng, H. Circ_0011460 upregulates HTRA1 expression by sponging miR-762 to suppress HTR8/SVneo cell growth, migration, and invasion. Am. J. Reprod. Immunol. 2021, 86, e13485. [Google Scholar] [CrossRef]
- Shan, L.; Hou, X. Circular RNA hsa_circ_0026552 inhibits the proliferation, migration and invasion of trophoblast cells via the miR-331-3p/TGF-betaR1 axis in pre-eclampsia. Mol. Med. Rep. 2021, 24. [Google Scholar] [CrossRef]
- Dishop, M. Developmental and pediatric lung disease. In Practical Pulmonary Pathology: A Diagnostic Approach, 3rd ed.; Leslie, K.O., Wick, M.R., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 99–124. [Google Scholar]
- Ran, Y.; Yin, N.; Huang, D.; Zhao, Y.; Yang, J.; Zhang, H.; Qi, H. Identification and Characterization of Circular RNA as a Novel Regulator and Biomarker in Preterm Birth. Front. Bioeng. Biotechnol. 2020, 8, 566984. [Google Scholar] [CrossRef]
- Coalson, J.J. Pathology of new bronchopulmonary dysplasia. Semin. Neonatol. 2003, 8, 73–81. [Google Scholar] [CrossRef]
- Abman, S.H. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am. J. Respir. Crit. Care Med. 2001, 164, 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Di Pietro, G.M.; Esposito, S. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 2018, 16, 36. [Google Scholar] [CrossRef]
- Hennelly, M.; Greenberg, R.G.; Aleem, S. An Update on the Prevention and Management of Bronchopulmonary Dysplasia. Pediatric Health Med. Ther. 2021, 12, 405–419. [Google Scholar] [CrossRef]
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L832–L887. [Google Scholar] [CrossRef]
- Chen, Y.F.; Feng, D.D.; Wu, S.H.; Lu, H.Y.; Banu Pasha, A.; Permall, D.L.; Chen, J.H.; Sun, Z.Y.; Li, B.J.; Zhou, H.; et al. Promotion of Bronchopulmonary Dysplasia Progression Using Circular RNA circabcc4 via Facilitating PLA2G6 Expression by Sequestering miR-663a. Front. Cell. Dev. Biol. 2020, 8, 585541. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Fang, Y.; Huo, C. Long non-coding RNA Rian protects against experimental bronchopulmonary dysplasia by sponging miR-421. Exp. Ther. Med. 2021, 22, 781. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, J.; Kan, Q.; Zhou, X.G.; Zhou, X.Y. MicroRNA expression profiling studies on bronchopulmonary dysplasia: A systematic review and meta-analysis. Genet. Mol. Res. 2013, 12, 5195–5206. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, B.; Wang, L.; Hu, T.; Deng, Z.; Li, D. Insights into the expression profiles and functions of circRNAs in a newborn hyperoxia-induced rat bronchopulmonary dysplasia model. J. Gene Med. 2020, 22, e3163. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, W.; Zhang, S.; Wang, C.; He, X.; Zhang, Z.; Zhu, L.; Wang, Y.; Feng, Z. MicroRNA expression profile in hyperoxia-exposed newborn mice during the development of bronchopulmonary dysplasia. Respir. Care 2011, 56, 1009–1015. [Google Scholar] [CrossRef]
- Dong, J.; Carey, W.A.; Abel, S.; Collura, C.; Jiang, G.; Tomaszek, S.; Sutor, S.; Roden, A.C.; Asmann, Y.W.; Prakash, Y.S.; et al. MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics 2012, 13, 204. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, L.; Yu, J.; Fu, H.; Zhou, D.; Liu, H. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol. Med. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hong, H.; Li, X.X.; Li, J.; Zhang, Z.Q. Involvement of Hdac3-mediated inhibition of microRNA cluster 17-92 in bronchopulmonary dysplasia development. Mol. Med. 2020, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kai, G.; Pu, X.D.; Qing, K.; Guo, X.R.; Zhou, X.Y. Expression profile of microRNAs in fetal lung development of Sprague-Dawley rats. Int. J. Mol. Med. 2012, 29, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, S.; Nielsen, H.C.; Volpe, M.V. MiR-221 and miR-130a regulate lung airway and vascular development. PLoS ONE 2013, 8, e55911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Lv, Y.; Yu, W.; Liu, X.; Liu, C. MiR-130a-5p/Foxa2 axis modulates fetal lung development in congenital diaphragmatic hernia by activating the Shh/Gli1 signaling pathway. Life Sci. 2020, 241, 117166. [Google Scholar] [CrossRef]
- Ma, H.; Lin, Y.; Zhao, Z.A.; Lu, X.; Yu, Y.; Zhang, X.; Wang, Q.; Li, L. MicroRNA-127 Promotes Mesendoderm Differentiation of Mouse Embryonic Stem Cells by Targeting Left-Right Determination Factor 2. J. Biol. Chem. 2016, 291, 12126–12135. [Google Scholar] [CrossRef]
- Komarovsky Gulman, N.; Armon, L.; Shalit, T.; Urbach, A. Heterochronic regulation of lung development via the Lin28-Let-7 pathway. FASEB J. 2019, 33, 12008–12018. [Google Scholar] [CrossRef]
- Carraro, G.; El-Hashash, A.; Guidolin, D.; Tiozzo, C.; Turcatel, G.; Young, B.M.; De Langhe, S.P.; Bellusci, S.; Shi, W.; Parnigotto, P.P.; et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev. Biol. 2009, 333, 238–250. [Google Scholar] [CrossRef]
- Ruiz-Camp, J.; Quantius, J.; Lignelli, E.; Arndt, P.F.; Palumbo, F.; Nardiello, C.; Surate Solaligue, D.E.; Sakkas, E.; Mizikova, I.; Rodriguez-Castillo, J.A.; et al. Targeting miR-34a/Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Zhou, H.; Chanda, B.; Chen, Y.F.; Wang, X.J.; You, M.Y.; Zhang, Y.H.; Cheng, R.; Yang, Y.; Chen, X.Q. Microarray and Bioinformatics Analysis of Circular RNA Differential Expression in Newborns With Acute Respiratory Distress Syndrome. Front. Pediatr. 2021, 9, 728462. [Google Scholar] [CrossRef]
- De Luca, D.; van Kaam, A.H.; Tingay, D.G.; Courtney, S.E.; Danhaive, O.; Carnielli, V.P.; Zimmermann, L.J.; Kneyber, M.C.J.; Tissieres, P.; Brierley, J.; et al. The Montreux definition of neonatal ARDS: Biological and clinical background behind the description of a new entity. Lancet Respir. Med. 2017, 5, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Aggarwal, T.; Fredriksson, R. SLC38A10 Transporter Plays a Role in Cell Survival Under Oxidative Stress and Glutamate Toxicity. Front. Mol. Biosci. 2021, 8, 671865. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Olave, N.; Travers, C.; Rezonzew, G.; Dolma, K.; Simpson, A.; Halloran, B.; Aghai, Z.; Das, P.; Sharma, N.; et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Xu, Q.; Yin, J.; Wang, H.; Zhang, L. CircRNA, lncRNA, and mRNA profiles of umbilical cord blood exosomes from preterm newborns showing bronchopulmonary dysplasia. Eur. J. Pediatr. 2022, 181, 3345–3365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Du, W.; Liu, J.; Ma, W.; Zhang, L.; Du, Z.; Cai, B. Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front. Pharmacol. 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yu, T.; Jing, X.; Ma, L.; Fan, Y.; Yang, F.; Ma, P.; Jiang, H.; Wu, X.; Shu, Y.; et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer 2020, 19, 112. [Google Scholar] [CrossRef]
- Jost, I.; Shalamova, L.A.; Gerresheim, G.K.; Niepmann, M.; Bindereif, A.; Rossbach, O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018, 15, 1032–1039. [Google Scholar] [CrossRef]
- Lavenniah, A.; Luu, T.D.A.; Li, Y.P.; Lim, T.B.; Jiang, J.; Ackers-Johnson, M.; Foo, R.S. Engineered Circular RNA Sponges Act as miRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol. Ther. 2020, 28, 1506–1517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).