Do Serum Galectin-9 Levels in Women with Gestational Diabetes and Healthy Ones Differ before or after Delivery? A Pilot Study
Abstract
1. Introduction
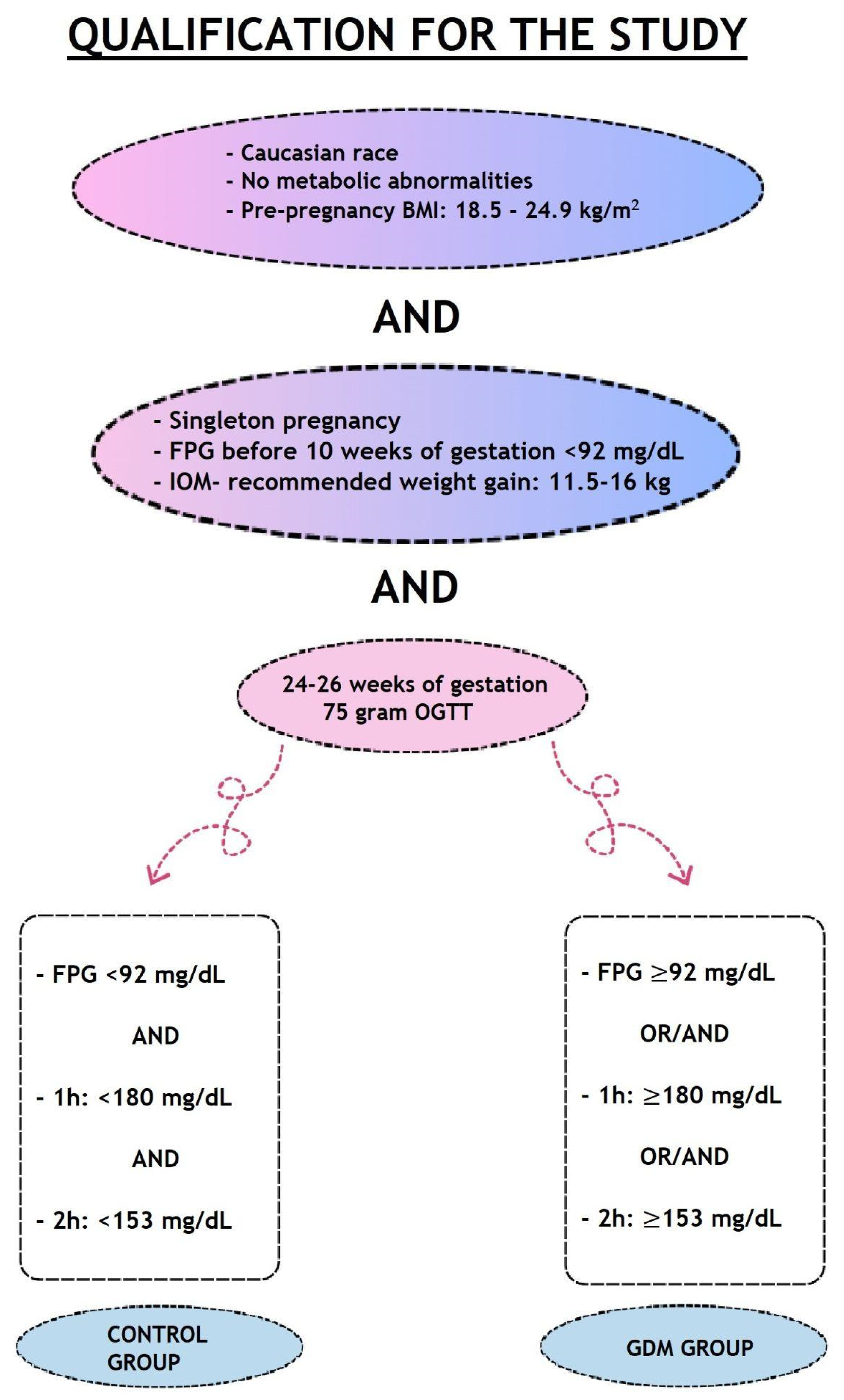
2. Materials and Methods
3. Results
3.1. Comparison of Galectin-9 Concentration in GDM Patients and Healthy Controls
3.2. Correlations of Galectin-9 Determinations
3.2.1. Prepartum Serum Galectin-9 and Postpartum Serum Galectin-9
3.2.2. Prepartum Serum Galectin-9 and Postpartum Urine Galectin-9
3.2.3. Postpartum Serum Galectin-9 and Postpartum Urine Galectin-9
3.3. Correlations with Galectin-9 Levels
3.4. Multiple Linear Regression Analyses
3.5. Correlations with Galectin-9 Levels and the Sex of the Child
3.6. Correlations with Galectin-9 Levels and the Mode of Delivery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hepp, P.; Unverdorben, L.; Hutter, S.; Kuhn, C.; Ditsch, N.; Groß, E.; Mahner, S.; Jeschke, U.; Knabl, J.; Heidegger, H.H. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. Int. J. Mol. Sci. 2020, 21, 2404. [Google Scholar] [CrossRef] [PubMed]
- Modzelewski, R.; Stefanowicz-Rutkowska, M.M.; Matuszewski, W.; Bandurska-Stankiewicz, E.M. Gestational Diabetes Mellitus—Recent Literature Review. J. Clin. Med. 2022, 11, 5736. [Google Scholar] [CrossRef]
- Jarmuzek, P.; Wielgos, M.; Bomba-Opon, D. Placental pathologic changes in gestational diabetes mellitus. Neuroendocrinol. Lett. 2015, 36, 101–105. [Google Scholar] [PubMed]
- Zhang, Z.; Kang, X.; Guo, Y.; Zhang, J.; Xie, J.; Shao, S.; Xiang, Y.; Chen, G.; Yu, X. Association of circulating galectin-3 with gestational diabetes mellitus, progesterone, and insulin resistance. J. Diabetes 2021, 13, 54–62. [Google Scholar] [CrossRef]
- Blois, S.M.; Gueuvoghlanian-Silva, B.Y.; Tirado-González, I.; Torloni, M.R.; Freitag, N.; Mattar, R.; Conrad, M.L.; Unverdorben, L.; Barrientos, G.; Knabl, J.; et al. Getting too sweet: Galectin-1 dysregulation in gestational diabetes mellitus. Mol. Hum. Reprod. 2014, 20, 644–649. [Google Scholar] [CrossRef]
- Pankiewicz, K.; Szczerba, E.; Fijałkowska, A.; Sierdziński, J.; Issat, T.; Maciejewski, T.M. The Impact of Coexisting Gestational Diabetes Mellitus on the Course of Preeclampsia. J. Clin. Med. 2022, 11, 6390. [Google Scholar] [CrossRef]
- Damm, P.; Houshmand-Oeregaard, A.; Kelstrup, L.; Lauenborg, J.; Mathiesen, E.R.; Clausen, T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: A view from Denmark. Diabetologia 2016, 59, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Heusler, I.; Biron-Shental, T.; Farladansky-Gershnabel, S.; Pasternak, Y.; Kidron, D.; Vulih-Shuitsman, I.; Einbinder, Y.; Cohen-Hagai, K.; Benchetrit, S.; Zitman-Gal, T. Enhanced expression of Galectin-3 in gestational diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1791–1797. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, L.; Wang, M.; Chen, D.; Chen, Z.; Jiang, S.W. A Critical Review of Proteomic Studies in Gestational Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 6450352. [Google Scholar] [CrossRef]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Chezar-Azerrad, C.; Kruchin, B.; Leshem-Lev, D.; Levi, A.; Hadar, E.; Kornowski, R.; Tenenbaum-Gavish, K.; Porter, A. Elevated galectin-3 in women with gestational diabetes mellitus, a new surrogate for cardiovascular disease in women. PLoS ONE 2020, 15, e0234732. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Ruszała, M.; Niebrzydowska, M.; Pilszyk, A.; Kimber-Trojnar, Ż.; Trojnar, M.; Leszczyńska-Gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 11578. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Hüttenbrenner, R.; Knabl, J.; Jeschke, U.; Hutter, S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta 2015, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lyu, Y.; Li, C.; Liu, P.; Li, J.; Ma, R.C.; Yang, X. Long-term risk of diabetes in women at varying durations after gestational diabetes: A systematic review and meta-analysis with more than 2 million women. Obes. Rev. 2018, 19, 421–429. [Google Scholar] [CrossRef]
- Mansour, A.A.; Krautter, F.; Zhi, Z.; Iqbal, A.J.; Recio, C. The interplay of galectins-1, -3, and -9 in the immune-inflammatory response underlying cardiovascular and metabolic disease. Cardiovasc. Diabetol. 2022, 21, 253. [Google Scholar] [CrossRef]
- Moar, P.; Tandon, R. Galectin-9 as a biomarker of disease severity. Cell. Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.A.; Rabinovich, G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef]
- Boroń, D.G.; Świetlicki, A.; Potograbski, M.; Kurzawińska, G.; Wirstlein, P.; Boroń, D.; Drews, K.; Seremak-Mrozikiewicz, A. Galectin-1 and Galectin-9 Concentration in Maternal Serum: Implications in Pregnancies Complicated with Preterm Prelabor Rupture of Membranes. J. Clin. Med. 2022, 11, 6330. [Google Scholar] [CrossRef]
- Schnabel, A.; Blois, S.M.; Meint, P.; Freitag, N.; Ernst, W.; Barrientos, G.; Conrad, M.L.; Rose, M.; Seelbach-Göbel, B. Elevated systemic galectin-1 levels characterize HELLP syndrome. J. Reprod. Immunol. 2016, 114, 38–43. [Google Scholar] [CrossRef]
- Zhao, B.; Han, X.; Meng, Q.; Luo, Q. Early second trimester maternal serum markers in the prediction of gestational diabetes mellitus. J Diabetes Investig. 2018, 9, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Unverdorben, L.; Hutter, S.; Knabl, J.; Schmoeckel, E.; Meister, S.; Zehni, A.Z.; Beyer, S.; Vilsmaier, T.; Mahner, S.; et al. Overexpression of galectin-4 in placentas of women with gestational diabetes. J. Reprod. Immunol. 2022, 151, 103629. [Google Scholar] [CrossRef] [PubMed]
- Freitag, N.; Tirado-González, I.; Barrientos, G.; Cohen, M.; Daher, S.; Goldman-Wohl, D.; Mincheva-Nilsson, L.; John, C.M.; Jeschke, U.; Blois, S.M. The chimera-type galectin-3 is a positive modulator of trophoblast functions with dysregulated expression in gestational diabetes mellitus. Am. J. Reprod. Immunol. 2020, 84, e13311. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Harrington, S.M.; Creedon, D.J.; Ruano, R.; Markovic, S.N.; Dong, H.; Dronca, R.S. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12795. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases. Biomolecules 2021, 11, 430. [Google Scholar] [CrossRef]
- Sun, L.; Zou, S.; Ding, S.; Du, X.; Shen, Y.; Liu, C.; Shi, B.; Zhang, X. Circulating T Cells Exhibit Different TIM3/Galectin-9 Expression in Patients with Obesity and Obesity-Related Diabetes. J. Diabetes Res. 2020, 2020, 2583257. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Mishra, R. Galectin-9: From cell biology to complex disease dynamics. J. Biosci. 2016, 41, 507–534. [Google Scholar] [CrossRef]
- Jeschke, U.; Hutter, S.; Heublein, S.; Vrekoussis, T.; Andergassen, U.; Unverdorben, L.; Papadakis, G.; Makrigiannakis, A. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 2013, 34, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Dziennik Ustaw Rzeczypospolitej Polskiej. Rozporządzenie Ministra Zdrowia z Dnia 16 Sierpnia 2018 r. w Sprawie Standardu Organizacyjnego Opieki Okołoporodowej. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180001756/O/D20181756.pdf (accessed on 8 April 2023).
- Li, X.; Li, G.; Cheng, T.; Liu, J.; Song, G.; Ma, H. Association between Triglyceride-Glucose Index and Risk of Incident Diabetes: A Secondary Analysis Based on a Chinese Cohort Study. Lipids Health Dis. 2020, 19, 236. [Google Scholar] [CrossRef]
- Baycan, O.F.; Barman, H.A.; Atici, A.; Tatlisu, A.; Bolen, F.; Ergen, P.; Icten, S.; Gungor, B.; Caliskan, M. Evaluation of Biventricular Function in Patients with COVID-19 Using Speckle Tracking Echocardiography. Int. J. Cardiovasc. Imaging 2020, 37, 135–144. [Google Scholar] [CrossRef]
- Tudoran, C.; Bende, R.; Bende, F.; Giurgi-Oncu, C.; Enache, A.; Dumache, R.; Tudoran, M. Connections between Diabetes Mellitus and Metabolic Syndrome and the Outcome of Cardiac Dysfunctions Diagnosed during the Recovery from COVID-19 in Patients without a Previous History of Cardiovascular Diseases. Biology 2023, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Kurose, Y.; Wada, J.; Kanzaki, M.; Teshigawara, S.; Nakatsuka, A.; Murakami, K.; Inoue, K.; Terami, T.; Katayama, A.; Watanabe, M.; et al. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol. 2013, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Zhu, R.R.; Zhao, L.; Zhong, Y.C.; Zeng, Q.T. Down-regulation and Clinical Implication of Galectin-9 Levels in Patients with Acute Coronary Syndrome and Chronic Kidney Disease. Curr. Med. Sci. 2020, 40, 662–670. [Google Scholar] [CrossRef]
- Jia, J.; Bissa, B.; Brecht, L.; Allers, L.; Choi, S.W.; Gu, Y.; Zbinden, M.; Burge, M.R.; Timmins, G.; Hallows, K.; et al. AMPK, a Regulator of Metabolism and Autophagy, Is Activated by Lysosomal Damage via a Novel Galectin-Directed Ubiquitin Signal Transduction System. Mol. Cell 2020, 57, 951–969. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Patro-Małysza, J.; Trojnar, M.; Skórzyńska-Dziduszko, K.E.; Bartosiewicz, J.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fatty Acid-Binding Protein 4—An “Inauspicious” Adipokine—In Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain and Gestational Diabetes Mellitus. J. Clin. Med. 2018, 7, 505. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Patro-Małysza, J.; Skórzyńska-Dziduszko, K.E.; Oleszczuk, J.; Trojnar, M.; Mierzyński, R.; Leszczyńska-Gorzelak, B. Ghrelin in Serum and Urine of Post-Partum Women with Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3001. [Google Scholar] [CrossRef]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Czuba, M.; Mosiewicz, J.; Leszczyńska-Gorzelak, B. Vaspin in Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain. Medicina 2019, 55, 76. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Singh, P.; Aggarwal, A. Serum and urinary galectin-9 and C-X-C motif chemokine ligand 10. Lupus 2022, 31, 482–487. [Google Scholar] [CrossRef]

| Variables in OGTT | Glycemia | |
|---|---|---|
| FPG (mg/dL) | Mean (SD) | 100.72 |
| Median (quartile) | 96.8 | |
| Range | 80–112 | |
| After 1 h (mg/dL) | Mean (SD) | 159.84 |
| Median (quartile) | 171 | |
| Range | 100–205 | |
| After 2 h (mg/dL) | Mean (SD) | 125.86 |
| Median (quartile) | 147 | |
| Range | 97–173 |
| Variables | Control Group (n = 23) | GDM Group (n = 18) | p | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 31.65 (3.91) | 31.94 (6.06) | p = 0.612 |
| Median (quartile) | 31.62 (28.98–34.07) | 32.95 (27.31–36.3) | ||
| Range | 24.02–38.97 | 20.15–40.43 | ||
| Week of pregnancy termination (weeks) | Mean (SD) | 39 (1.31) | 38.39 (1.2) | p = 0.215 |
| Median (quartile) | 39 (38–40) | 38 (38–39) | ||
| Range | 37–42 | 36–40 | ||
| Mode of delivery | Caesarean section | 9 (39.13%) | 6 (33.33%) | p = 0.956 |
| Natural labour | 14 (60.87%) | 12 (66.67%) | ||
| Sex of the child | Female | 6 (26.09%) | 6 (33.33%) | p = 0.873 |
| Male | 17 (73.91%) | 12 (66.67%) | ||
| Birth weight (g) | Mean (SD) | 3451.74 (499.37) | 3315.56 (397.75) | p = 0.312 |
| Median (quartile) | 3440 (3040–3570) | 3290 (3132.5–3432.5) | ||
| Range | 2800–4800 | 2630–4480 | ||
| Parameters obtained between 36 and 48 h after delivery | ||||
| BMI (kg/m2) | Mean (SD) | 28.04 (3.88) | 31.26 (8.84) | p = 0.18 |
| Median (quartile) | 27.3 (25.56–28.8) | 29.75 (25.4–34.12) | ||
| Range | 22.2–37.2 | 19.77–59.16 | ||
| Fasting glycemia (mg/dL) | Mean (SD) | 74.48 (10.81) | 84.06 (13.25) | p = 0.008 * |
| Median (quartile) | 75 (72–80.5) | 83.5 (78.25–94.5) | ||
| Range | 37–92 | 54–105 | ||
| Insulin (mU/L) | Mean (SD) | 10.32 (4.41) | 11.95 (6.7) | p = 0.608 |
| Median (quartile) | 9.2 (7.65–13.9) | 12 (6.32–18.4) | ||
| Range | 3–19.6 | 2.1–22.7 | ||
| HbA1c (IFCC) (mmol/mol) | Mean (SD) | 34.83 (3.59) | 35.61 (6.34) | p = 0.607 |
| Median (quartile) | 34 (32.5–36.5) | 36 (30–39.5) | ||
| Range | 30–43 | 25–48 | ||
| Creatinine (mg/dL) | Mean (SD) | 0.54 (0.07) | 0.55 (0.09) | p = 0.907 |
| Median (quartile) | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | ||
| Range | 0.4–0.7 | 0.4–0.7 | ||
| Total cholesterol (mg/dL) | Mean (SD) | 269.83 (45.88) | 276.11 (50.23) | p = 0.646 |
| Median (quartile) | 264 (241–299) | 271.5 (241.25–315.75) | ||
| Range | 206–387 | 188–354 | ||
| HDL (mg/dL) | Mean (SD) | 73.43 (15.51) | 77.22 (23.92) | p = 1 |
| Median (quartile) | 69 (64.5–78) | 72.5 (57–93.75) | ||
| Range | 48–108 | 50–132 | ||
| LDL (mg/dL) | Mean (SD) | 129.26 (54.24) | 147.06 (34.66) | p = 0.379 |
| Median (quartile) | 139 (106–160) | 148 (126–176.25) | ||
| Range | 0–222 | 92–207 | ||
| Triglycerides (mg/dL) | Mean (SD) | 275.35 (93.08) | 259 (58.99) | p = 0.674 |
| Median (quartile) | 258 (214.5–328.5) | 250 (214.75–304.25) | ||
| Range | 138–517 | 167–353 | ||
| TyG | Mean (SD) | 9.17 (0.41) | 9.26 (0.33) | p = 0.543 |
| Median (quartile) | 9.2 (8.95–9.4) | 9.25 (9.2–9.4) | ||
| Range | 8.2–9.9 | 8.6–9.8 | ||
| E/I | Mean (SD) | 0.89 (0.13) | 0.95 (0.2) | p = 0.494 |
| Median (quartile) | 0.9 (0.86–0.95) | 0.92 (0.83–0.97) | ||
| Range | 0.59–1.15 | 0.61–1.4 | ||
| ATM (kg) | Mean (SD) | 37.46 (22.65) | 46.23 (26.59) | p = 0.145 |
| Median (quartile) | 32 (26.35–40.45) | 42 (30.58–60.35) | ||
| Range | 15.4–125.6 | 7.3–125.6 | ||
| FTI (kg/m2) | Mean (SD) | 12.95 (7.92) | 17.11 (9.4) | p = 0.04 * |
| Median (quartile) | 11.3 (9.4–14.25) | 15.1 (12.38–21.9) | ||
| Range | 5.5–45 | 2.5–45 | ||
| BCM (kg) | Mean (SD) | 20.88 (6.79) | 17.51 (4.49) | p = 0.148 |
| Median (quartile) | 18.7 (16.6–23.85) | 17.05 (14.53–19.73) | ||
| Range | 13.9–43.5 | 8.6–27.3 | ||
| Parameter | GDM | N | Mean | SD | Median | p |
|---|---|---|---|---|---|---|
| Galectin-9 in serum before delivery (ng/mL) | Absent | 23 | 7.80 | 2.23 | 7.12 | p = 0.074 |
| Occurring | 18 | 9.39 | 3.02 | 8.80 | ||
| Galectin-9 in serum after delivery (ng/mL) | Absent | 23 | 7.74 | 3.50 | 6.66 | p = 0.253 |
| Occurring | 18 | 8.40 | 3.09 | 7.52 | ||
| Galectin-9 in urine after delivery (ng/mL) | Absent | 23 | 72.59 | 58.91 | 65.58 | p = 1 |
| Occurring | 18 | 78.31 | 71.77 | 72.19 |
| Parameter | Galectin-9 in Serum before Delivery | Galectin-9 in Serum after Delivery | Galectin-9 in Urine after Delivery |
|---|---|---|---|
| HbA1c (IFCC) | r = −0.064. p = 0.692 | r = −0.179. p = 0.264 | r = 0.002. p = 0.992 |
| HOMA-IR | r = 0.298. p = 0.059 | r = 0.13. p = 0.419 | r = 0.043. p = 0.787 |
| TyG | r = 0.161. p = 0.314 | r = 0.194. p = 0.225 | r = 0.137. p = 0.393 |
| Total cholesterol | r = −0.037. p = 0.819 | r = 0.157. p = 0.327 | r = −0.076. p = 0.636 |
| HDL | r = −0.217. p = 0.174 | r = 0.06. p = 0.711 | r = −0.126. p = 0.434 |
| LDL | r = −0.032. p = 0.841 | r = 0.117. p = 0.468 | r = −0.215. p = 0.176 |
| Non-HDL | r = 0.003. p = 0.984 | r = 0.111. p = 0.489 | r = −0.08. p = 0.618 |
| Triglycerides | r = 0.06. p = 0.71 | r = 0.173. p = 0.279 | r = 0.128. p = 0.426 |
| Homocysteine | r = 0.165. p = 0.304 | r = 0.187. p = 0.241 | r = −0.169. p = 0.292 |
| BMI | r = 0.311. p = 0.048 * | r = 0.082. p = 0.612 | r = 0.164. p = 0.304 |
| E/I | r = 0.447. p = 0.003 * | r = 0.203. p = 0.202 | r = −0.154. p = 0.335 |
| ATM | r = 0.498. p = 0.001 * | r = 0.19. p = 0.234 | r = 0.047. p = 0.772 |
| FTI | r = 0.47. p = 0.002 * | r = 0.174. p = 0.276 | r = 0.099. p = 0.537 |
| BCM | r = −0.201. p = 0.208 | r = −0.024. p = 0.88 | r = 0.217. p = 0.173 |
| Parameter | Parameter | 95%CI | p | |
|---|---|---|---|---|
| HbA1c (IFCC) | −0.104 | −0.317 | 0.11 | 0.351 |
| HOMA | 0.013 | −0.046 | 0.072 | 0.671 |
| TyG | 1.922 | −5.474 | 9.319 | 0.615 |
| Total cholesterol | 0.007 | −0.052 | 0.065 | 0.819 |
| HDL | 0.001 | −0.097 | 0.099 | 0.991 |
| LDL | −0.01 | −0.052 | 0.032 | 0.642 |
| Triglycerides | −0.011 | −0.055 | 0.032 | 0.619 |
| Homocysteine | 0.233 | −0.126 | 0.592 | 0.214 |
| BMI | 0.003 | −0.283 | 0.288 | 0.985 |
| E/I | 1.84 | −8.018 | 11.697 | 0.717 |
| ATM | 0.059 | −0.102 | 0.22 | 0.479 |
| FTI | −0.067 | −0.527 | 0.392 | 0.776 |
| BCM | −0.015 | −0.244 | 0.214 | 0.9 |
| Parameter | Parameter | 95%CI | p | |
|---|---|---|---|---|
| HbA1c (IFCC) | −0.095 | −0.378 | 0.189 | 0.518 |
| HOMA | 0.021 | −0.057 | 0.098 | 0.604 |
| TyG | 3.562 | −6.232 | 13.356 | 0.482 |
| Total cholesterol | −0.041 | −0.118 | 0.037 | 0.314 |
| HDL | 0.082 | −0.048 | 0.211 | 0.228 |
| LDL | 0.025 | −0.03 | 0.081 | 0.38 |
| Triglycerides | 0.008 | −0.05 | 0.066 | 0.79 |
| Homocysteine | 0.244 | −0.231 | 0.719 | 0.323 |
| BMI | −0.011 | −0.389 | 0.367 | 0.955 |
| E/I | 6.654 | −6.4 | 19.707 | 0.327 |
| ATM | 0.043 | −0.17 | 0.256 | 0.696 |
| FTI | −0.183 | −0.791 | 0.426 | 0.561 |
| BCM | 0.096 | −0.207 | 0.399 | 0.134 |
| Parameter | Parameter | 95%CI | p | |
|---|---|---|---|---|
| HbA1c (IFCC) | −1.274 | −6.781 | 4.233 | 0.654 |
| HOMA | 0.397 | −1.113 | 1.907 | 0.611 |
| TyG | −0.997 | −191.449 | 189.454 | 0.992 |
| Total cholesterol | −1.188 | −2.695 | 0.319 | 0.134 |
| HDL | 1.672 | −0.851 | 4.195 | 0.205 |
| LDL | 0.392 | −0.693 | 1.477 | 0.485 |
| Triglycerides | 0.52 | −0.602 | 1.642 | 0.372 |
| Homocysteine | 1.397 | −7.844 | 10.637 | 0.769 |
| BMI | 3.474 | −3.874 | 10.821 | 0.362 |
| E/I | −32.736 | −286.576 | 221.105 | 0.802 |
| ATM | −3.031 | −7.179 | 1.116 | 0.163 |
| FTI | 6.123 | −5.707 | 17.953 | 0.319 |
| BCM | 0.895 | −4.997 | 6.787 | 0.768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pełech, A.; Ruszała, M.; Niebrzydowska-Tatus, M.; Bień, K.; Kimber-Trojnar, Ż.; Czuba, M.; Świstowska, M.; Leszczyńska-Gorzelak, B. Do Serum Galectin-9 Levels in Women with Gestational Diabetes and Healthy Ones Differ before or after Delivery? A Pilot Study. Biomolecules 2023, 13, 697. https://doi.org/10.3390/biom13040697
Pełech A, Ruszała M, Niebrzydowska-Tatus M, Bień K, Kimber-Trojnar Ż, Czuba M, Świstowska M, Leszczyńska-Gorzelak B. Do Serum Galectin-9 Levels in Women with Gestational Diabetes and Healthy Ones Differ before or after Delivery? A Pilot Study. Biomolecules. 2023; 13(4):697. https://doi.org/10.3390/biom13040697
Chicago/Turabian StylePełech, Aleksandra, Monika Ruszała, Magdalena Niebrzydowska-Tatus, Katarzyna Bień, Żaneta Kimber-Trojnar, Monika Czuba, Małgorzata Świstowska, and Bożena Leszczyńska-Gorzelak. 2023. "Do Serum Galectin-9 Levels in Women with Gestational Diabetes and Healthy Ones Differ before or after Delivery? A Pilot Study" Biomolecules 13, no. 4: 697. https://doi.org/10.3390/biom13040697
APA StylePełech, A., Ruszała, M., Niebrzydowska-Tatus, M., Bień, K., Kimber-Trojnar, Ż., Czuba, M., Świstowska, M., & Leszczyńska-Gorzelak, B. (2023). Do Serum Galectin-9 Levels in Women with Gestational Diabetes and Healthy Ones Differ before or after Delivery? A Pilot Study. Biomolecules, 13(4), 697. https://doi.org/10.3390/biom13040697






