Regulation of Cx43 Gap Junction Intercellular Communication by Bruton’s Tyrosine Kinase and Interleukin-2-Inducible T-Cell Kinase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Detection Reagents
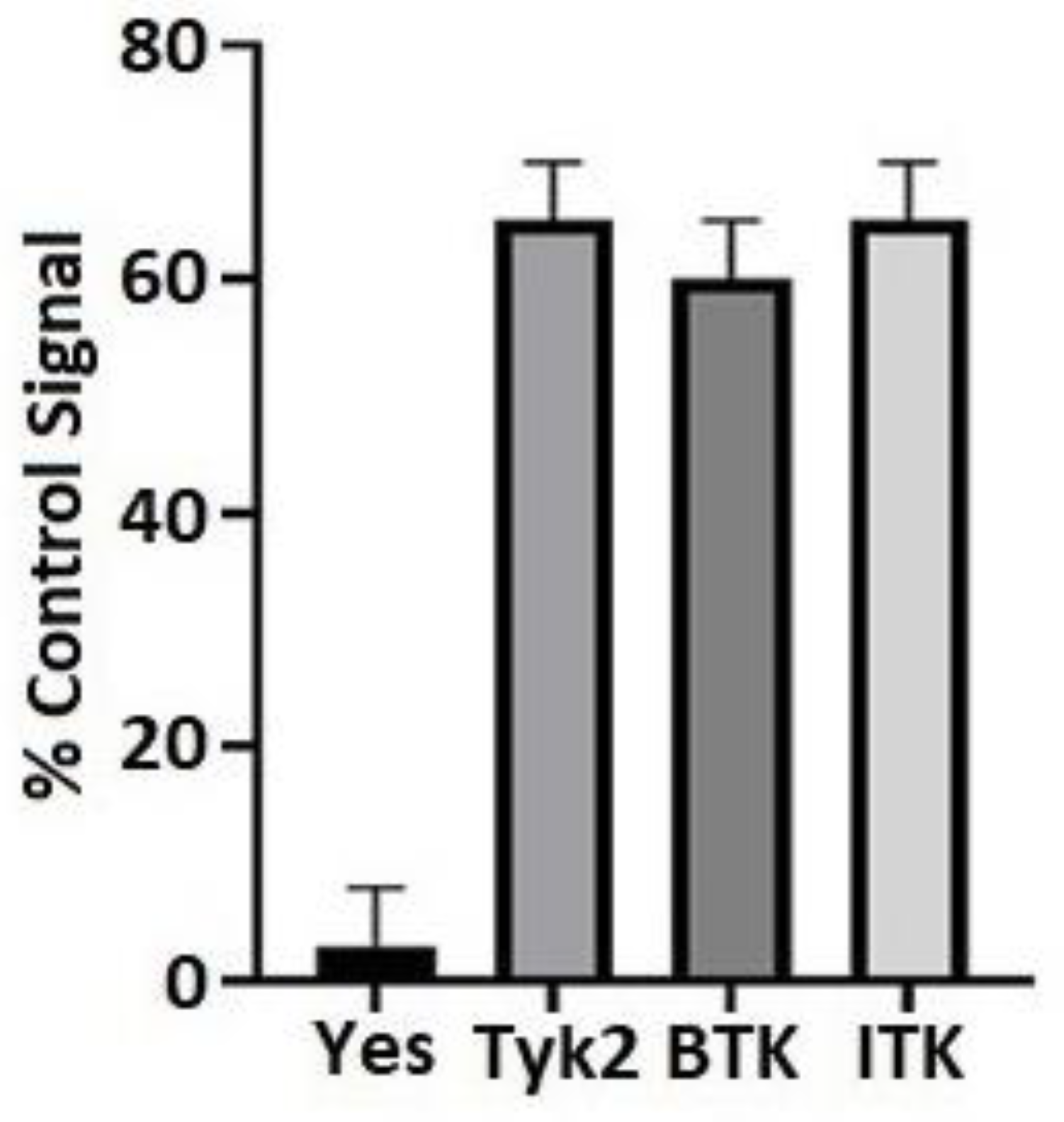
2.2. Kinase Screen
2.3. Mass Spectrometry
2.4. Cell Culture
2.5. Stable Clone Generation
2.6. Activation of Endogenous BTK and ITK
2.7. GST Pull-Down Assay
2.8. Western Blot
2.9. Triton X-100 Solubility Assay
2.10. Immunofluorescence
2.11. Confocal Imaging
2.12. Dye Transfer Assay
2.13. Parachute Assay
2.14. Statistical Analysis
3. Results
3.1. BTK and ITK Directly Interact with and Phosphorylate the Cx43CT Domain
3.2. BTK and ITK Phosphorylate Cx43CT Tyrosine Residues
3.3. BTK and ITK Phosphorylation of Cx43 Decreases the P2 Isoform and Increases Tyrosine Phosphorylation in HEK Cells
3.4. BTK and ITK Phosphorylation Decreases the Cx43 Plaque Area in HEK-293T Cells
3.5. BTK and ITK Phosphorylation of Cx43 Decreases Gap Junction Intercellular Communication in HEK-293T Cells
3.6. Tyrosine Phosphorylation of Cx43 Is Increased after Activation of B and T Lymphoblasts
3.7. Cx43 Co-Localizes with BTK and ITK in Lymphocytes
3.8. Activation of B and T Lymphocytes Decreases Gap Junction Intercellular Communication
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein–Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauf, U.; Giepmans, B.N.; Lopez, P.; Braconnot, S.; Chen, S.C.; Falk, M.M. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 10446–10451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giepmans, B.N.; Verlaan, I.; Hengeveld, T.; Janssen, H.; Calafat, J.; Falk, M.M.; Moolenaar, W.H. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001, 11, 1364–1368. [Google Scholar] [CrossRef] [Green Version]
- Fort, A.G.; Murray, J.W.; Dandachi, N.; Davidson, M.W.; Dermietzel, R.; Wolkoff, A.W.; Spray, D.C. In vitro motility of liver connexin vesicles along microtubules utilizes kinesin motors. J. Biol. Chem. 2011, 286, 22875–22885. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.M.; Fay, A.J.; Puthenveedu, M.A.; von Zastrow, M.; Jan, Y.N.; Jan, L.Y. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007, 128, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Smyth, J.W.; Vogan, J.M.; Buch, P.J.; Zhang, S.S.; Fong, T.S.; Hong, T.T.; Shaw, R.M. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 2012, 110, 978–989. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.W.; Barker, R.J.; Zhu, C.; Gourdie, R.G. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell 2005, 16, 5686–5698. [Google Scholar] [CrossRef] [Green Version]
- Pidoux, G.; Gerbaud, P.; Dompierre, J.; Lygren, B.; Solstad, T.; Evain-Brion, D.; Tasken, K. A PKA-ezrin-Cx43 signaling complex controls gap junction communication and thereby trophoblast cell fusion. J. Cell Sci. 2014, 127, 4172–4185. [Google Scholar] [CrossRef] [Green Version]
- Dukic, A.R.; Gerbaud, P.; Guibourdenche, J.; Thiede, B.; Tasken, K.; Pidoux, G. Ezrin-anchored PKA phosphorylates serine 369 and 373 on connexin 43 to enhance gap junction assembly, communication, and cell fusion. Biochem. J. 2018, 475, 455–476. [Google Scholar] [CrossRef] [Green Version]
- Butkevich, E.; Hulsmann, S.; Wenzel, D.; Shirao, T.; Duden, R.; Majoul, I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 2004, 14, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W. The life cycle of a connexin: Gap junction formation, removal, and degradation. J. Bioenerg. Biomembr. 1996, 28, 311–318. [Google Scholar] [CrossRef]
- Laird, D.W.; Naus, C.C.; Lampe, P.D. SnapShot: Connexins and Disease. Cell 2017, 170, 1260–1260.e1. [Google Scholar] [CrossRef]
- Laird, D.W.; Lampe, P.D. Cellular mechanisms of connexin-based inherited diseases. Trends Cell Biol. 2022, 32, 58–69. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014, 588, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta 2005, 1711, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.T.; Kells, R.M.; Gumpert, A.M.; Marzillier, J.Y.; Davidson, M.W.; Falk, M.M. Internalized gap junctions are degraded by autophagy. Autophagy 2012, 8, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Falk, M.M.; Kells, R.M.; Berthoud, V.M. Degradation of connexins and gap junctions. FEBS Lett. 2014, 588, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Epifantseva, I.; Shaw, R.M. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim. Biophys. Acta Biomembr. 2018, 1860, 40–47. [Google Scholar] [CrossRef]
- Leithe, E. Regulation of connexins by the ubiquitin system: Implications for intercellular communication and cancer. Biochim. Biophys. Acta 2016, 1865, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.I.; Piwnica-Worms, H.; McNamee, H.; Paul, D.L. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul. 1990, 1, 989–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Kasperek, E.M.; Nicholson, B.J. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J. Cell Biol. 1999, 144, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Li, H.; Cannon, A.; Trease, A.J.; Spagnol, G.; Zheng, H.; Radio, S.; Patel, K.; Batra, S.; Sorgen, P.L. Phosphorylation of Cx43 residue Y313 by Src contributes to blocking the interaction with Drebrin and disassembling gap junctions. J. Mol. Cell. Cardiol. 2019, 126, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Hengeveld, T.; Postma, F.R.; Moolenaar, W.H. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J. Biol. Chem. 2001, 276, 8544–8549. [Google Scholar] [CrossRef] [Green Version]
- Gilleron, J.; Fiorini, C.; Carette, D.; Avondet, C.; Falk, M.M.; Segretain, D.; Pointis, G. Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J. Cell Sci. 2008, 121, 4069–4078. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.S.; Xu, J.; Nicholson, B.J. Coregulation of multiple signaling mechanisms in pp60v-Src-induced closure of Cx43 gap junction channels. J. Membr. Biol. 2012, 245, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Pahujaa, M.; Anikin, M.; Goldberg, G.S. Phosphorylation of connexin43 induced by Src: Regulation of gap junctional communication between transformed cells. Exp. Cell Res. 2007, 313, 4083–4090. [Google Scholar] [CrossRef]
- Toyofuku, T.; Akamatsu, Y.; Zhang, H.; Kuzuya, T.; Tada, M.; Hori, M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 2001, 276, 1780–1788. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Warn-Cramer, B.J.; Kurata, W.E.; Lau, A.F. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 2001, 154, 815–827. [Google Scholar] [CrossRef]
- Spagnol, G.; Kieken, F.; Kopanic, J.L.; Li, H.; Zach, S.; Stauch, K.L.; Grosely, R.; Sorgen, P.L. Structural Studies of the Nedd4 WW Domains and Their Selectivity for the Connexin43 (Cx43) Carboxyl Terminus. J. Biol. Chem. 2016, 291, 7637–7650. [Google Scholar] [CrossRef] [Green Version]
- Leykauf, K.; Salek, M.; Bomke, J.; Frech, M.; Lehmann, W.D.; Durst, M.; Alonso, A. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J. Cell Sci. 2006, 119, 3634–3642. [Google Scholar] [CrossRef] [Green Version]
- Totland, M.Z.; Bergsland, C.H.; Fykerud, T.A.; Knudsen, L.M.; Rasmussen, N.L.; Eide, P.W.; Yohannes, Z.; Sorensen, V.; Brech, A.; Lothe, R.A.; et al. The E3 ubiquitin ligase NEDD4 induces endocytosis and lysosomal sorting of connexin 43 to promote loss of gap junctions. J. Cell Sci. 2017, 130, 2867–2882. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, G.T.; Lin, R.; Warn-Cramer, B.J.; Lau, A.F.; Burt, J.M. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am. J. Physiol. Cell Physiol. 2003, 284, C511–C520. [Google Scholar] [CrossRef]
- Ambrosi, C.; Ren, C.; Spagnol, G.; Cavin, G.; Cone, A.; Grintsevich, E.E.; Sosinsky, G.E.; Sorgen, P.L. Connexin43 Forms Supramolecular Complexes through Non-Overlapping Binding Sites for Drebrin, Tubulin, and ZO-1. PLoS ONE 2016, 11, e0157073. [Google Scholar] [CrossRef] [Green Version]
- Saidi Brikci-Nigassa, A.; Clement, M.J.; Ha-Duong, T.; Adjadj, E.; Ziani, L.; Pastre, D.; Curmi, P.A.; Savarin, P. Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules. Biochemistry 2012, 51, 4331–4342. [Google Scholar] [CrossRef]
- Kieken, F.; Mutsaers, N.; Dolmatova, E.; Virgil, K.; Wit, A.L.; Kellezi, A.; Hirst-Jensen, B.J.; Duffy, H.S.; Sorgen, P.L. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ. Res. 2009, 104, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Dunn, C.A.; Lampe, P.D. Injury-triggered Akt phosphorylation of Cx43: A ZO-1-driven molecular switch that regulates gap junction size. J. Cell Sci. 2014, 127, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.T.; Kells, R.M.; Falk, M.M. Two tyrosine-based sorting signals in the Cx43 C-terminus cooperate to mediate gap junction endocytosis. Mol. Biol. Cell 2013, 24, 2834–2848. [Google Scholar] [CrossRef]
- Kittler, J.T.; Chen, G.; Kukhtina, V.; Vahedi-Faridi, A.; Gu, Z.; Tretter, V.; Smith, K.R.; McAinsh, K.; Arancibia-Carcamo, I.L.; Saenger, W.; et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc. Natl. Acad. Sci. USA 2008, 105, 3616–3621. [Google Scholar] [CrossRef] [Green Version]
- Girao, H.; Catarino, S.; Pereira, P. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp. Cell Res. 2009, 315, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Iyyathurai, J.; Decuypere, J.P.; Leybaert, L.; D’Hondt, C.; Bultynck, G. Connexins: Substrates and regulators of autophagy. BMC Cell. Biol. 2016, 17 (Suppl. S1), 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solan, J.L.; Lampe, P.D. Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover. Biomolecules 2020, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Girao, H.; Yuste, A.; Patel, B.; Marques, C.; Spray, D.C.; Pereira, P.; Cuervo, A.M. Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol. Biol. Cell 2012, 23, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Mitra, S.; Katoch, P.; Kelsey, L.S.; Johnson, K.R.; Mehta, P.P. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol. Biol. Cell 2013, 24, 715–733. [Google Scholar] [CrossRef]
- Totland, M.Z.; Rasmussen, N.L.; Knudsen, L.M.; Leithe, E. Regulation of gap junction intercellular communication by connexin ubiquitination: Physiological and pathophysiological implications. Cell. Mol. Life Sci. 2020, 77, 573–591. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Spagnol, G.; Zheng, L.; Stauch, K.L.; Sorgen, P.L. Regulation of Connexin43 Function and Expression by Tyrosine Kinase 2. J. Biol. Chem. 2016, 291, 15867–15880. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Trease, A.J.; Katsurada, K.; Spagnol, G.; Li, H.; Shi, W.; Duan, B.; Patel, K.P.; Sorgen, P.L. Inhibition of Pyk2 and Src activity improves Cx43 gap junction intercellular communication. J. Mol. Cell. Cardiol. 2020, 149, 27–40. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Morrow, T.A.; Desiderio, S.V. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc. Natl. Acad. Sci. USA 1992, 89, 11194–11198. [Google Scholar] [CrossRef] [Green Version]
- Vetrie, D.; Vorechovsky, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarstrom, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef]
- Felices, M.; Falk, M.; Kosaka, Y.; Berg, L.J. Tec kinases in T cell and mast cell signaling. Adv. Immunol. 2007, 93, 145–184. [Google Scholar] [CrossRef]
- Oviedo-Orta, E.; Evans, W.H. Gap junctions and connexins: Potential contributors to the immunological synapse. J. Leukoc. Biol. 2002, 72, 636–642. [Google Scholar] [CrossRef]
- Tittarelli, A.; Navarrete, M.; Gleisner, M.A.; Gebicke-Haerter, P.; Salazar-Onfray, F. Connexin-Mediated Signaling at the Immunological Synapse. Int. J. Mol. Sci. 2020, 21, 3736. [Google Scholar] [CrossRef]
- Roman-Garcia, S.; Merino-Cortes, S.V.; Gardeta, S.R.; de Bruijn, M.J.W.; Hendriks, R.W.; Carrasco, Y.R. Distinct Roles for Bruton’s Tyrosine Kinase in B Cell Immune Synapse Formation. Front. Immunol. 2018, 9, 2027. [Google Scholar] [CrossRef]
- Singleton, K.L.; Gosh, M.; Dandekar, R.D.; Au-Yeung, B.B.; Ksionda, O.; Tybulewicz, V.L.; Altman, A.; Fowell, D.J.; Wulfing, C. Itk controls the spatiotemporal organization of T cell activation. Sci. Signal. 2011, 4, ra66. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Kannan, A.K.; August, A. Structure and function of Tec family kinase Itk. Biomol. Concepts 2011, 2, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Falk, L.; Dang-Lawson, M.; Vega, J.L.; Pournia, F.; Choi, K.; Jang, C.; Naus, C.C.; Matsuuchi, L. Mutations of Cx43 that affect B cell spreading in response to BCR signaling. Biol. Open 2014, 3, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Grosely, R.; Kopanic, J.L.; Nabors, S.; Kieken, F.; Spagnol, G.; Al-Mugotir, M.; Zach, S.; Sorgen, P.L. Effects of phosphorylation on the structure and backbone dynamics of the intrinsically disordered connexin43 C-terminal domain. J. Biol. Chem. 2013, 288, 24857–24870. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Spagnol, G.; Naslavsky, N.; Caplan, S.; Sorgen, P.L. TC-PTP directly interacts with connexin43 to regulate gap junction intercellular communication. J. Cell Sci. 2014, 127, 3269–3279. [Google Scholar] [CrossRef] [Green Version]
- Brunner, C.; Avots, A.; Kreth, H.W.; Serfling, E.; Schuster, V. Bruton’s tyrosine kinase is activated upon CD40 stimulation in human B lymphocytes. Immunobiology 2002, 206, 432–440. [Google Scholar] [CrossRef]
- Shan, X.; Wange, R.L. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J. Biol. Chem. 1999, 274, 29323–29330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Dong, S.; Strattan, E.; Ren, L.; Butchar, J.P.; Thornton, K.; Mishra, A.; Porcu, P.; Bradshaw, J.M.; Bisconte, A.; et al. Targeting interleukin-2-inducible T-cell kinase (ITK) and resting lymphocyte kinase (RLK) using a novel covalent inhibitor PRN694. J. Biol. Chem. 2015, 290, 5960–5978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdeuil, K.; Taneyhill, L.A. The gap junction protein connexin 43 controls multiple aspects of cranial neural crest cell development. J. Cell Sci. 2020, 133, jcs235440. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; Barberi-Heyob, M.; Blondel, W.; Guillemin, F.; Didelon, J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques 2008, 45, 33–62. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.Y.; Chen, J.; Gross, M.L.; Pakrasi, H.B. Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates. Proc. Natl. Acad. Sci. USA 2011, 108, 18536–18541. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.D.; Solan, J.L.; Dolejsi, M.K.; Lampe, P.D. Analysis of connexin phosphorylation sites. Methods 2000, 20, 196–204. [Google Scholar] [CrossRef]
- Sirnes, S.; Leithe, E.; Rivedal, E. The detergent resistance of Connexin43 is lost upon TPA or EGF treatment and is an early step in gap junction endocytosis. Biochem. Biophys. Res. Commun. 2008, 373, 597–601. [Google Scholar] [CrossRef]
- Musil, L.S.; Goodenough, D.A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 1991, 115, 1357–1374. [Google Scholar] [CrossRef] [Green Version]
- Oviedo-Orta, E.; Perreau, M.; Evans, W.H.; Potolicchio, I. Control of the proliferation of activated CD4+ T cells by connexins. J. Leukoc. Biol. 2010, 88, 79–86. [Google Scholar] [CrossRef]
- Saez, P.J.; Shoji, K.F.; Aguirre, A.; Saez, J.C. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediat. Inflamm. 2014, 2014, 742734. [Google Scholar] [CrossRef] [Green Version]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Bryceson, Y.T.; Meckel, T.; Vasiliver-Shamis, G.; Dustin, M.L.; Long, E.O. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity 2009, 31, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Yuseff, M.I.; Reversat, A.; Lankar, D.; Diaz, J.; Fanget, I.; Pierobon, P.; Randrian, V.; Larochette, N.; Vascotto, F.; Desdouets, C.; et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity 2011, 35, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Naranjo, A.; Bouma, G.; Pereda, C.; Ramirez, M.; Webb, K.F.; Tittarelli, A.; Lopez, M.N.; Kalergis, A.M.; Thrasher, A.J.; Becker, D.L.; et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J. Immunol. 2011, 187, 3121–3132. [Google Scholar] [CrossRef] [Green Version]
- Glass, A.M.; Snyder, E.G.; Taffet, S.M. Connexins and pannexins in the immune system and lymphatic organs. Cell. Mol. Life Sci. 2015, 72, 2899–2910. [Google Scholar] [CrossRef]
- Matsuuchi, L.; Naus, C.C. Gap junction proteins on the move: Connexins, the cytoskeleton and migration. Biochim. Biophys. Acta 2013, 1828, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Solan, J.L.; Lampe, P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 2018, 1860, 83–90. [Google Scholar] [CrossRef]
- Rocha-Perugini, V.; Gordon-Alonso, M.; Sanchez-Madrid, F. Role of Drebrin at the Immunological Synapse. Adv. Exp. Med. Biol. 2017, 1006, 271–280. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Martz, R.; Dorward, D.; Waisberg, M.; Pierce, S.K. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat. Immunol. 2011, 12, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Altan-Bonnet, G.; Germain, R.N. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005, 3, e356. [Google Scholar] [CrossRef] [Green Version]
- Machtaler, S.; Dang-Lawson, M.; Choi, K.; Jang, C.; Naus, C.C.; Matsuuchi, L. The gap junction protein Cx43 regulates B-lymphocyte spreading and adhesion. J. Cell Sci. 2011, 124, 2611–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onabajo, O.O.; Seeley, M.K.; Kale, A.; Qualmann, B.; Kessels, M.; Han, J.; Tan, T.H.; Song, W. Actin-binding protein 1 regulates B cell receptor-mediated antigen processing and presentation in response to B cell receptor activation. J. Immunol. 2008, 180, 6685–6695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Lampe, P.D. Identification of connexin-43 interacting proteins. Cell Commun. Adhes. 2003, 10, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solan, J.L.; Lampe, P.D. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 2007, 217, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Fontes, M.S.; van Veen, T.A.; de Bakker, J.M.; van Rijen, H.V. Functional consequences of abnormal Cx43 expression in the heart. Biochim. Biophys. Acta 2012, 1818, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Lampe, P.D.; Kurata, W.E.; Warn-Cramer, B.J.; Lau, A.F. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J. Cell Sci. 1998, 111 Pt 6, 833–841. [Google Scholar] [CrossRef]








| Peptide Sequence | Start–End | Peptide Mass | Peptide Mass | Number of Phospho-Peptides | Phosphorylation Site |
|---|---|---|---|---|---|
| (Residue Number) | (Calculated) | (Actual) | |||
| SDPyHATTGPLSPSK | 244–258 | 1557.6 | 1637.72 | 110 | Y247 |
| yAYYNGcSSPTAPLSPMSPPGYK | 265–287 | 2435.7 | 2588.09 | 39 | Y265 |
| YAyFNGcSSPTAPLSPMSPPGYK | 265–287 | 2435.7 | 2588.09 | 12 | Y267 |
| YAYFNGcSSPTAPLSPMSPPGyK | 265–287 | 2435.7 | 2588.09 | 9 | Y286 |
| yAYFNGcSSPTAPLSPMSPPGyK | 265–287 | 2435.7 | 2668.05 | 1 | Y265/Y286 |
| QASEQNWANySAEQNR | 304–319 | 1895.9 | 1975.79 | 80 | Y313 |
| Peptide Sequence | Start–End | Peptide Mass | Peptide Mass | Number of Phospho-Peptides | Phosphorylation Site |
|---|---|---|---|---|---|
| (Residue Number) | (Calculated) | (Actual) | |||
| SDPyHATTGPLSPSK | 244–258 | 1557.6 | 1637.72 | 50 | Y247 |
| yAYYNGcSSPTAPLSPMSPPGYK | 265–287 | 2435.7 | 2588.09 | 33 | Y265 |
| YAyFNGcSSPTAPLSPMSPPGYK | 265–287 | 2435.7 | 2588.09 | 16 | Y267 |
| YAYFNGcSSPTAPLSPMSPPGyK | 265–287 | 2435.7 | 2588.09 | 24 | Y286 |
| yAyFNGcSSPTAPLSPMSPPGYK | 265–287 | 2435.7 | 2588.09 | 5 | Y265/Y267 |
| yAYFNGcSSPTAPLSPMSPPGyK | 265–287 | 2435.7 | 2668.05 | 16 | Y265/Y286 |
| QASEQNWANySAEQNR | 304–319 | 1895.9 | 1975.79 | 80 | Y313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, I.; Li, H.; Trease, A.J.; Sorgen, P.L. Regulation of Cx43 Gap Junction Intercellular Communication by Bruton’s Tyrosine Kinase and Interleukin-2-Inducible T-Cell Kinase. Biomolecules 2023, 13, 660. https://doi.org/10.3390/biom13040660
Basu I, Li H, Trease AJ, Sorgen PL. Regulation of Cx43 Gap Junction Intercellular Communication by Bruton’s Tyrosine Kinase and Interleukin-2-Inducible T-Cell Kinase. Biomolecules. 2023; 13(4):660. https://doi.org/10.3390/biom13040660
Chicago/Turabian StyleBasu, Ishika, Hanjun Li, Andrew J. Trease, and Paul L. Sorgen. 2023. "Regulation of Cx43 Gap Junction Intercellular Communication by Bruton’s Tyrosine Kinase and Interleukin-2-Inducible T-Cell Kinase" Biomolecules 13, no. 4: 660. https://doi.org/10.3390/biom13040660





