Senescence-Related lncRNA Signature Predicts Prognosis, Response to Immunotherapy and Chemotherapy in Skin Cutaneous Melanoma
Abstract
1. Introduction
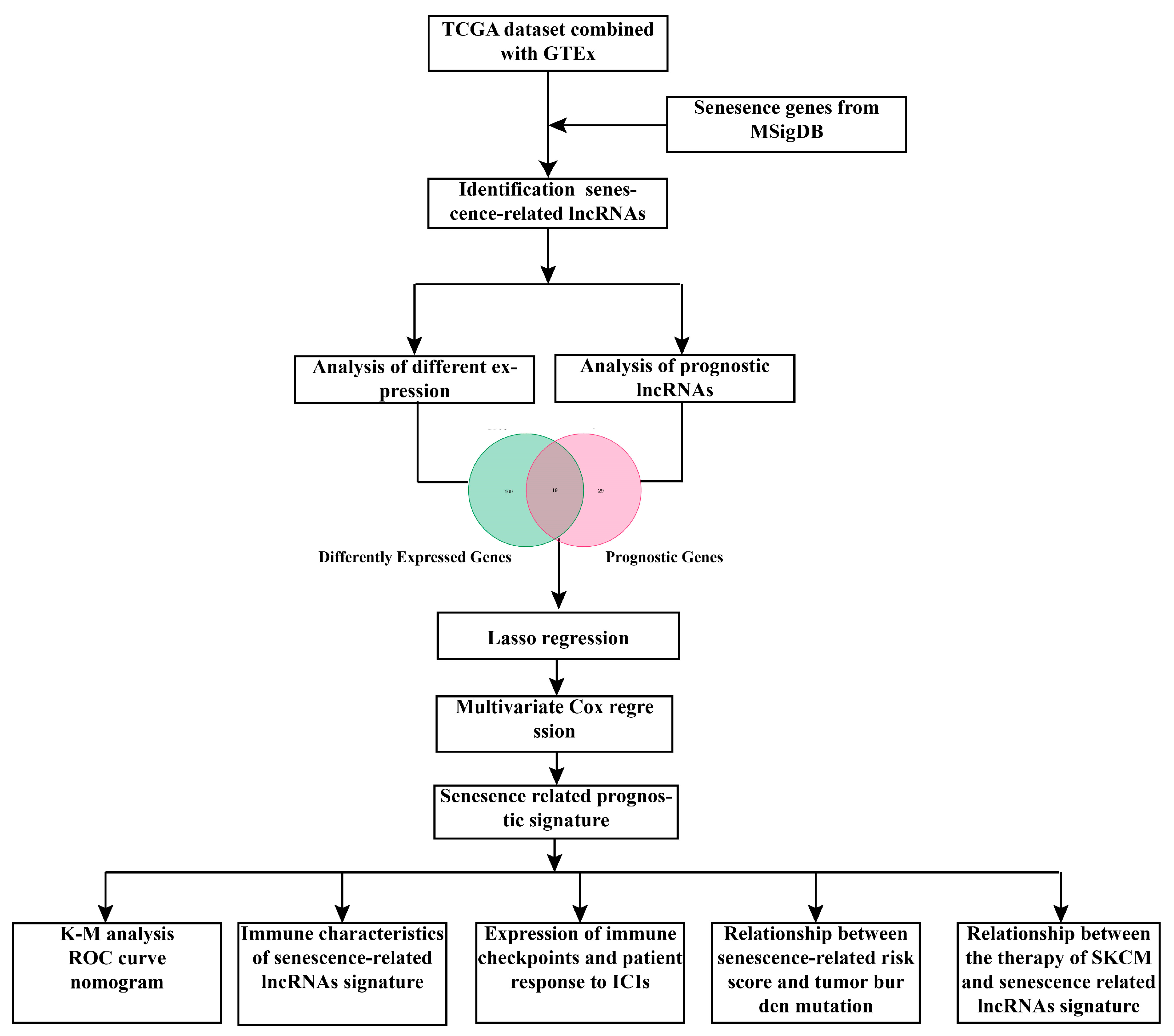
2. Materials and Methods
2.1. Data Acquisition and Normalization
2.2. Prognosis-Related lncRNAs and Differentially Expressed lncRNAs
2.3. Development of Senescence-Related lncRNAs Signature
2.4. Gene Set Enrichment Analysis and Construction of ceRNA Network
2.5. Assessment of Tumor Immune Microenvironment and Calculation of Tumor Burden Mutation
2.6. Predicting Patient Response to ICIs and the Assessment of Immune Checkpoint Molecules
2.7. Prediction of Chemotherapy Drug Sensitivity
2.8. Immunohistochemistry Staining
2.9. Real-Time Quantitative PCR
2.10. Statistical Analysis
3. Results
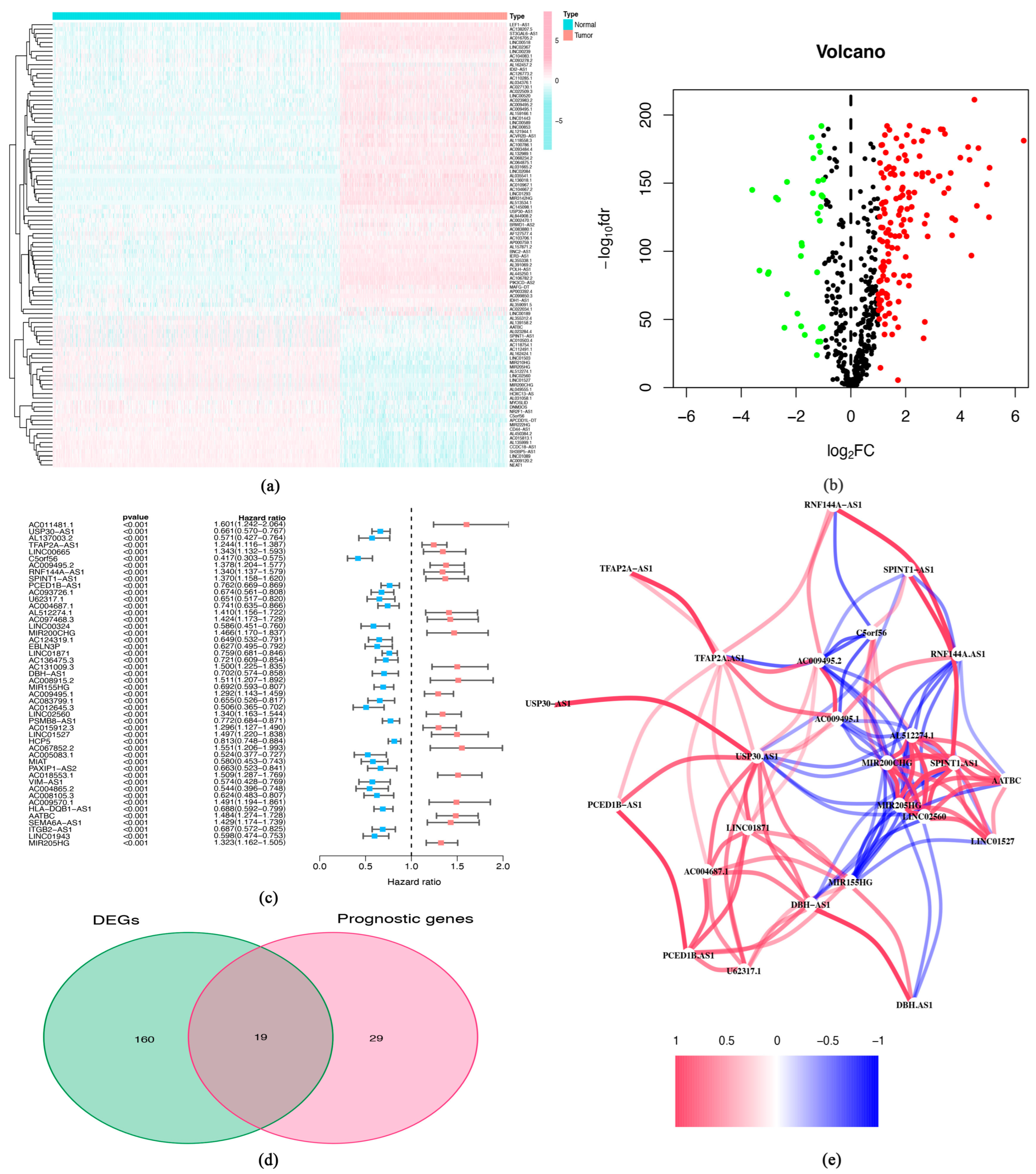
3.1. Identification of Differently Expressed lncRNAs and Prognosis-Related lncRNAs
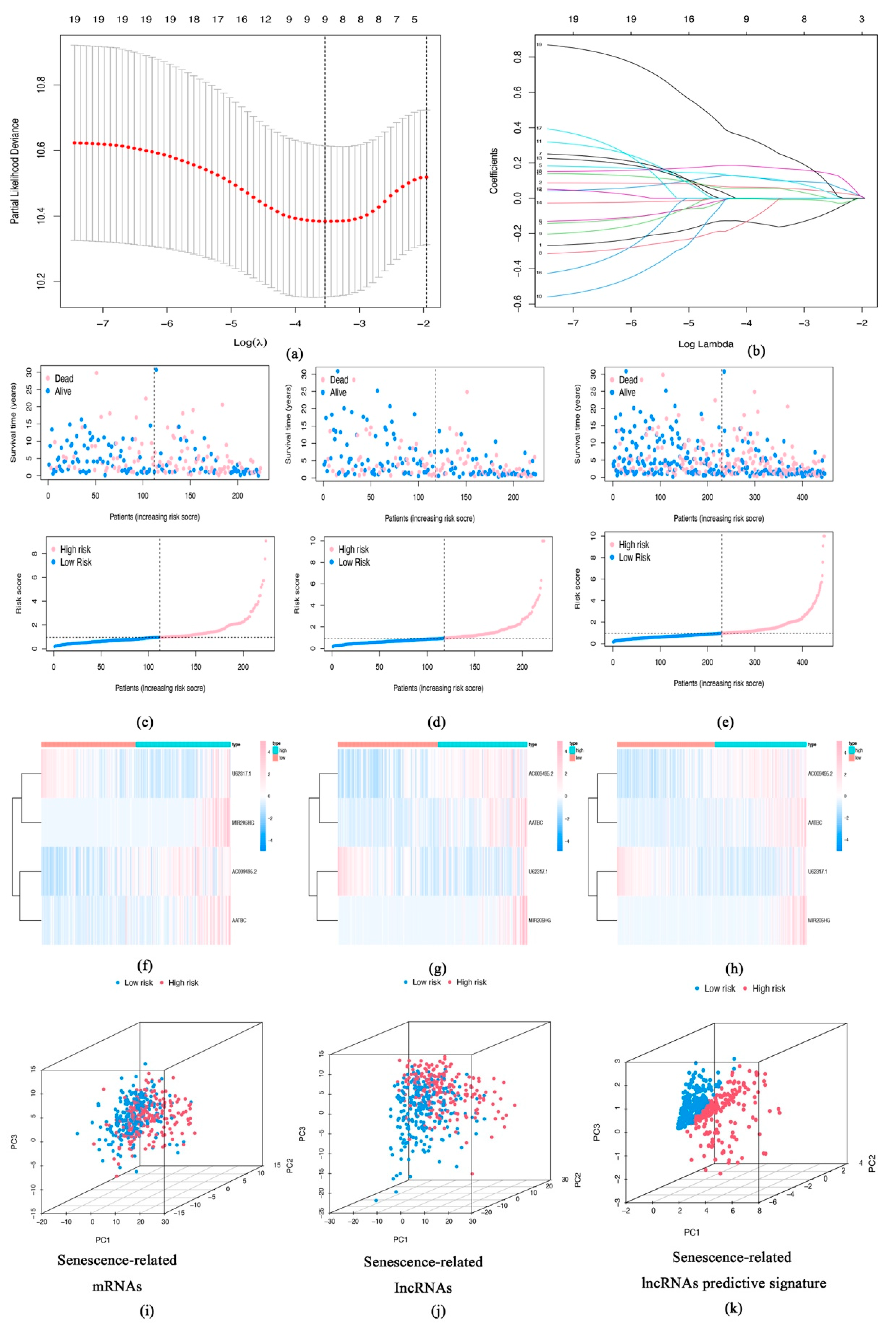
3.2. Construction of Senescence-Related lncRNA Signature and Evaluation of Predictive Effects
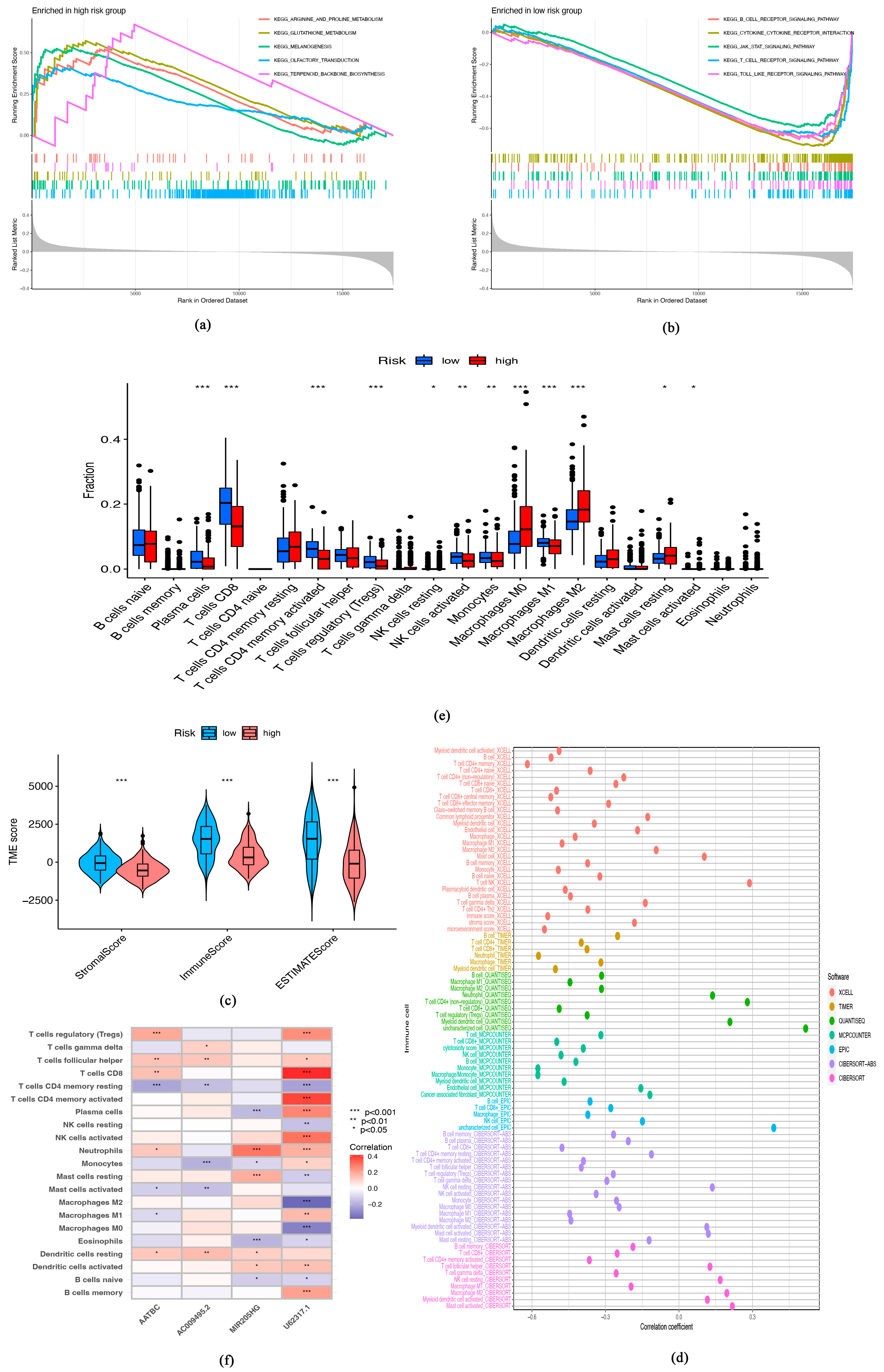
3.3. Immune Characteristics of Senescence-Related lncRNA Signature
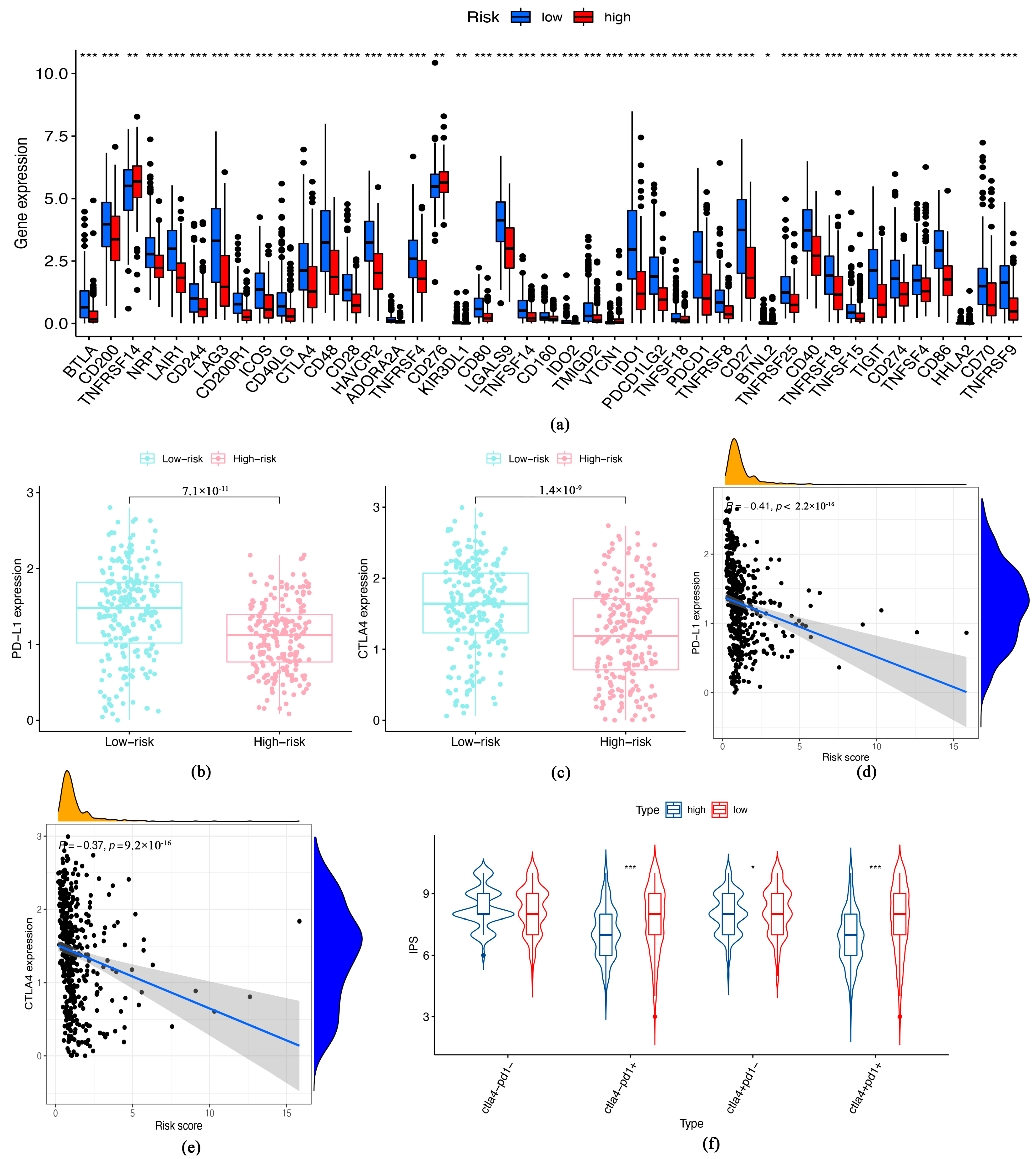
3.4. Immune Checkpoints Expression and Response of Patients to ICIs
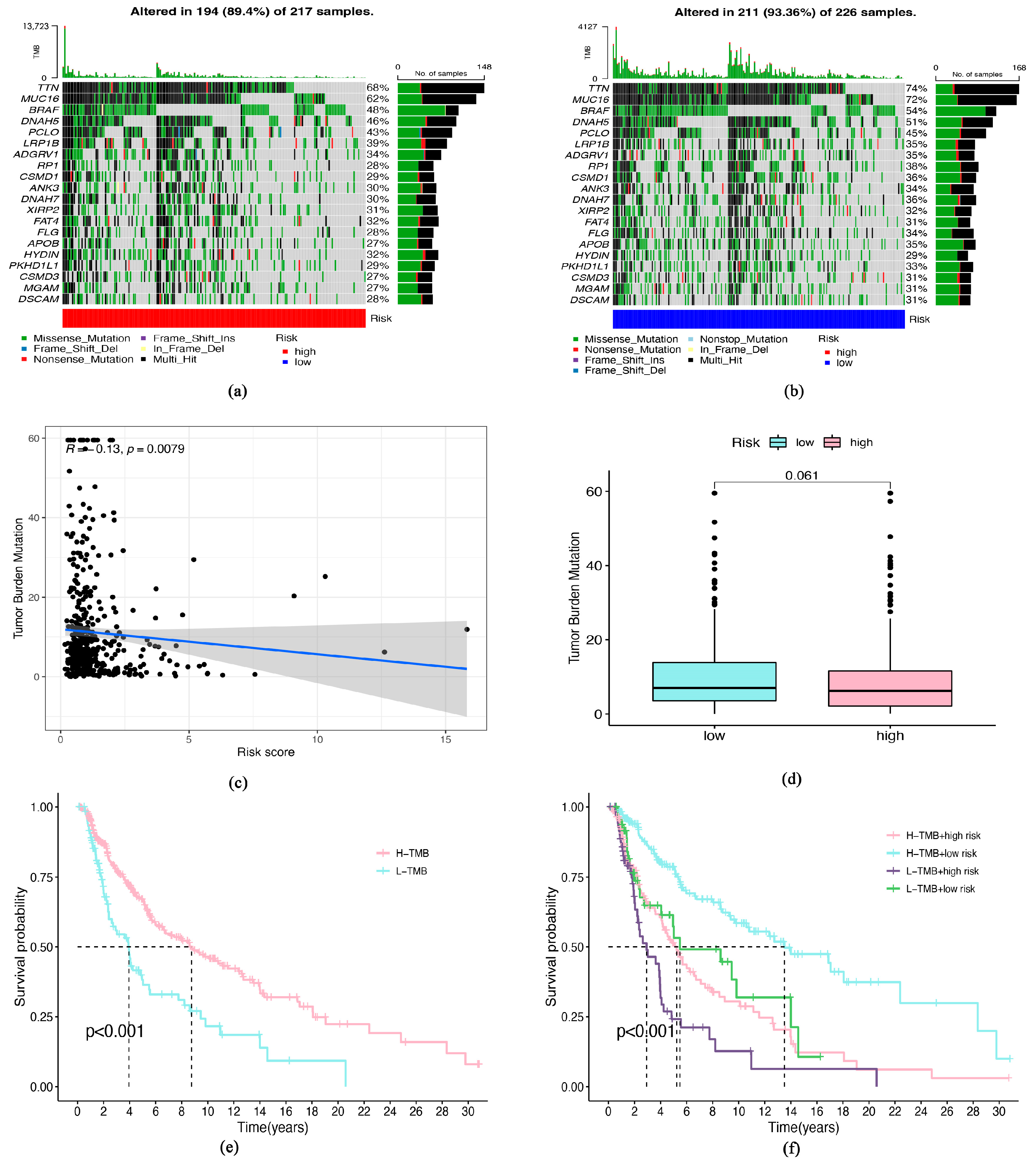
3.5. Tumor Burden Mutation (TMB) and Senescence-Related lncRNA Signature
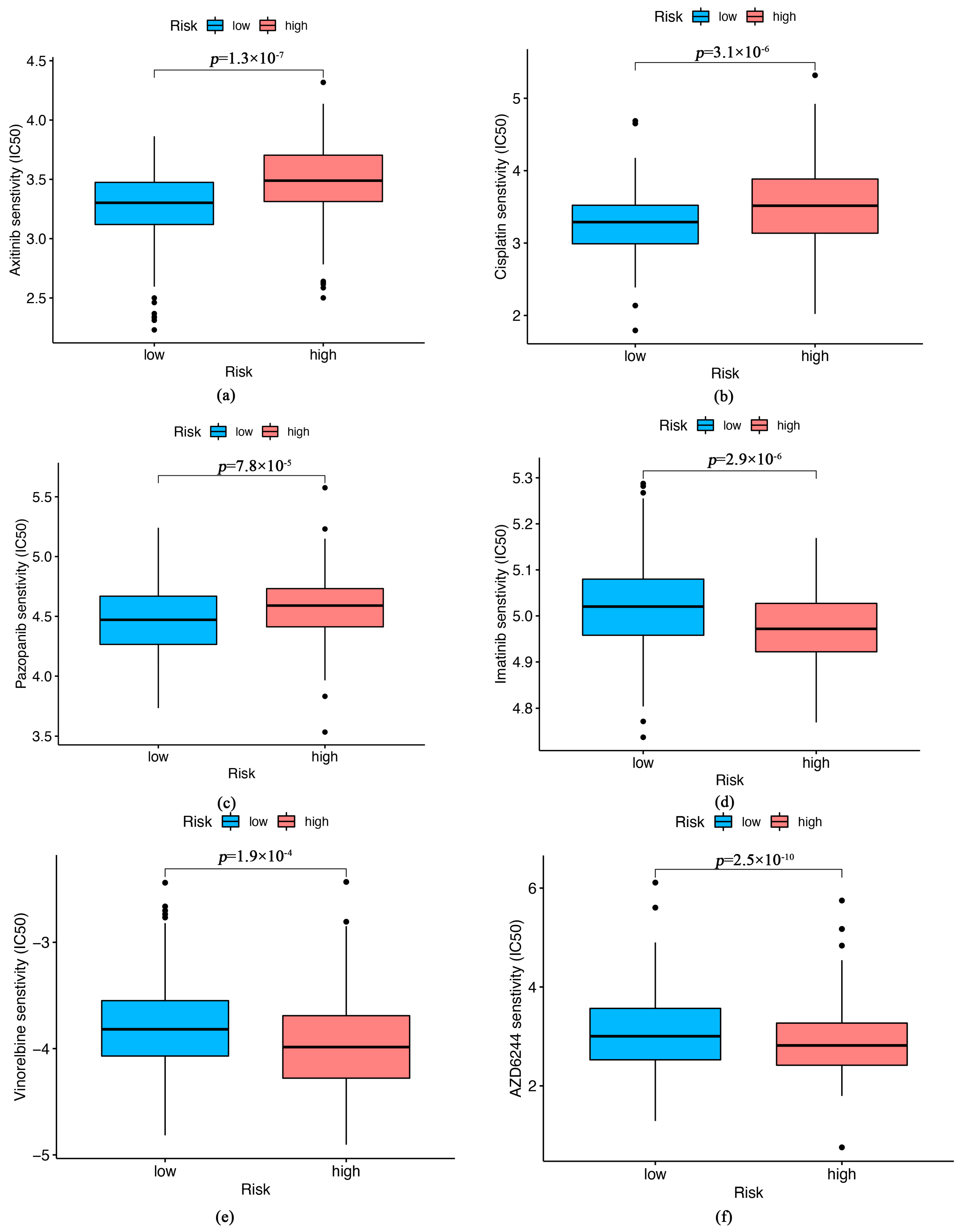
3.6. Relationship between the Therapeutic and Senescence-Related Risk Signature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastian, B.C. The Molecular Pathology of Melanoma: An Integrated Taxonomy of Melanocytic Neoplasia. Annu. Rev. Pathol. 2014, 9, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.I.; Gershenwald, J.E. Evidence-Based Treatment of Early-Stage Melanoma. J. Surg. Oncol. 2011, 104, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-Clinical Modeling of Cutaneous Melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-L.; Hoffmann, R.; González-López, C.; Doherty, G.J.; Korkola, J.E.; Muñoz-Espín, D. Cellular Senescence in Cancer: From Mechanisms to Detection. Mol. Oncol. 2021, 15, 2634. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Non-Coding RNA (LncRNA) and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef]
- Sun, L.; Guan, Z.; Wei, S.; Tan, R.; Li, P.; Yan, L. Identification of Long Non-Coding and Messenger RNAs Differentially Expressed Between Primary and Metastatic Melanoma. Front. Genet. 2019, 10, 292. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Y.; He, R.; Lu, X.; Dai, Y.; Qi, G.; Liu, J.; Deng, J.; Lu, W.; Jin, J.; et al. Identification and Validation of a Novel Cellular Senescence-Related LncRNA Prognostic Signature for Predicting Immunotherapy Response in Stomach Adenocarcinoma. Front. Genet. 2022, 13, 935056. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Ma, T.; Zhou, J.; Ma, N.; Yang, W.; Liu, C.; Hou, Z.; Chen, S.; Zong, Z.; Zeng, B.; et al. A Novel Senescence-Associated LncRNA Signature Predicts the Prognosis and Tumor Microenvironment of Patients with Colorectal Cancer: A Bioinformatics Analysis. J. Gastrointest. Oncol. 2022, 13, 1842–1863. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, W.; Ni, S.; Sang, M.; Wu, S.; Mu, Y.; Liu, K.; Li, N.; Zhu, L.; Xu, G. Construction of an Immune-Related LncRNA Signature as a Novel Prognosis Biomarker for LUAD. Aging 2021, 13, 20684–20697. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ringnér, M. What Is Principal Component Analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Mayakonda, A.; Lin, D.-C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and Comprehensive Analysis of Somatic Variants in Cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Profitós-Pelejà, N.; Santos, J.C.; Marín-Niebla, A.; Roué, G.; Ribeiro, M.L. Regulation of B-Cell Receptor Signaling and Its Therapeutic Relevance in Aggressive B-Cell Lymphomas. Cancers 2022, 14, 860. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Kloor, M.; von Knebel Doeberitz, M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer 2016, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Schmitt, C.A. The Dynamic Nature of Senescence in Cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting Senescence for the Treatment of Cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Yan, K.; Wang, Y.; Shao, Y.; Xiao, T. Gene Instability-Related LncRNA Prognostic Model of Melanoma Patients via Machine Learning Strategy. J. Oncol. 2021, 2021, 5582920. [Google Scholar] [CrossRef]
- Guo, J.; Gan, Q.; Gan, C.; Zhang, X.; Ma, X.; Dong, M. LncRNA MIR205HG Regulates Melanomagenesis via the MiR-299-3p/VEGFA Axis. Aging 2021, 13, 5297–5311. [Google Scholar] [CrossRef]
- Li, H.; Jia, J.; Yang, L.; Chu, J.; Sheng, J.; Wang, C.; Meng, W.; Jia, Z.; Yin, H.; Wan, J.; et al. LncRNA MIR205HG Drives Esophageal Squamous Cell Carcinoma Progression by Regulating MiR-214/SOX4 Axis. Onco Targets Ther. 2020, 13, 13097–13109. [Google Scholar] [CrossRef]
- Li, F.-W.; Luo, S.-K. Identification and Construction of a Predictive Immune-Related LncRNA Signature Model for Melanoma. Int. J. Gen. Med. 2021, 14, 9227–9235. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Liu, F.; Wu, X.; Wen, Z. An Immune-Related Long Noncoding RNA Pair as a New Biomarker to Predict the Prognosis of Patients in Breast Cancer. Front. Genet. 2022, 13, 895200. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Herlyn, M. Melanoma and the Tumor Microenvironment. Curr. Oncol. Rep. 2008, 10, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T Cell Metabolism in Infection and Cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Zhou, Y.; Lin, Y.; Feng, Y.; Chen, Y.; Cai, L. Senescence-Related lncRNA Signature Predicts Prognosis, Response to Immunotherapy and Chemotherapy in Skin Cutaneous Melanoma. Biomolecules 2023, 13, 661. https://doi.org/10.3390/biom13040661
Lin K, Zhou Y, Lin Y, Feng Y, Chen Y, Cai L. Senescence-Related lncRNA Signature Predicts Prognosis, Response to Immunotherapy and Chemotherapy in Skin Cutaneous Melanoma. Biomolecules. 2023; 13(4):661. https://doi.org/10.3390/biom13040661
Chicago/Turabian StyleLin, Kefan, Yingtong Zhou, Yanling Lin, Yuanyuan Feng, Yuting Chen, and Longmei Cai. 2023. "Senescence-Related lncRNA Signature Predicts Prognosis, Response to Immunotherapy and Chemotherapy in Skin Cutaneous Melanoma" Biomolecules 13, no. 4: 661. https://doi.org/10.3390/biom13040661
APA StyleLin, K., Zhou, Y., Lin, Y., Feng, Y., Chen, Y., & Cai, L. (2023). Senescence-Related lncRNA Signature Predicts Prognosis, Response to Immunotherapy and Chemotherapy in Skin Cutaneous Melanoma. Biomolecules, 13(4), 661. https://doi.org/10.3390/biom13040661





