The Roles of sPLA2s in Skin Homeostasis and Disease
Abstract
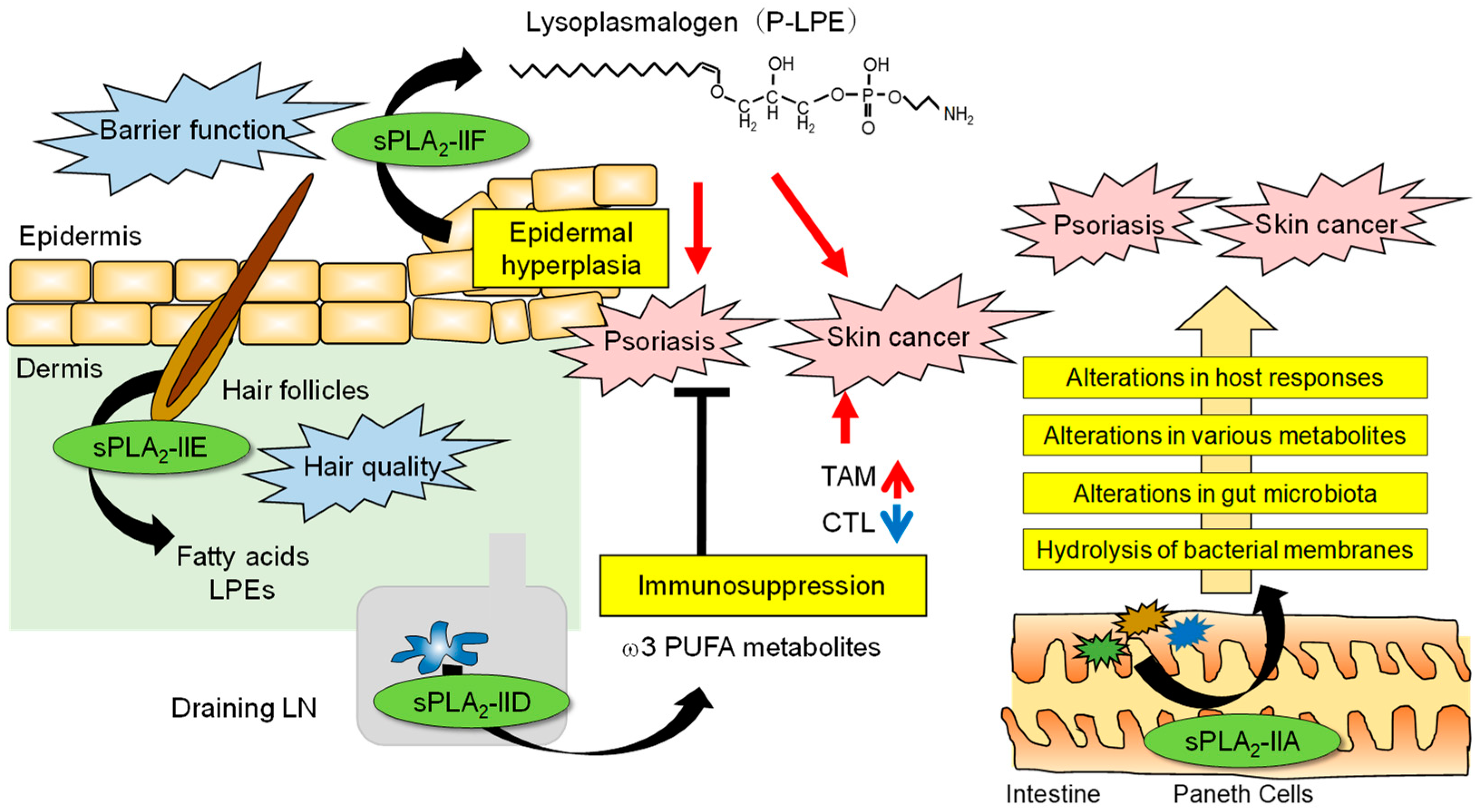
:1. Introduction
2. The Roles of Lipids in the Skin
3. sPLA2 in the Epidermis
4. sPLA2 in Hair Follicles
5. sPLA2 in Lymphoid Tissues That Affects Skin Diseases by Regulating Adaptive Immune Responses
6. sPLA2 Involved in an Alteration of the Intestinal Microbiota That Secondarily Affects Skin Diseases
7. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murakami, M. Novel functions of phospholipase A2s: Overview. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Gelb, M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008, 77, 495–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.Y. Group 1B phospholipase A2 in metabolic and inflammatory disease modulation. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Dore, E.; Boilard, E. Roles of secreted phospholipase A2 group IIA in inflammation and host defense. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 789–802. [Google Scholar] [CrossRef]
- Seilhamer, J.J.; Randall, T.L.; Yamanaka, M.; Johnson, L.K. Pancreatic phospholipase A2: Isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA 1986, 5, 519–527. [Google Scholar] [CrossRef]
- Kramer, R.M.; Hession, C.; Johansen, B.; Hayes, G.; McGray, P.; Chow, E.P.; Tizard, R.; Pepinsky, R.B. Structure and properties of a human non-pancreatic phospholipase A2. J. Biol. Chem. 1989, 264, 5768–5775. [Google Scholar] [CrossRef]
- Seilhamer, J.J.; Pruzanski, W.; Vadas, P.; Plant, S.; Miller, J.A.; Kloss, J.; Johnson, L.K. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J. Biol. Chem. 1989, 264, 5335–5338. [Google Scholar] [CrossRef]
- Chen, J.; Engle, S.J.; Seilhamer, J.J.; Tischfield, J.A. Cloning and recombinant expression of a novel human low molecular weight Ca2+-dependent phospholipase A2. J. Biol. Chem. 1994, 269, 2365–2368. [Google Scholar] [CrossRef]
- Chen, J.; Engle, S.J.; Seilhamer, J.J.; Tischfield, J.A. Cloning and characterization of novel rat and mouse low molecular weight Ca2+-dependent phospholipase A2s containing 16 cysteines. J. Biol. Chem. 1994, 269, 23018–23024. [Google Scholar] [CrossRef]
- Cupillard, L.; Koumanov, K.; Mattéi, M.-G.; Lazdunski, M.; Lambeau, G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 1997, 272, 15745–15752. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, J.; Suzuki, N.; Higashino, K.-i.; Yokota, Y.; Ono, T.; Kawamoto, K.; Fujii, N.; Arita, H.; Hanasaki, K. Cloning and characterization of novel mouse and human secretory phospholipase A2s. J. Biol. Chem. 1999, 274, 24973–24979. [Google Scholar] [CrossRef] [Green Version]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. On the Diversity of Secreted Phospholipases A2: Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999, 274, 31195–31202. [Google Scholar] [CrossRef]
- Valentin, E.; Koduri, R.S.; Scimeca, J.-C.; Carle, G.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. Cloning and recombinant expression of a novel mouse secreted phospholipase A2. J. Biol. Chem. 1999, 274, 19152–19160. [Google Scholar] [CrossRef] [Green Version]
- Shakhov, A.N.; Rubtsov, A.V.; Lyakhov, I.G.; Tumanov, A.V.; Nedospasov, S.A. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immun. 2000, 1, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Ishizaki, J.; Yokota, Y.; Higashino, K.-i.; Ono, T.; Ikeda, M.; Fujii, N.; Kawamoto, K.; Hanasaki, K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A2s. J. Biol. Chem. 2000, 275, 5785–5793. [Google Scholar] [CrossRef] [Green Version]
- Valentin, E.; Singer, A.G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H.; Lambeau, G. Cloning and recombinant expression of human group IIF-secreted phospholipase A2. Biochem. Biophys. Res. Commun. 2000, 279, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 2000, 275, 7492–7496. [Google Scholar] [CrossRef] [Green Version]
- Gelb, M.H.; Valentin, E.; Ghomashchi, F.; Lazdunski, M.; Lambeau, G. Cloning and recombinant expression of a structurally novel human secreted phospholipase A2. J. Biol. Chem. 2000, 275, 39823–39826. [Google Scholar] [CrossRef] [Green Version]
- Ho, I.C.; Arm, J.P.; Bingham, C.O.; Choi, A.; Austen, K.F.; Glimcher, L.H. A Novel Group of Phospholipase A2s Preferentially expressed in type 2 helper T Cells. J. Biol. Chem. 2001, 276, 18321–18326. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Kambe, T.; Shimbara, S.; Kudo, I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999, 274, 3103–3115. [Google Scholar] [CrossRef] [Green Version]
- Bezzine, S.; Koduri, R.S.; Valentin, E.; Murakami, M.; Kudo, I.; Ghomashchi, F.; Sadilek, M.; Lambeau, G.; Gelb, M.H. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 2000, 275, 3179–3191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Koduri, R.S.; Enomoto, A.; Shimbara, S.; Seki, M.; Yoshihara, K.; Singer, A.; Valentin, E.; Ghomashchi, F.; Lambeau, G.; et al. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 2001, 276, 10083–10096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, K.W.; Boileau, A.C.; Hui, D.Y. Protection against diet-induced obesity and obesity- related insulin resistance in Group 1B PLA2-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2002, 283, e994–e1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labonté, E.D.; Kirby, R.J.; Schildmeyer, N.M.; Cannon, A.M.; Huggins, K.W.; Hui, D.Y. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes 2006, 55, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsuki, M.; Taketomi, Y.; Arata, S.; Masuda, S.; Ishikawa, Y.; Ishii, T.; Takanezawa, Y.; Aoki, J.; Arai, H.; Yamamoto, K.; et al. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 2006, 281, 36420–36433. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Taketomi, Y.; Ushida, A.; Isogai, Y.; Kojima, T.; Hirabayashi, T.; Miki, Y.; Yamamoto, K.; Nishito, Y.; Kobayashi, T.; et al. The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity. Cell Metab. 2014, 20, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, L.H.; Duchez, A.C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [Green Version]
- Kudo, K.; Miki, Y.; Carreras, J.; Nakayama, S.; Nakamoto, Y.; Ito, M.; Nagashima, E.; Yamamoto, K.; Higuchi, H.; Morita, S.Y.; et al. Secreted phospholipase A2 modifies extracellular vesicles and accelerates B cell lymphoma. Cell Metab. 2022, 34, 615–633.e618. [Google Scholar] [CrossRef]
- Yokota, Y.; Hanasaki, K.; Ono, T.; Nakazato, H.; Kobayashi, T.; Arita, H. Suppression of murine endotoxic shock by sPLA2 inhibitor, indoxam, through group IIA sPLA2-independent mechanisms. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 1999, 1438, 213–222. [Google Scholar] [CrossRef]
- Tamaru, S.; Mishina, H.; Watanabe, Y.; Watanabe, K.; Fujioka, D.; Takahashi, S.; Suzuki, K.; Nakamura, T.; Obata, J.E.; Kawabata, K.; et al. Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J. Immunol. 2013, 191, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Sato, H.; Taketomi, Y.; Yamamoto, K. Integrated lipidomics in the secreted phospholipase A2 biology. Int. J. Mol. Sci. 2011, 12, 1474–1495. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Yamamoto, K.; Miki, Y.; Murase, R.; Sato, H.; Taketomi, Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016, 132, 91–134. [Google Scholar] [CrossRef]
- Murakami, M.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Yamamoto, K. Group IID, IIE, IIF and III secreted phospholipase A2s. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 803–818. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef]
- Taketomi, Y.; Miki, Y.; Murakami, M. Old but new: Group IIA phospholipase A2 as a modulator of gut microbiota. Metabolites 2022, 12, 352. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Radner, F.P.; Fischer, J. The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2014, 1841, 409–415. [Google Scholar] [CrossRef]
- Elias, P.M.; Gruber, R.; Crumrine, D.; Menon, G.; Williams, M.L.; Wakefield, J.S.; Holleran, W.M.; Uchida, Y. Formation and functions of the corneocyte lipid envelope (CLE). Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2014, 1841, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihara, A. Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog. Lipid Res. 2016, 63, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Anjo, T.; Kaneko, A.; Senoo, Y.; Shibata, A.; Takama, H.; Yokoyama, K.; Nishito, Y.; Ono, T.; Taya, C.; et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017, 8, 14609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, T.; Murakami, M.; Kihara, A. The role of PNPLA1 in ω-O-acylceramide synthesis and skin barrier function. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 869–879. [Google Scholar] [CrossRef]
- Krieg, P.; Fürstenberger, G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2014, 1841, 390–400. [Google Scholar] [CrossRef]
- Takeichi, T.; Hirabayashi, T.; Miyasaka, Y.; Kawamoto, A.; Okuno, Y.; Taguchi, S.; Tanahashi, K.; Murase, C.; Takama, H.; Tanaka, K.; et al. SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation. J. Clin. Investig. 2020, 130, 890–903. [Google Scholar] [CrossRef]
- Elias, P.M.; Brown, B.E. The mammalian cutaneous permeability barrier: Defective barrier function is essential fatty acid deficiency correlates with abnormal intercellular lipid deposition. Lab. Investig. 1978, 39, 574–583. [Google Scholar]
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Brash, A.R. Mutations associated with a congenital form of ichthyosis (NCIE) inactivate the epidermal lipoxygenases 12R-LOX and eLOX3. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2005, 1686, 238–247. [Google Scholar] [CrossRef]
- Kazantseva, A.; Goltsov, A.; Zinchenko, R.; Grigorenko, A.P.; Abrukova, A.V.; Moliaka, Y.K.; Kirillov, A.G.; Guo, Z.; Lyle, S.; Ginter, E.K.; et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science 2006, 314, 982–985. [Google Scholar] [CrossRef] [Green Version]
- Vasireddy, V.; Uchida, Y.; Salem, N., Jr.; Kim, S.Y.; Mandal, M.N.; Reddy, G.B.; Bodepudi, R.; Alderson, N.L.; Brown, J.C.; Hama, H.; et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 2007, 16, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Grall, A.; Guaguere, E.; Planchais, S.; Grond, S.; Bourrat, E.; Hausser, I.; Hitte, C.; Le Gallo, M.; Derbois, C.; Kim, G.J.; et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat. Genet. 2012, 44, 140–147. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Jain, M.; Feingold, K.R.; Elias, P.M. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J. Investig. Dermatol. 1996, 106, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Fluhr, J.W.; Kao, J.; Jain, M.; Ahn, S.K.; Feingold, K.R.; Elias, P.M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Fluhr, J.W.; Mao-Qiang, M.; Brown, B.E.; Hachem, J.P.; Moskowitz, D.G.; Demerjian, M.; Haftek, M.; Serre, G.; Crumrine, D.; Mauro, T.M.; et al. Functional consequences of a neutral pH in neonatal rat stratum corneum. J. Investig. Dermatol. 2004, 123, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Arima, N.; Ishiguro, J.; Prestwich, G.D.; Arai, H.; Aoki, J. LPA-producing enzyme PA-PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J. 2011, 30, 4248–4260. [Google Scholar] [CrossRef] [Green Version]
- Nagamachi, M.; Sakata, D.; Kabashima, K.; Furuyashiki, T.; Murata, T.; Segi-Nishida, E.; Soontrapa, K.; Matsuoka, T.; Miyachi, Y.; Narumiya, S. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J. Exp. Med. 2007, 204, 2865–2874. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Kabashima, K. Prostanoids and leukotrienes in the pathophysiology of atopic dermatitis and psoriasis. Int. Immunol. 2019, 31, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Takamiya, R.; Miki, Y.; Heike, K.; Taketomi, Y.; Sugimoto, N.; Yamaguchi, M.; Shitara, H.; Nishito, Y.; Kobayashi, T.; et al. Group IVE cytosolic phospholipase A2 limits psoriatic inflammation by mobilizing the anti-inflammatory lipid N-acylethanolamine. FASEB J. 2022, 36, e22301. [Google Scholar] [CrossRef]
- Ilic, D.; Bollinger, J.M.; Gelb, M.; Mauro, T.M. sPLA2 and the epidermal barrier. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2014, 1841, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Grass, D.S.; Felkner, R.H.; Chiang, M.Y.; Wallace, R.E.; Nevalainen, T.J.; Bennett, C.F.; Swanson, M.E. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Investig. 1996, 97, 2233–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Taketomi, Y.; Isogai, Y.; Miki, Y.; Sato, H.; Masuda, S.; Nishito, Y.; Morioka, K.; Ishimoto, Y.; Suzuki, N.; et al. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 2011, 286, 11616–11631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulherkar, R.; Kirtane, B.M.; Ramchandani, A.; Mansukhani, N.P.; Kannan, S.; Naresh, K.N. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene 2003, 22, 1936–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chovatiya, G.L.; Sunkara, R.R.; Roy, S.; Godbole, S.R.; Waghmare, S.K. Context-dependent effect of sPLA2-IIA induced proliferation on murine hair follicle stem cells and human epithelial cancer. EBioMedicine 2019, 48, 364–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Miki, Y.; Sato, M.; Taketomi, Y.; Nishito, Y.; Taya, C.; Muramatsu, K.; Ikeda, K.; Nakanishi, H.; Taguchi, R.; et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 2015, 212, 1901–1919. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Miki, Y.; Sato, H.; Nishito, Y.; Gelb, M.H.; Taketomi, Y.; Murakami, M. Expression and function of group IIE phospholipase A2 in mouse skin. J. Biol. Chem. 2016, 291, 15602–15613. [Google Scholar] [CrossRef] [Green Version]
- Weinmuellner, R.; Kryeziu, K.; Zbiral, B.; Tav, K.; Schoenhacker-Alte, B.; Groza, D.; Wimmer, L.; Schosserer, M.; Nagelreiter, F.; Rösinger, S.; et al. Long-term exposure of immortalized keratinocytes to arsenic induces EMT, impairs differentiation in organotypic skin models and mimics aspects of human skin derangements. Arch. Toxicol. 2018, 92, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Miki, Y.; Yamamoto, K.; Taketomi, Y.; Sato, H.; Shimo, K.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; Nakanishi, H.; Ikeda, K.; et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013, 210, 1217–1234. [Google Scholar] [CrossRef] [Green Version]
- Miki, Y.; Kidoguchi, Y.; Sato, M.; Taketomi, Y.; Taya, C.; Muramatsu, K.; Gelb, M.H.; Yamamoto, K.; Murakami, M. Dual roles of group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 2016, 291, 15588–15601. [Google Scholar] [CrossRef] [Green Version]
- Von Allmen, C.E.; Schmitz, N.; Bauer, M.; Hinton, H.J.; Kurrer, M.O.; Buser, R.B.; Gwerder, M.; Muntwiler, S.; Sparwasser, T.; Beerli, R.R.; et al. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 11673–11678. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Murakami, M. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 2017, 583, 101–117. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiu, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.W.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166. [Google Scholar] [CrossRef]
- Titos, E.; Rius, B.; Gonzalez-Periz, A.; Lopez-Vicario, C.; Moran-Salvador, E.; Martinez-Clemente, M.; Arroyo, V.; Claria, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [Green Version]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggstrom, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef] [Green Version]
- Vijay, R.; Hua, X.; Meyerholz, D.K.; Miki, Y.; Yamamoto, K.; Gelb, M.; Murakami, M.; Perlman, S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J. Exp. Med. 2015, 212, 1851–1868. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Meyerholz, D.; Wong, L.R.; Gelb, M.; Murakami, M.; Perlman, S. Coronavirus-specific antibody production in middle-aged mice requires phospholipase A2G2D. J. Clin. Investig. 2021, 131, e147201. [Google Scholar] [CrossRef]
- Wong, L.R.; Zheng, J.; Wilhelmsen, K.; Li, K.; Ortiz, M.E.; Schnicker, N.J.; Thurman, A.; Pezzulo, A.A.; Szachowicz, P.J.; Li, P.; et al. Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature 2022, 605, 146–151. [Google Scholar] [CrossRef]
- MacPhee, M.; Chepenik, K.P.; Liddell, R.A.; Nelson, K.K.; Siracusa, L.D.; Buchberg, A.M. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell 1995, 81, 957–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulherkar, R.; Rao, R.S.; Wagle, A.S.; Patki, V.; Deo, M.G. Enhancing factor, a Paneth cell specific protein from mouse small intestines: Predicted amino acid sequence from RT-PCR amplified cDNA and its expression. Biochem. Biophys. Res. Commun. 1993, 195, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Murakami, M.; Enomoto, A.; Shimbara, S.; Kudo, I. Regulation of type V phospholipase A2 expression and function by proinflammatory stimuli. Eur. J. Biochem. 1999, 263, 826–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, Y.; Taketomi, Y.; Kidoguchi, Y.; Yamamoto, K.; Muramatsu, K.; Nishito, Y.; Park, J.; Hosomi, K.; Mizuguchi, K.; Kunisawa, J.; et al. Group IIA secreted phospholipase A2 controls skin carcinogenesis and psoriasis by shaping the gut microbiota. JCI Insight 2022, 7, e152611. [Google Scholar] [CrossRef]
- Doré, E.; Joly-Beauparlant, C.; Morozumi, S.; Mathieu, A.; Lévesque, T.; Allaeys, I.; Duchez, A.-C.; Cloutier, N.; Leclercq, M.; Bodein, A.; et al. The interaction of secreted phospholipase A2-IIA with the microbiota alters its lipidome and promotes inflammation. JCI Insight 2022, 7, e152638. [Google Scholar] [CrossRef]
- Džavík, V.; Lavi, S.; Thorpe, K.; Yip, P.M.; Plante, S.; Ing, D.; Overgaard, C.B.; Osten, M.D.; Lan, J.; Robbins, K.; et al. The sPLA2 Inhibition to Decrease Enzyme Release after Percutaneous Coronary Intervention (SPIDER-PCI) trial. Circulation 2010, 122, 2411–2418. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, S.J.; Kastelein, J.J.; Schwartz, G.G.; Bash, D.; Rosenson, R.S.; Cavender, M.A.; Brennan, D.M.; Koenig, W.; Jukema, J.W.; Nambi, V.; et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: The VISTA-16 randomized clinical trial. JAMA 2014, 311, 252–262. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, M.L. Acute coronary syndromes: Targeting inflammation-what has the VISTA-16 trial taught us? Nat. Rev. Cardiol. 2014, 11, 130–132. [Google Scholar] [CrossRef]
- Hui, D.Y.; Cope, M.J.; Labonté, E.D.; Chang, H.T.; Shao, J.; Goka, E.; Abousalham, A.; Charmot, D.; Buysse, J. The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br. J. Pharmacol. 2009, 157, 1263–1269. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Hakoi, H.; Nomura, S.; Murakami, M. The Roles of sPLA2s in Skin Homeostasis and Disease. Biomolecules 2023, 13, 668. https://doi.org/10.3390/biom13040668
Yamamoto K, Hakoi H, Nomura S, Murakami M. The Roles of sPLA2s in Skin Homeostasis and Disease. Biomolecules. 2023; 13(4):668. https://doi.org/10.3390/biom13040668
Chicago/Turabian StyleYamamoto, Kei, Haruka Hakoi, Saki Nomura, and Makoto Murakami. 2023. "The Roles of sPLA2s in Skin Homeostasis and Disease" Biomolecules 13, no. 4: 668. https://doi.org/10.3390/biom13040668




