The Emerging Role of IL-9 in the Anticancer Effects of Anti-PD-1 Therapy
Abstract
1. Introduction: The Success of PD-1 Blockade Relies on Effector Cytokines
2. Human IL-9+ Cells in Cancer and Associations with the Response to Anti-PD-1
3. TH9 Cells in the Immune Response Elicited by PD-1 Blockade
4. The Role of the PD-1 Signaling in the Regulation of IL-9 Expression
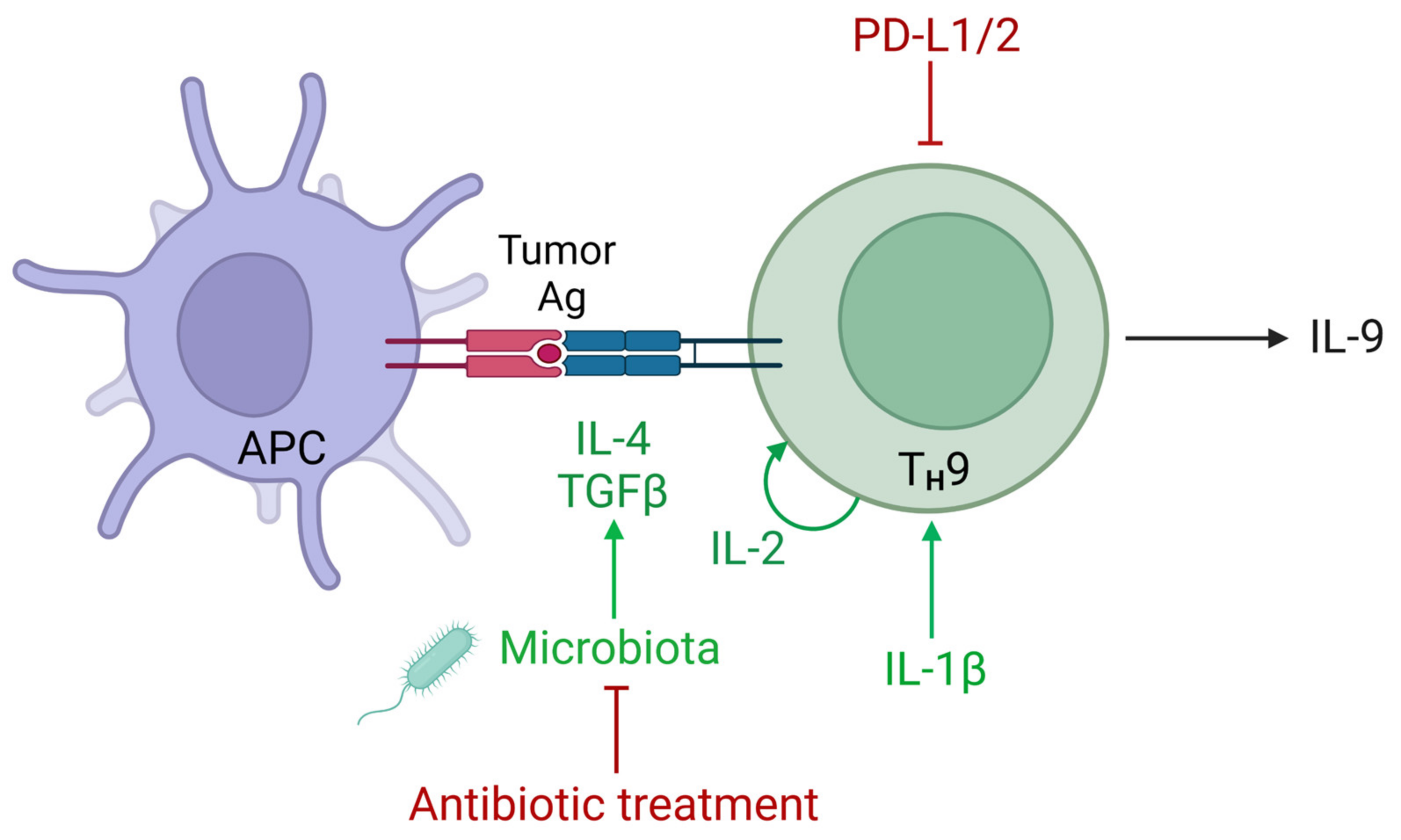
5. Regulation of IL-9 in the Tumor Microenvironment
6. The Role of IL-9 in Combination Therapies including PD-1 Blockade
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apetoh, L.; Smyth, M.J.; Drake, C.G.; Abastado, J.P.; Apte, R.N.; Ayyoub, M.; Blay, J.Y.; Bonneville, M.; Butterfield, L.H.; Caignard, A.; et al. Consensus nomenclature for CD8 T cell phenotypes in cancer. Oncoimmunology 2015, 4, e998538. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Martínez-Sabadell, A.; Arenas, E.J.; Arribas, J. IFNγ Signaling in Natural and Therapy-Induced Antitumor Responses. Clin. Cancer Res. 2022, 28, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8 T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Hoeres, T.; Holzmann, E.; Smetak, M.; Birkmann, J.; Wilhelm, M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. Oncoimmunology 2019, 8, 1550618. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, L.; Ding, G.; Huang, H.; Cao, G.; Sun, X.; Lou, N.; Wei, Q.; Shen, T.; Xu, X.; et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J. Immunother. Cancer 2020, 8, e000308. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Koblish, H.K.; Horton, B.; Scherle, P.A.; Newton, R.; Gajewski, T.F. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J. Immunother. Cancer 2014, 2, 3. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Tannir, N.M.; Bentebibel, S.E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef]
- Schmitt, E.; Germann, T.; Goedert, S.; Hoehn, P.; Huels, C.; Koelsch, S.; Kühn, R.; Müller, W.; Palm, N.; Rüde, E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J. Immunol. 1994, 153, 3989–3996. [Google Scholar] [CrossRef]
- Liao, W.; Spolski, R.; Li, P.; Du, N.; West, E.E.; Ren, M.; Mitra, S.; Leonard, W.J. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3508–3513. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, J.; Panangipalli, G.; Ulrich, B.J.; Koh, B.; Xu, C.; Kharwadkar, R.; Chu, X.; Wang, Y.; Gao, H.; et al. STAT5 promotes accessibility and is required for BATF-mediated plasticity at the Il9 locus. Nat. Commun. 2020, 11, 4882. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, X.; Bai, Q.; Qin, C.; Mohamud, A.O.; Zhu, Z.; Ball, T.W.; Ruth, C.M.; Newcomer, D.R.; Herrick, E.J.; et al. IL-9 inhibits HTB-72 melanoma cell growth through upregulation of p21 and TRAIL. J. Surg. Oncol. 2015, 111, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.J.; Zhou, Q.; Yin, W.; Yuan, M.L.; Yang, W.B.; Xiong, X.Z.; Zhang, J.C.; Shi, H.Z. Differentiation and immune regulation of IL-9-producing CD4+ T cells in malignant pleural effusion. Am. J. Respir. Crit. Care Med. 2012, 186, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Qiu-Lan, H.; Lei, R.E.; Shi, C.; Jiang, H.X.; Qin, S.Y. Interleukin-9 Promotes Pancreatic Cancer Cells Proliferation and Migration via the miR-200a/Beta-Catenin Axis. Biomed Res. Int. 2017, 2017, 2831056. [Google Scholar] [CrossRef] [PubMed]
- Benoit-Lizon, I.; Apetoh, L. Harnessing TH9 cells in cancer immunotherapy. Semin. Immunol. 2021, 52, 101477. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hong, S.; Li, H.; Park, J.; Hong, B.; Wang, L.; Zheng, Y.; Liu, Z.; Xu, J.; He, J.; et al. Th9 cells promote antitumor immune responses in vivo. J. Clin. Investig. 2012, 122, 4160–4171. [Google Scholar] [CrossRef]
- You, F.P.; Zhang, J.; Cui, T.; Zhu, R.; Lv, C.Q.; Tang, H.T.; Sun, D.W. Th9 cells promote antitumor immunity via IL-9 and IL-21 and demonstrate atypical cytokine expression in breast cancer. Int. Immunopharmacol. 2017, 52, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Purwar, R.; Schlapbach, C.; Xiao, S.; Kang, H.S.; Elyaman, W.; Jiang, X.; Jetten, A.M.; Khoury, S.J.; Fuhlbrigge, R.C.; Kuchroo, V.K.; et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 2012, 18, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahid, A.; Cydzik, M.; Prodeus, A.; Alwash, M.; Stanojcic, M.; Thompson, M.; Huang, E.H.; Shively, J.E.; Gray-Owen, S.D.; Gariépy, J. Induction of antigen-specific TH 9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int. J. Cancer 2016, 139, 841–853. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Dong, C. IL-9-producing T cells: Potential players in allergy and cancer. Nat. Rev. Immunol. 2021, 21, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, Z.; Lu, X.; Ma, Z.; Lu, C.; Zhang, B.; Chen, Y.; Duan, M.; Apetoh, L.; Li, X.; et al. Fas signaling-mediated TH9 cell differentiation favors bowel inflammation and antitumor functions. Nat. Commun. 2019, 10, 2924. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, Y.; Chen, L.; Gao, T.; Yang, Q.; Zhu, C.; Chen, Y. Th9 cells are subjected to PD-1/PD-L1-mediated inhibition and are capable of promoting CD8 T cell expansion through IL-9R in colorectal cancer. Int. Immunopharmacol. 2020, 78, 106019. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Chang, R.; Zhang, P.; Zhang, J.; Huo, L. Lentiviral vector-mediated IL-9 overexpression stimulates cell proliferation by targeting c-myc and cyclin D1 in colitis-associated cancer. Oncol. Lett. 2019, 17, 175–182. [Google Scholar] [CrossRef]
- Chauhan, S.R.; Singhal, P.G.; Sharma, U.; Bandil, K.; Chakraborty, K.; Bharadwaj, M. Th9 cytokines curb cervical cancer progression and immune evasion. Hum. Immunol. 2019, 80, 1020–1025. [Google Scholar] [CrossRef]
- Tong, H.; Feng, H.; Hu, X.; Wang, M.F.; Song, Y.F.; Wen, X.L.; Li, Y.R.; Wan, X.P. Identification of Interleukin-9 Producing Immune Cells in Endometrial Carcinoma and Establishment of a Prognostic Nomogram. Front. Immunol. 2020, 11, 544248. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Wang, Z.; Zeng, H.; Liu, Z.; Huang, Q.; Lin, Z.; Qu, Y.; Xiong, Y.; Wang, J.; et al. Poor clinical outcomes and immunoevasive contexture in interleukin-9 abundant muscle-invasive bladder cancer. Int. J. Cancer 2020, 147, 3539–3549. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Q.; Ouyang, J.; Pu, J.; Hou, J.; Zhang, J. Expression and clinical significance of interleukin-9 in renal tumors. Transl. Androl. Urol. 2020, 9, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, R.; Gu, Y.; Fei, Y.; Jin, K.; Chen, Y.; Cao, Y.; Liu, X.; Lv, K.; Wang, J.; et al. Intratumoral interleukin-9 delineates a distinct immunogenic class of gastric cancer patients with better prognosis and adjuvant chemotherapeutic response. Oncoimmunology 2020, 9, 1856468. [Google Scholar] [CrossRef] [PubMed]
- Heim, L.; Yang, Z.; Tausche, P.; Hohenberger, K.; Chiriac, M.T.; Koelle, J.; Geppert, C.I.; Kachler, K.; Miksch, S.; Graser, A.; et al. IL-9 Producing Tumor-Infiltrating Lymphocytes and Treg Subsets Drive Immune Escape of Tumor Cells in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 859738. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, Y.; Otsuka, A.; Nakashima, C.; Seidel, J.A.; Kitoh, A.; Dainichi, T.; Nakajima, S.; Sawada, Y.; Matsushita, S.; Aoki, M.; et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology 2016, 5, e1248327. [Google Scholar] [CrossRef]
- Forget, M.A.; Haymaker, C.; Hess, K.R.; Meng, Y.J.; Creasy, C.; Karpinets, T.; Fulbright, O.J.; Roszik, J.; Woodman, S.E.; Kim, Y.U.; et al. Prospective Analysis of Adoptive TIL Therapy in Patients with Metastatic Melanoma: Response, Impact of Anti-CTLA4, and Biomarkers to Predict Clinical Outcome. Clin. Cancer Res. 2018, 24, 4416–4428. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, S.; Lam, S.K.; Ko, F.C.F.; Chen, C.; Khan, M.; Ho, J.C. IL-9 stimulates an anti-tumor immune response and facilitates immune checkpoint blockade in the CMT167 mouse model. Lung Cancer 2022, 174, 14–26. [Google Scholar] [CrossRef]
- Davis, M.R.; Zhu, Z.; Hansen, D.M.; Bai, Q.; Fang, Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015, 358, 107–114. [Google Scholar] [CrossRef]
- Vegran, F.; Berger, H.; Boidot, R.; Mignot, G.; Bruchard, M.; Dosset, M.; Chalmin, F.; Rebe, C.; Derangere, V.; Ryffel, B.; et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol. 2014, 15, 758–766. [Google Scholar] [CrossRef]
- Lewis, K.E.; Selby, M.J.; Masters, G.; Valle, J.; Dito, G.; Curtis, W.R.; Garcia, R.; Mink, K.A.; Waggie, K.S.; Holdren, M.S.; et al. Interleukin-21 combined with PD-1 or CTLA-4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology 2017, 7, e1377873. [Google Scholar] [CrossRef]
- Deng, S.; Sun, Z.; Qiao, J.; Liang, Y.; Liu, L.; Dong, C.; Shen, A.; Wang, Y.; Tang, H.; Fu, Y.X.; et al. Targeting tumors with IL-21 reshapes the tumor microenvironment by proliferating PD-1intTim-3-CD8+ T cells. JCI Insight 2020, 5, e132000. [Google Scholar] [CrossRef]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4 T Cell Help Is Required for the Formation of a Cytolytic CD8 T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e4. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, B.S.; Bae, E.A.; Min, B.S.; Han, Y.D.; Shin, S.J.; Kang, C.Y. IL21 Therapy Combined with PD-1 and Tim-3 Blockade Provides Enhanced NK Cell Antitumor Activity against MHC Class I-Deficient Tumors. Cancer Immunol. Res. 2018, 6, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Yimingjiang, M.; Tuergan, T.; Chen, X.; Wen, H.; Shao, Y.; Zhang, R.; Aihaiti, K.; Xue, J.; Aji, T.; Zhang, W. Comparative Analysis of Immunoactivation by Nanosecond Pulsed Electric Fields and PD-1 Blockade in Murine Hepatocellular Carcinoma. Anal. Cell. Pathol. 2020, 2020, 9582731. [Google Scholar] [CrossRef]
- Kerzerho, J.; Maazi, H.; Speak, A.O.; Szely, N.; Lombardi, V.; Khoo, B.; Geryak, S.; Lam, J.; Soroosh, P.; Van Snick, J.; et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J. Allergy Clin. Immunol. 2013, 131, 1048–1057.e2. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Shafiei-Jahani, P.; Lo, R.; Howard, E.; Hurrell, B.P.; Galle-Treger, L.; Painter, J.D.; Lewis, G.; Soroosh, P.; Sharpe, A.H.; et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 2020, 11, 3998. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Hirota, K.; Stieglitz, B.; Van Snick, J.; Tolaini, M.; Lahl, K.; Sparwasser, T.; Helmby, H.; Stockinger, B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 2011, 12, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Moral, J.A.; Leung, J.; Rojas, L.A.; Ruan, J.; Zhao, J.; Sethna, Z.; Ramnarain, A.; Gasmi, B.; Gururajan, M.; Redmond, D.; et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020, 579, 130–135. [Google Scholar] [CrossRef]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef]
- Roy, S.; Awasthi, A. ATP Triggers Human Th9 Cell Differentiation via Nitric Oxide-Mediated mTOR-HIF1α Pathway. Front. Immunol. 2019, 10, 1120. [Google Scholar] [CrossRef]
- Roy, S.; Rizvi, Z.A.; Clarke, A.J.; Macdonald, F.; Pandey, A.; Zaiss, D.M.W.; Simon, A.K.; Awasthi, A. EGFR-HIF1α signaling positively regulates the differentiation of IL-9 producing T helper cells. Nat. Commun. 2021, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Huang, M.; Huang, J.; Zhu, X.; Wang, H.; Romano, S.; Deng, X.; Wang, Y.; Luo, Y.; Hao, S.; et al. BFAR coordinates TGFβ signaling to modulate Th9-mediated cancer immunotherapy. J. Exp. Med. 2021, 218, e20202144. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Almeida, R.R.; Vieira, R.S.; Castoldi, A.; Terra, F.F.; Melo, A.C.L.; Canesso, M.C.C.; Lemos, L.; Cipelli, M.; Rana, N.; Hiyane, M.I.; et al. Host dysbiosis negatively impacts IL-9-producing T-cell differentiation and antitumour immunity. Br. J. Cancer 2020, 123, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Jin, G.; Fang, J.; Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef]
- Apetoh, L. Anticancer effects of the microbiota: How the microbiome shapes the development of IL-9-producing T cells. Br. J. Cancer 2020, 123, 497–498. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Ting, N.L.; Lau, H.C.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Buzzatti, G.; Dellepiane, C.; Del Mastro, L. New emerging targets in cancer immunotherapy: The role of GITR. ESMO Open 2020, 4 (Suppl. S3), e000738. [Google Scholar] [CrossRef] [PubMed]
- Rakké, Y.S.; Campos Carrascosa, L.; van Beek, A.A.; de Ruiter, V.; van Gemerden, R.S.; Doukas, M.; Doornebosch, P.G.; Vermaas, M.; Ter Borg, S.; van der Harst, E.; et al. GITR Ligation Improves Anti-PD1-Mediated Restoration of Human MMR-Proficient Colorectal Carcinoma Tumor-Derived T Cells. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Kim, B.S.; Koh, C.H.; Seok, J.W.; Park, J.S.; Shin, K.S.; Bae, E.A.; Lee, G.E.; Jeon, H.; Cho, J.; et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat. Med. 2015, 21, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Hamid, O.; Thompson, J.A.; Ros, W.; Eskens, F.A.L.M.; Doi, T.; Hu-Lieskovan, S.; Klempner, S.J.; Ganguly, B.; Fleener, C.; et al. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clin. Cancer Res. 2022, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Wang, H.; Chiu, Y.; Kingsley, C.V.; Fry, D.; Delaney, S.N.; Wei, S.C.; Zhang, J.; Maitra, A.; et al. Combination of PD-1 Inhibitor and OX40 Agonist Induces Tumor Rejection and Immune Memory in Mouse Models of Pancreatic Cancer. Gastroenterology 2020, 159, 306–319.e12. [Google Scholar] [CrossRef]
- Xiao, X.; Balasubramanian, S.; Liu, W.; Chu, X.; Wang, H.; Taparowsky, E.J.; Fu, Y.X.; Choi, Y.; Walsh, M.C.; Li, X.C. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 2012, 13, 981–990. [Google Scholar] [CrossRef]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Benoit-Lizon, I.; Jacquin, E.; Rivera Vargas, T.; Richard, C.; Roussey, A.; Dal Zuffo, L.; Martin, T.; Melis, A.; Vinokurova, D.; Shahoei, S.H.; et al. CD4 T cell-intrinsic STING signaling controls the differentiation and effector functions of TH1 and TH9 cells. J. Immunother. Cancer 2022, 10, e003459. [Google Scholar] [CrossRef] [PubMed]
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Mifune, Y.; Shi, H.; Wang, K.W.; Zhan, X.; Liu, A.; Wang, J.; et al. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti-PD-L1 to eliminate melanoma in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E8698–E8706. [Google Scholar] [CrossRef]
- Karim, A.F.; Reba, S.M.; Li, Q.; Boom, W.H.; Rojas, R.E. Toll like Receptor 2 engagement on CD4+ T cells promotes TH9 differentiation and function. Eur. J. Immunol. 2017, 47, 1513–1524. [Google Scholar] [CrossRef]

| Tumor Type (Number of Patients) | Location of IL-9 Expression | IL-9- Producing Cell Types | Correlation of IL-9 Expression with Survival | Effects of IL-9 on Tumor Cells and Immune Cells | Reference |
|---|---|---|---|---|---|
| NSCLC (32) | Malignant pleural effusion Blood | CD4 | Negative correlation with OS | Promotes proliferation, survival, and migration of tumor cells (in vitro) | [17] |
| Metastatic melanoma (8) | TME Blood | CD4 | Not investigated | Not investigated | [22] |
| Colitis- associated colorectal cancer (12) | TME | Immune cells, tumor cells | Not investigated | Promotes proliferation of tumor cells (in vitro) | [27] |
| Breast cancer (12) | TME Blood | CD4 | Not investigated | Promotes cytotoxic capacity of CD8 T cells (ex vivo) | [21] |
| NSCLC (36) | TME | CD4 | Positive correlation with RF survival | Not investigated | [25] |
| Cervical squamous cell carcinomas (28) | TME | CD4 | Not investigated | Promotes MHC I expression in tumor cells, inhibits proliferation and survival of tumor cells (in vitro) | [28] |
| Endometrial carcinoma (143 + genomic data of 1274 patients) | TME | ILC2s, Vδ2 γδT cells, mast cells, macrophages, and TH9 cells | Positive correlation with OS | Correlates with higher tumor cell differentiation | [29] |
| Colorectal carcinoma (20) | TME Blood | CD4 | Not investigated | Correlates with CD8 T-cell infiltration Correlates with high PD-1 expression | [26] |
| Muscle-invasive bladder cancer (259) | TME | Immune cells | Negative correlation with OS and RF survival | Correlates with high PD-1 expression and low granzyme and perforin expression in CD8 T cells | [30] |
| Renal clear cell carcinoma (66 + 537 patients from TCGA) | TME | Not investigated | Positive correlation with OS | Correlates with high tumor cell differentiation and lower (better) pathological score | [31] |
| Gastric cancer (453) | TME | Immune cells | Positive correlation with OS | Correlates with CD8 T-cell infiltration and expression of granzyme, perforin, and IFNγ by CD8 T cells Enhances tumor-killing capacity of CD8 T cells (ex vivo) | [32] |
| NSCLC (63) | TME Blood | CD4: TH9 and Treg, tumor cells | Not investigated | Correlates with low IFNγ and TNFα expression and high IL-21 expression | [33] |
| Cancer Type (Number of Patients) | Location of IL-9 Expression | IL-9- Producing Cell Types | Correlation of IL-9 with Therapy Response | Effects of IL-9 on Anticancer Immunity | Reference |
|---|---|---|---|---|---|
| Metastatic melanoma (46) | TME Blood | CD4 | Positive correlation with response to nivolumab | Co-localizes with CD8 T cells in tumors | [34] |
| Metastatic melanoma (76) | Blood | Not investigated (serum levels measured) | Positive correlation with response to TIL ACT | Not investigated | [35] |
| Muscle-invasive bladder cancer (259) | TME | Not investigated | Not investigated | Correlates with expansion and cytotoxic function of CD8 T cells in response to nivolumab | [30] |
| Gastric cancer (453) | TME | Immune cells | Not investigated | Synergizes with anti-PD-1 in promoting cytotoxic activity of CD8 T cells against tumor cells (ex vivo) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinokurova, D.; Apetoh, L. The Emerging Role of IL-9 in the Anticancer Effects of Anti-PD-1 Therapy. Biomolecules 2023, 13, 670. https://doi.org/10.3390/biom13040670
Vinokurova D, Apetoh L. The Emerging Role of IL-9 in the Anticancer Effects of Anti-PD-1 Therapy. Biomolecules. 2023; 13(4):670. https://doi.org/10.3390/biom13040670
Chicago/Turabian StyleVinokurova, Daria, and Lionel Apetoh. 2023. "The Emerging Role of IL-9 in the Anticancer Effects of Anti-PD-1 Therapy" Biomolecules 13, no. 4: 670. https://doi.org/10.3390/biom13040670
APA StyleVinokurova, D., & Apetoh, L. (2023). The Emerging Role of IL-9 in the Anticancer Effects of Anti-PD-1 Therapy. Biomolecules, 13(4), 670. https://doi.org/10.3390/biom13040670






