High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
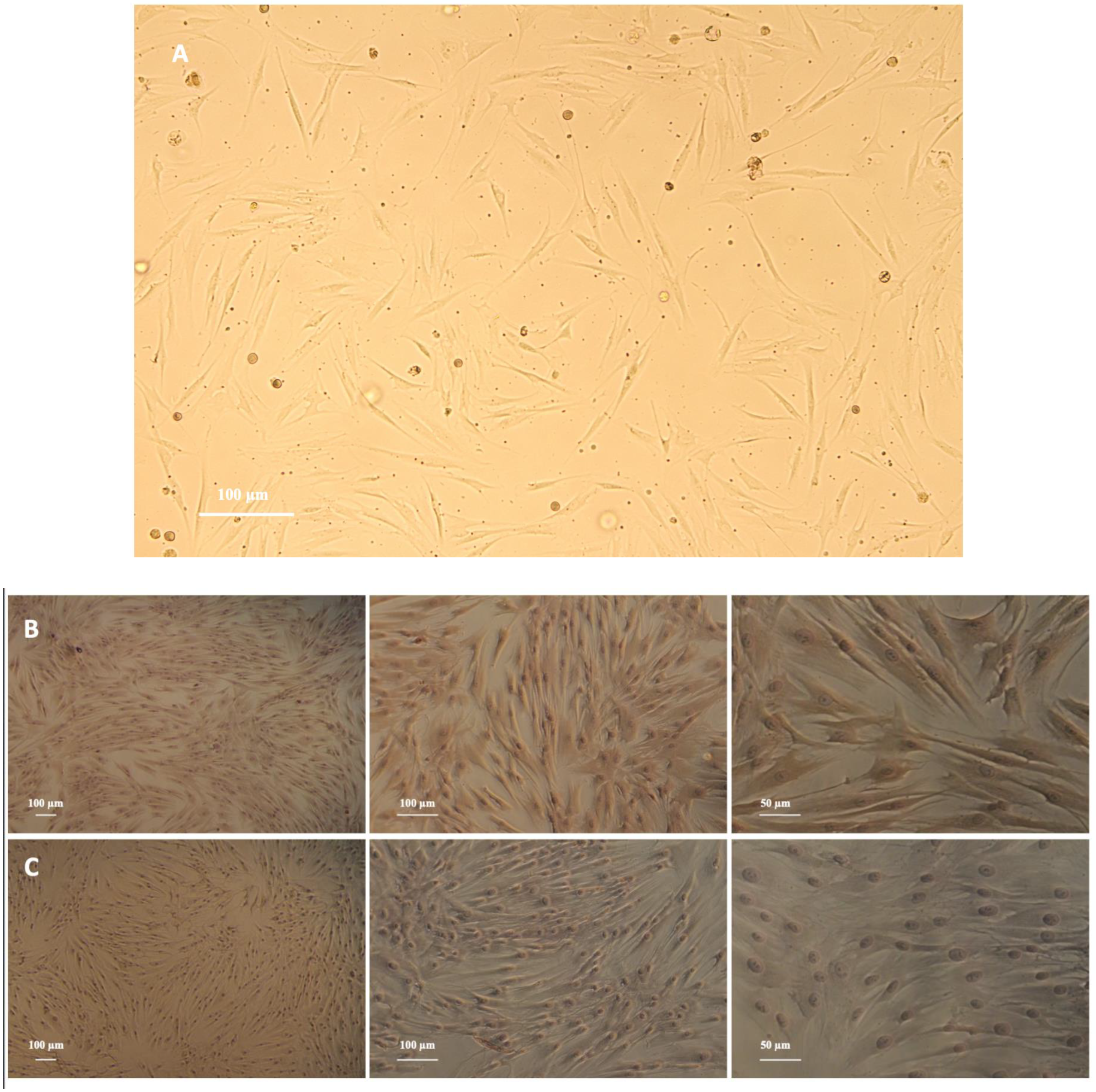
2.1. Isolation and Culturing of Human Periodontal Ligament Fibroblasts (PDLFs)
2.2. Immunohistochemistry
2.3. Stimulation of PDLFs with/without Lipopolysaccharide in Different Glucose Concentrations
2.4. Alamar Blue Assay
2.5. Lactic Acid Dehydrogenase (LDH) Cytotoxicity Assay
2.6. Scratch Migration Assay
2.7. Quantification of Inflammatory Cytokines Levels by Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Quantification of Interleukin-6 and Interleukin-10 by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. PDLFs Express Vimentin and Lack Keratin
3.2. The Proliferation of PDLFs Was Affected by Different Glucose Concentrations
3.3. High-Glucose Concentrations Amplified the Cytotoxicity of PDLFs
3.4. High-Glucose Concentrations Have Adverse Effects on PDLFs Wound Closure
3.5. LPS Induces the Expression of Interleukin-6, Interleukin-23, and Toll-like Receptor-4 but Not Interleukin-10
3.6. LPS and High-Glucose Concentrations Induce Interleukin-6 Secretion but Not Interleukin-10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442. [Google Scholar] [CrossRef]
- Lamster, I.B.; Lalla, E.; Borgnakke, W.S. Taylor GW the relationship between oral health and diabetes mellitus. J. Am. Dent. Assoc. 2008, 139, 19S–24S. [Google Scholar] [CrossRef]
- Segura-Egea, J.J.; Castellanos-Cosano, L.; Machuca, G.; López-López, J.; Martín-González, J.; Velasco-Ortega, E.; Sánchez-Domínguez, B.; López-Frías, F.J. Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Med. Oral Patol. Oral Cirugía Buccal 2012, 17, e356–e361. [Google Scholar] [CrossRef]
- Flemmig, T.F. Periodontitis. Ann. Periodontol. 1999, 4, 32–37. [Google Scholar] [CrossRef]
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health 2016, 17, 31. [Google Scholar] [CrossRef]
- de Miguel-Infante, A.; Martinez-Huedo, M.A.; Mora-Zamorano, E.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; de Burgos-Lunar, C.; Cardenas Valladolid, J.; Jiménez-García, R.; Lopez-de-Andrés, A. Periodontal disease in adults with diabetes, prevalence and risk factors. Results of an observational study. Int. J. Clin. Pract. 2018, 73, e13294. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q. Periodontitis aggravated pancreatic β-cell dysfunction in diabetic mice through interleukin-12 regulation on Klotho. J. Diabetes Investig. 2016, 7, 303–311. [Google Scholar] [CrossRef]
- Chen, T.L.; Xu, E.L.; Lu, H.J.; Xu, H.; Wang, S.F.; Zhao, H.J.; Liu, Y.M. The influence of diabetes enhanced inflammation on cell apoptosis and periodontitis. Adv. Biosci. Biotechnol. 2012, 3, 712–719. [Google Scholar] [CrossRef]
- Faria-Almeida, R.; Navarro, A.; Bascones, A. Clinical and Metabolic Changes after Conventional Treatment of Type 2 Diabetic Patients with Chronic Periodontitis. J. Periodontol. 2006, 77, 591–598. [Google Scholar] [CrossRef]
- Montoya-Carralero, J.M.; Saura-Pérez, M.; Canteras-Jordana, M.; Morata-Murcia, I.M. Reduction of HbA1c levels following nonsurgical treatment of periodontal disease in type 2 diabetics. Med. Oral Patol. Oral Cirugía Buccal 2010, 15, e808-12. [Google Scholar] [CrossRef]
- Engebretson, S.; Kocher, T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S153–S163. [Google Scholar] [CrossRef]
- Beertsen, W.; Mcculloch, C.A.G.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, X.D.; Wang, Q.; Zhang, L.; Wang, Y.; Li, X.Y.; Huang, D.M. Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch. Oral Biol. 2011, 56, 1064–1072. [Google Scholar] [CrossRef]
- Morandini, A.C.; Chaves Souza, P.P.; Ramos-Junior, E.S.; Souza Costa, C.A.; Santos, C.F. MyD88 or TRAM Knockdown Regulates Interleukin (IL)-6, IL-8, and CXCL12 mRNA Expression in Human Gingival and Periodontal Ligament Fibroblasts. J. Periodontol. 2013, 84, 1353–1360. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Wang, X.; Zhang, P.; Tan, Y. Endotoxin tolerance induction in human periodontal ligament fibroblasts stimulated with different bacterial lipopolysaccharides. Arch. Oral Biol. 2015, 60, 463–470. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ren, W.; Liu, J.; Yang, P.; Chen, Z.; Zhang, Q.; Yang, F. Lipopolysaccharide-induced suppression of periodontal ligament cell proliferation and apoptosis are strengthened under high glucose conditions. Arch. Oral Biol. 2017, 79, 70–76. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, F.; Zhang, X.; Shu, L. Insulin modulates cytokines expression in human periodontal ligament cells. Arch. Oral Biol. 2014, 59, 1301–1306. [Google Scholar] [CrossRef]
- Sigma-Aldrich (Glucose in Cell Culture|Glucose in Cell Culture|Sigma-Aldrich). Available online: https://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/glucose.html (accessed on 24 February 2020).
- Kim, H.S.; Park, J.W.; Yeo, S.I.; Choi, B.J.; Suh, J.Y. Effects of high glucose on cellular activity of periodontal ligament cells in vitro. Diabetes Res. Clin. Pract. 2006, 74, 41–47. [Google Scholar] [CrossRef]
- Li, M.; Li, C.Z. High glucose improves healing of periodontal wound by inhibiting proliferation and osteogenetic differentiation of human PDL cells. Int. Wound J. 2016, 13, 39–43. [Google Scholar] [CrossRef]
- Seubbuk, S.; Sritanaudomchai, H.; Kasetsuwan, J.; Surarit, R. High glucose promotes the osteogenic differentiation capability of human periodontal ligament fbroblasts. Mol. Med. Rep. 2017, 15, 2788–2794. [Google Scholar] [CrossRef]
- Nilsson, B.O. Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: Implications in periodontitis. J. Periodontal. Res. 2021, 56, 249–255. [Google Scholar] [CrossRef]
- Jones, K.J.; Ekhlassi, S.; Montufar-Solis, D.; Klein, J.R.; Schaefer, J.S. Differential cytokine patterns in mouse macrophages and gingival fibroblasts after stimulation with porphyromonas gingivalis or Escherichia coli lipopolysaccharide. J. Periodontol. 2010, 81, 1850–1857. [Google Scholar] [CrossRef]
- Nebel, D.; Arvidsson, J.; Lillqvist, J.; Holm, A.; Nilsson, B.O. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL-6 production in human periodontal ligament cells. Acta Odontol. Scand. 2013, 71, 892–898. [Google Scholar] [CrossRef]
- Andrukhov, O.; Ertlschweiger, S.; Moritz, A.; Bantleon, H.P.; Rausch-Fan, X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontol. Scand. 2014, 72, 337–345. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Qiu, C.; Zhang, J.; Zhang, T.; Zhou, W.; Lu, Z.; Rausch-Fan, X.; Liu, Z. Escin inhibits lipopolysaccharide-induced inflammation in human periodontal ligament cells. Mol. Med. Rep. 2012, 6, 1150–1154. [Google Scholar] [CrossRef][Green Version]
- Engebretson, S.P.; Hey-Hadavi, J.; Ehrhardt, F.J.; Hsu, D.; Celenti, R.S.; Grbic, J.T.; Lamster, I.B. Gingival Crevicular Fluid Levels of Interleukin-1β β and Glycemic Control in Patients With Chronic Periodontitis and Type 2 Diabetes. J. Periodontol. 2004, 75, 1203–1208. [Google Scholar] [CrossRef]
- Albiero, M.L.; Amorim, B.R.; Martins, L.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr.; Silvério, K.G. Exposure of periodontal ligament progenitor cells to lipopolysaccharide from Escherichia coli changes osteoblast differentiation pattern. J. Appl. Oral Sci. 2015, 23, 145–152. [Google Scholar] [CrossRef]
- Alsalleeh, F.; Young, A.; Algarawi, Z.; Petro, T.C. Albicans Biofilm Formation is Restricted by Periodontal Ligament Cells and Induces Differential Cytokines Response Compared to Planktonic, C. Albicans J. Dent. Appl. Open 2014, 1, 139–144. [Google Scholar]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased Expression of Interleukin 23 p19 and p40 in Lesional Skin of Patients with Psoriasis Vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Okiji, T.; Kaneko, R.; Sunakawa, M.; Kaneko, M.; Suda, H. Gene Expression Analysis of Acutely Traumatized Pulps. J. Endod. 2010, 36, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Carollo-Bittel, B.; Lang, N.P. Effects of diabetes mellitus on periodontal and peri-implant conditions: Update on associations and risks. J. Clin. Periodontol. 2008, 35, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Borgnakke, W.S. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008, 14, 191–203. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Chen, Y.; Zhao, P.; Liu, X.; Dong, H.; Zheng, W.; Feng, S.; Mao, X.; Li, C. Mechanisms of the lipopolysaccharide-induced inflammatory response in alveolar epithelial cell/macrophage co-culture. Exp. Ther. Med. 2020, 20, 76. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, W.; Mo, W.; Liang, M. High-glucose concentration aggravates TNF-alpha-induced cell viability reduction in human CD146-positive periodontal ligament cells via TNFR-1 gene demethylation. Cell Biol. Int. 2020, 44, 2383–2394. [Google Scholar] [CrossRef]
- Zhu, W.; Qiu, Q.; Luo, H.; Zhang, F.; Wu, J.; Zhu, X.; Liang, M. High Glucose Exacerbates TNF- α -Induced Proliferative Inhibition in Human Periodontal Ligament Stem Cells through Upregulation and Activation of TNF Receptor 1. Stem Cells Int. 2020, 2020, 4910767. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Wang, B.; Yuan, X.; Fang, B. High Levels of Glucose Induced the Caspase-3/PARP Signaling Pathway, Leading to Apoptosis in Human Periodontal Ligament Fibroblasts. Cell Biochem. Biophys. 2013, 66, 229–237. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Mao, J.; Gu, B.; Liu, H.; Fang, B. High levels of glucose induces a dose-dependent apoptosis in human periodontal ligament fibroblasts by activating caspase-3 signaling pathway. Appl. Biochem. Biotechnol. 2013, 170, 1458–1471. [Google Scholar] [CrossRef]
- Maruyama, K.; Sato, S. Effect of high-glucose conditions on human periodontal ligament endothelial cells: In vitro analysis. Odontology 2017, 105, 76–83. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon Na Mahasarakham, C.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Kimura, D.; Yamawaki, I.; Noguchi, M.; Yamauchi, N.; Tamura, I.; Tanaka, A.; Umeda, M. High Glucose Concentrations Suppress the Proliferation of Human Periodontal Ligament Stem Cells and Their Differentiation Into Osteoblasts. J. Periodontol. 2016, 87, e44–e51. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Morran, M.; Slotterbeck, B.; Leaman, D.W.; Sun, Y.; Grafenstein, H.V.; Hong, S.C.; McInerney, M.F. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int. Immunol. 2006, 18, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, H.; Wu, Y.; Sun, Z.; Wu, Y. Clinical significance of IL-23 regulating IL-17A and/or IL-17F positive Th17 cells in chronic periodontitis. Mediat. Inflamm. 2014, 2014, 627959. [Google Scholar] [CrossRef]






| Target | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| IL-6 | 5′-AGGAGACTTGCCTGGTGAAA-3′ | 5′-CAGGGGTGGTTATTGCATCT-3′ |
| IL-10 | 5′-TGGTGAAACCCCGTCTCTAC-3′ | 5′-CTGGAGTACAGGGGCATGAT-3′ |
| IL-23 P19 | 5′-GTTCCCCATATCCAGTGTGG-3′ | 5′-TTTTGAAGCGGAGAAGGAGA-3′ |
| IL-23 P40 | 5′-ACGGACAAGACCTCAGCCAC-3′ | 5′-GGGCCCGCACGCTAA-3′ |
| TLR-4 | 5′-ACAACCTCCCCTTCTCAACC-3′ | 5′-TGAGATGTCCAATGGGGAAG-3′ |
| GAPDH | 5′-CAGCCTCCCGCTTCGCTCTC-3′ | 5′-CCAGGCGCCCAATACGACCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldoss, A.; Lambarte, R.; Alsalleeh, F. High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts. Biomolecules 2023, 13, 690. https://doi.org/10.3390/biom13040690
Aldoss A, Lambarte R, Alsalleeh F. High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts. Biomolecules. 2023; 13(4):690. https://doi.org/10.3390/biom13040690
Chicago/Turabian StyleAldoss, Alaa, Rhodanne Lambarte, and Fahd Alsalleeh. 2023. "High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts" Biomolecules 13, no. 4: 690. https://doi.org/10.3390/biom13040690
APA StyleAldoss, A., Lambarte, R., & Alsalleeh, F. (2023). High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts. Biomolecules, 13(4), 690. https://doi.org/10.3390/biom13040690






