Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance
Abstract
:1. Introduction: Sestrin, Its Structure and Biochemical Roles
2. Regulation of Sestrin Expression in Skeletal Muscle
3. Regulation of Sestrin Expression in the Heart
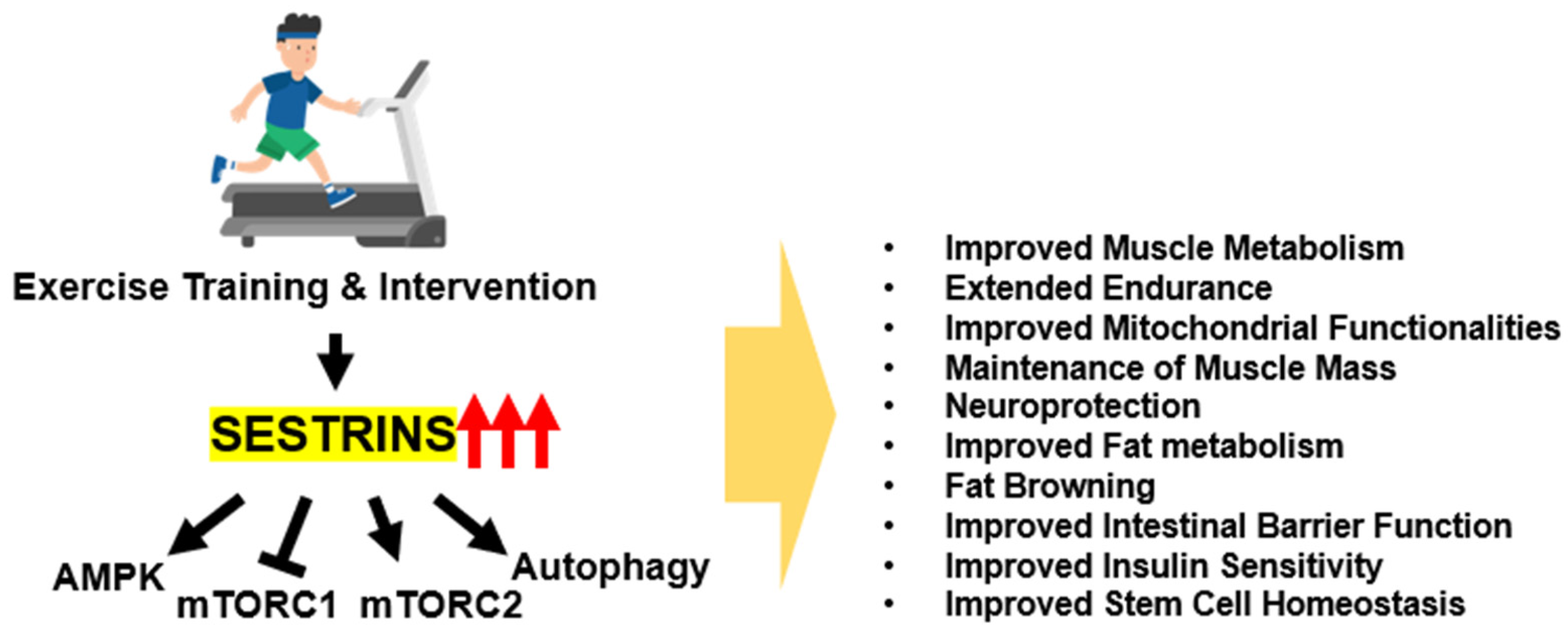
4. The Role of Sestrins in Exercise Adaptation and Benefits
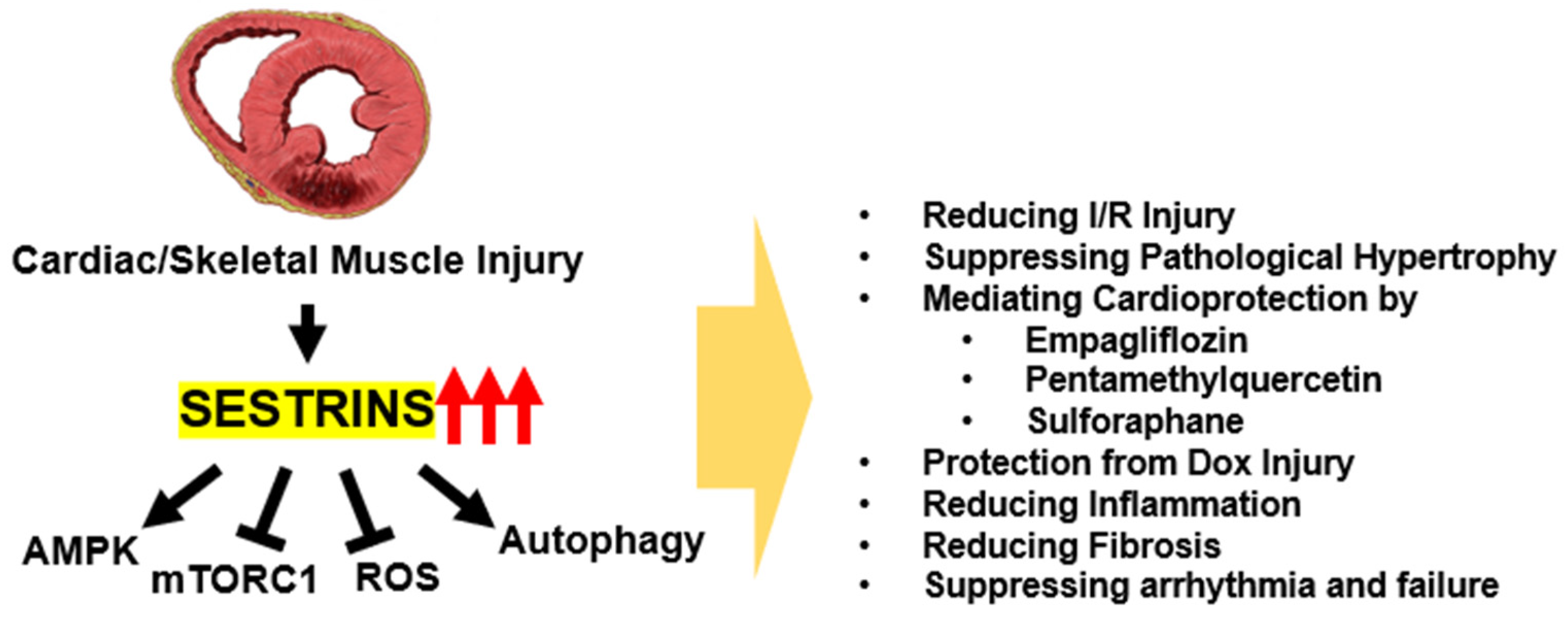
5. The Role of Sestrins in Cardiac Stress Resistance and Protection
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.; Kowalsky, A.H.; Lee, J.H. Sestrins in Physiological Stress Responses. Annu. Rev. Physiol. 2021, 83, 381–403. [Google Scholar] [CrossRef]
- Velasco-Miguel, S.; Buckbinder, L.; Jean, P.; Gelbert, L.; Talbott, R.; Laidlaw, J.; Seizinger, B.; Kley, N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 1999, 18, 127–137. [Google Scholar] [CrossRef]
- Ho, A.; Cho, C.S.; Namkoong, S.; Cho, U.S.; Lee, J.H. Biochemical Basis of Sestrin Physiological Activities. Trends Biochem. Sci. 2016, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Haidurov, A.; Budanov, A.V. Sestrin family—The stem controlling healthy ageing. Mech. Ageing Dev. 2020, 192, 111379. [Google Scholar] [CrossRef] [PubMed]
- Ro, S.H.; Fay, J.; Cyuzuzo, C.I.; Jang, Y.; Lee, N.; Song, H.S.; Harris, E.N. SESTRINs: Emerging Dynamic Stress-Sensors in Metabolic and Environmental Health. Front. Cell Dev. Biol. 2020, 8, 603421. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef]
- Woo, H.A.; Bae, S.H.; Park, S.; Rhee, S.G. Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins. Antioxid. Redox Signal. 2009, 11, 739–745. [Google Scholar] [CrossRef]
- Kim, H.; An, S.; Ro, S.H.; Teixeira, F.; Park, G.J.; Kim, C.; Cho, C.S.; Kim, J.S.; Jakob, U.; Lee, J.H.; et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat. Commun. 2015, 6, 10025. [Google Scholar] [CrossRef]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef]
- Kim, J.S.; Ro, S.H.; Kim, M.; Park, H.W.; Semple, I.A.; Park, H.; Cho, U.S.; Wang, W.; Guan, K.L.; Karin, M.; et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci. Rep. 2015, 5, 9502. [Google Scholar] [CrossRef]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Parmigiani, A.; Nourbakhsh, A.; Ding, B.; Wang, W.; Kim, Y.C.; Akopiants, K.; Guan, K.L.; Karin, M.; Budanov, A.V. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014, 9, 1281–1291. [Google Scholar] [CrossRef]
- Wong, P.M.; Puente, C.; Ganley, I.G.; Jiang, X. The ULK1 complex: Sensing nutrient signals for autophagy activation. Autophagy 2013, 9, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Peeters, H.; Debeer, P.; Bairoch, A.; Wilquet, V.; Huysmans, C.; Parthoens, E.; Fryns, J.P.; Gewillig, M.; Nakamura, Y.; Niikawa, N.; et al. PA26 is a candidate gene for heterotaxia in humans: Identification of a novel PA26-related gene family in human and mouse. Hum. Genet. 2003, 112, 573–580. [Google Scholar] [CrossRef]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef]
- Lee, J.H.; Budanov, A.V.; Park, E.J.; Birse, R.; Kim, T.E.; Perkins, G.A.; Ocorr, K.; Ellisman, M.H.; Bodmer, R.; Bier, E.; et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010, 327, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Loh, K.S.; Liou, B.Y.; Chu, I.H.; Kuo, C.J.; Chen, H.D.; Chen, C.S. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp. Gerontol. 2013, 48, 371–379. [Google Scholar] [CrossRef]
- Wang, T.; Niu, Y.; Liu, S.; Yuan, H.; Liu, X.; Fu, L. Exercise improves glucose uptake in murine myotubes through the AMPKalpha2-mediated induction of Sestrins. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3368–3377. [Google Scholar] [CrossRef]
- Lenhare, L.; Crisol, B.M.; Silva, V.R.R.; Katashima, C.K.; Cordeiro, A.V.; Pereira, K.D.; Luchessi, A.D.; da Silva, A.S.R.; Cintra, D.E.; Moura, L.P.; et al. Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp. Gerontol. 2017, 97, 17–21. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Yuan, H.; Huang, J.; Fu, L. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism 2015, 64, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Crisol, B.M.; Lenhare, L.; Gaspar, R.S.; Gaspar, R.C.; Munoz, V.R.; da Silva, A.S.R.; Cintra, D.E.; de Moura, L.P.; Pauli, J.R.; Ropelle, E.R. The role of physical exercise on Sestrin1 and 2 accumulations in the skeletal muscle of mice. Life Sci. 2018, 194, 98–103. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Liu, S.; Niu, Y.; Shao, H.; Fu, L. Aerobic exercise ameliorates insulin resistance in C57BL/6 J mice via activating Sestrin 3. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166568. [Google Scholar] [CrossRef]
- Anwar, M.; Mallick, S.; Paliwal, D.; Shekhar, S.; Panda, S.K.; Dey, S.; Dey, A.B. Impact of physical activity on mitochondrial enzymes, muscle stem cell and anti-oxidant protein Sestrins in Sarcopenic mice. Exp. Gerontol. 2021, 150, 111358. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.; Niu, Y.; Fu, L. Exercise protects intestinal epithelial barrier from high fat diet- induced permeabilization through SESN2/AMPKalpha1/HIF-1alpha signaling. J. Nutr. Biochem. 2022, 107, 109059. [Google Scholar] [CrossRef]
- Gombos, Z.; Koltai, E.; Torma, F.; Bakonyi, P.; Kolonics, A.; Aczel, D.; Ditroi, T.; Nagy, P.; Kawamura, T.; Radak, Z. Hypertrophy of Rat Skeletal Muscle Is Associated with Increased SIRT1/Akt/mTOR/S6 and Suppressed Sestrin2/SIRT3/FOXO1 Levels. Int. J. Mol. Sci. 2021, 22, 7588. [Google Scholar] [CrossRef]
- Zeng, N.; D’Souza, R.F.; Figueiredo, V.C.; Markworth, J.F.; Roberts, L.A.; Peake, J.M.; Mitchell, C.J.; Cameron-Smith, D. Acute resistance exercise induces Sestrin2 phosphorylation and p62 dephosphorylation in human skeletal muscle. Physiol. Rep. 2017, 5, e13526. [Google Scholar] [CrossRef]
- Segales, J.; Perdiguero, E.; Serrano, A.L.; Sousa-Victor, P.; Ortet, L.; Jardi, M.; Budanov, A.V.; Garcia-Prat, L.; Sandri, M.; Thomson, D.M.; et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nat. Commun. 2020, 11, 189. [Google Scholar] [CrossRef]
- Vilchinskaya, N.; Altaeva, E.; Lomonosova, Y. Gaining insight into the role of FoxO1 in the progression of disuse-induced skeletal muscle atrophy. Adv. Biol. Regul. 2022, 85, 100903. [Google Scholar] [CrossRef]
- Zeng, N.; D’Souza, R.F.; MacRae, C.L.; Figueiredo, V.C.; Pileggi, C.A.; Markworth, J.F.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Daily protein supplementation attenuates immobilization-induced blunting of postabsorptive muscle mTORC1 activation in middle-aged men. Am. J. Physiol. Cell Physiol. 2021, 320, C591–C601. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; D’Souza, R.F.; Mitchell, C.J.; Cameron-Smith, D. Sestrins are differentially expressed with age in the skeletal muscle of men: A cross-sectional analysis. Exp. Gerontol. 2018, 110, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martos, R.; Aparicio-Ugarriza, R.; Alcazar, J.; Ramirez-Castillejo, C.; Reihmane, D.; Menendez-Rey, A.; Gonzalez-Gross, M.; Guadalupe-Grau, A. Circulating sestrins and force velocity profiling in older adults with type 2 diabetes. Eur. J. Sport Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Rajan, S.P.; Anwar, M.; Jain, B.; Khan, M.A.; Dey, S.; Dey, A.B. Serum sestrins: Potential predictive molecule in human sarcopenia. Aging Clin. Exp. Res. 2021, 33, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; Rezola-Pardo, C.; Arrieta, H.; Fraile-Bermudez, A.B.; Alonso-Puyo, J.; Molano, I.; Rodriguez-Larrad, A.; Irazusta, J. Serum Sestrin-1 Concentration Is Higher in Frail than Non-Frail Older People Living in Nursing Homes. Int. J. Environ. Res. Public Health 2022, 19, 1079. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Chen, L.; Wang, J.; Zhang, M.; Yang, H.; Ma, Y.; Budanov, A.; Lee, J.H.; Karin, M.; Li, J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015, 29, 408–417. [Google Scholar] [CrossRef]
- Yang, K.; Xu, C.; Zhang, Y.; He, S.; Li, D. Sestrin2 Suppresses Classically Activated Macrophages-Mediated Inflammatory Response in Myocardial Infarction through Inhibition of mTORC1 Signaling. Front. Immunol. 2017, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, W.; Zhang, Y.; Nong, J.; Zhang, L. Effects of ferroptosis in myocardial ischemia/reperfusion model of rat and its association with Sestrin 1. Adv. Clin. Exp. Med. 2023, 32, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liao, H.H.; Feng, H.; Mou, S.Q.; Li, W.J.; Aiyasiding, X.; Lin, Z.; Ding, W.; Zhou, Z.Y.; Yan, H.; et al. Knockout of AMPKalpha2 Blocked the Protection of Sestrin2 Overexpression Against Cardiac Hypertrophy Induced by Pressure Overload. Front. Pharmacol. 2021, 12, 716884. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Semple, I.; Kim, M.; Zhang, Z.; Lee, J.H. Cardioprotective roles of sestrin 1 and sestrin 2 against doxorubicin cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H39–H48. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Lu, J.; Hu, Y.; Wang, Q.; Li, Z.; Cai, S.; Liang, L.; Guo, K.; Xie, J.; et al. SESN2 protects against doxorubicin-induced cardiomyopathy via rescuing mitophagy and improving mitochondrial function. J. Mol. Cell Cardiol. 2019, 133, 125–137. [Google Scholar] [CrossRef]
- Wang, A.J.; Tang, Y.; Zhang, J.; Wang, B.J.; Xiao, M.; Lu, G.; Li, J.; Liu, Q.; Guo, Y.; Gu, J. Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol. 2022, 52, 102310. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Sun, W.; Wang, L.; Chen, X.; Bogan, J.S.; Zhou, X.; Cates, C.; Liu, Q.; Zheng, Y.; Li, J. Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism. FASEB J. 2017, 31, 4153–4167. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yang, Z.; Shi, L.; Zeng, T.; Shi, Y.; Liu, L.; Liu, H.; Lin, Y. Circulating Sestrin Levels Are Increased in Hypertension Patients. Dis. Markers 2020, 2020, 3787295. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lin, C.; Liu, Y.; Jin, H.; Wu, H.; Li, Z.; Sun, L.; Zhang, L.; Hu, X.; Wei, Y.; et al. Upregulation of sestrins protect atriums against oxidative damage and fibrosis in human and experimental atrial fibrillation. Sci. Rep. 2017, 7, 46307. [Google Scholar] [CrossRef]
- Wang, H.; Xi, J.; Zhang, Z.; Li, J.; Guo, L.; Li, N.; Sun, Y.; Li, X.; Han, X. Sestrin2 Is Increased in Calcific Aortic Disease and Inhibits Osteoblastic Differentiation in Valvular Interstitial Cells via the Nuclear Factor E2-related Factor 2 Pathway. J. Cardiovasc. Pharmacol. 2022, 80, 609–615. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Shao, X.; Li, J.; Guo, L.; Yu, X.; Sun, Y.; Hao, J.; Niu, H.; Xiang, J.; et al. Increased plasma sestrin2 concentrations in patients with chronic heart failure and predicted the occurrence of major adverse cardiac events: A 36-month follow-up cohort study. Clin. Chim. Acta 2019, 495, 338–344. [Google Scholar] [CrossRef]
- Ye, J.; Wang, M.; Xu, Y.; Liu, J.; Jiang, H.; Wang, Z.; Lin, Y.; Wan, J. Sestrins increase in patients with coronary artery disease and associate with the severity of coronary stenosis. Clin. Chim. Acta 2017, 472, 51–57. [Google Scholar] [CrossRef]
- Lee, J.H.; Budanov, A.V.; Karin, M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013, 18, 792–801. [Google Scholar] [CrossRef]
- Sujkowski, A.; Wessells, R. Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms. Cells 2021, 10, 2479. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Liu, Z.; Li, W.; Li, C.; Chen, Z. SESN2 prevents the slow-to-fast myofiber shift in denervated atrophy via AMPK/PGC-1alpha pathway. Cell Mol. Biol. Lett. 2022, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Martyn, J.A.J.; Kaneki, M. Muscle Atrophy and the Sestrins. N. Engl. J. Med. 2020, 383, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Yuan, H.; Niu, Y.; Fu, L. Sestrin 2 induces autophagy and attenuates insulin resistance by regulating AMPK signaling in C2C12 myotubes. Exp. Cell Res. 2017, 354, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, C.; Xie, L.; Niu, Y.; Fu, L. Aerobic Exercise Improves Mitochondrial Function in Sarcopenia Mice Through Sestrin2 in an AMPKalpha2-Dependent Manner. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Wessells, R.J.; Lee, J.H. Sestrin/FNDC5: An ancient axis connecting exercise and thermoregulation. Acta Physiol. 2022, 234, e13804. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Liu, S.; Niu, Y.; Fu, L. Sestrin2 ablation attenuates the exercise-induced browning of white adipose tissue in C57BL/6J mice. Acta Physiol. 2022, 234, e13785. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, W.; Niu, Y.; Liu, S.; Fu, L. Exercise improves lipid metabolism disorders induced by high-fat diet in a SESN2/JNK-independent manner. Appl. Physiol. Nutr. Metab. 2021, 46, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.A.; Castor-Macias, J.; Fraczek, P.; Cornett, A.; Brown, L.A.; Kim, M.; Brooks, S.V.; Lombaert, I.M.A.; Lee, J.H.; Aguilar, C.A. Sestrins regulate muscle stem cell metabolic homeostasis. Stem Cell Rep. 2021, 16, 2078–2088. [Google Scholar] [CrossRef]
- Sujkowski, A.; Richardson, K.; Prifti, M.V.; Wessells, R.J.; Todi, S.V. Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias. Elife 2022, 11, e75389. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Shao, H.; Liu, S.; Niu, Y.; Fu, L. Globular adiponectin ameliorates insulin resistance in skeletal muscle by enhancing the LKB1-mediated AMPK activation via SESN2. Sport. Med. Health Sci. 2023, 5, 34–41. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Sun, M.; Zhang, Y.; Li, X.; Sun, W.; Quan, N. Sestrin2 is an endogenous antioxidant that improves contractile function in the heart during exposure to ischemia and reperfusion stress. Free Radic. Biol. Med. 2021, 165, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; He, Z.; Fedorova, J.; Zhang, J.; Wood, E.; Zhang, X.; Kang, D.E.; Li, J. Sestrin2 maintains OXPHOS integrity to modulate cardiac substrate metabolism during ischemia and reperfusion. Redox Biol. 2021, 38, 101824. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Wang, L.; Chen, X.; Luckett, C.; Cates, C.; Rousselle, T.; Zheng, Y.; Li, J. Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1alpha pathway. J. Mol. Cell Cardiol. 2018, 115, 170–178. [Google Scholar] [CrossRef]
- Xue, R.; Zeng, J.; Chen, Y.; Chen, C.; Tan, W.; Zhao, J.; Dong, B.; Sun, Y.; Dong, Y.; Liu, C. Sestrin 1 ameliorates cardiac hypertrophy via autophagy activation. J. Cell Mol. Med. 2017, 21, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Li, X.; Zhang, J.; Han, Y.; Sun, W.; Ren, D.; Tong, Q.; Li, J. Substrate metabolism regulated by Sestrin2-mTORC1 alleviates pressure overload-induced cardiac hypertrophy in aged heart. Redox Biol. 2020, 36, 101637. [Google Scholar] [CrossRef]
- Sun, X.; Han, F.; Lu, Q.; Li, X.; Ren, D.; Zhang, J.; Han, Y.; Xiang, Y.K.; Li, J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet-Induced Obese Mice. Diabetes 2020, 69, 1292–1305. [Google Scholar] [CrossRef]
- Du, J.; He, W.; Zhang, C.; Wu, J.; Li, Z.; Wang, M.; Feng, S.; Liang, G. Pentamethylquercetin Attenuates Cardiac Remodeling via Activation of the Sestrins/Keap1/Nrf2 Pathway in MSG-Induced Obese Mice. BioMed Res. Int. 2020, 2020, 3243906. [Google Scholar] [CrossRef]
- Du, J.X.; Wu, J.Z.; Li, Z.; Zhang, C.; Shi, M.T.; Zhao, J.; Jin, M.W.; Liu, H. Pentamethylquercetin protects against cardiac remodeling via activation of Sestrin2. Biochem. Biophys. Res. Commun. 2019, 512, 412–420. [Google Scholar] [CrossRef]
- Krause-Hauch, M.; Fedorova, J.; Zoungrana, L.I.; Wang, H.; Fatmi, M.K.; Li, Z.; Iglesias, M.; Slotabec, L.; Li, J. Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity. Cells 2022, 11, 2614. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Xu, L.; Zhang, X.; Qiao, S.; Yuan, J. PGC1alpha activation by pterostilbene ameliorates acute doxorubicin cardiotoxicity by reducing oxidative stress via enhancing AMPK and SIRT1 cascades. Aging 2019, 11, 10061–10073. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, Z.; Liu, Q.; Gu, J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017, 8, e3018. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lan, R.; Guo, Z.; Cai, S.; Wang, J.; Wang, Q.; Li, Z.; Li, Z.; Wang, Q.; Li, J.; et al. Histone Demethylase JMJD3 Mediated Doxorubicin-Induced Cardiomyopathy by Suppressing SESN2 Expression. Front. Cell Dev. Biol. 2020, 8, 548605. [Google Scholar] [CrossRef]
- Yang, D.; Han, B.; Baiyun, R.; Lv, Z.; Wang, X.; Li, S.; Lv, Y.; Xue, J.; Liu, Y.; Zhang, Z. Sulforaphane attenuates hexavalent chromium-induced cardiotoxicity via the activation of the Sesn2/AMPK/Nrf2 signaling pathway. Metallomics 2020, 12, 2009–2020. [Google Scholar] [CrossRef]
- Iglesias, M.; Wang, H.; Krause-Hauch, M.; Ren, D.; Zoungrana, L.I.; Li, Z.; Zhang, J.; Wei, J.; Yadav, N.; Patel, K.; et al. Sestrin2 Mediates Metformin Rescued the Age-Related Cardiac Dysfunctions of Cardiorenal Syndrome Type 3. Cells 2023, 12, 845. [Google Scholar] [CrossRef]
- Ljubicic, V.; Khogali, S.; Renaud, J.M.; Jasmin, B.J. Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle. Am. J. Physiol. Cell Physiol. 2012, 302, C110–C121. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Jasmin, B.J. AMP-activated protein kinase at the nexus of therapeutic skeletal muscle plasticity in Duchenne muscular dystrophy. Trends Mol. Med. 2013, 19, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kay, D.I.; Rudra, R.T.; Chen, B.M.; Hsu, N.; Izumiya, Y.; Martinez, L.; Spencer, M.J.; Walsh, K.; Grinnell, A.D.; et al. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 1324–1338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.; Kim, M. Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance. Biomolecules 2023, 13, 722. https://doi.org/10.3390/biom13050722
Hwang I, Kim M. Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance. Biomolecules. 2023; 13(5):722. https://doi.org/10.3390/biom13050722
Chicago/Turabian StyleHwang, Irene, and Myungjin Kim. 2023. "Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance" Biomolecules 13, no. 5: 722. https://doi.org/10.3390/biom13050722
APA StyleHwang, I., & Kim, M. (2023). Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance. Biomolecules, 13(5), 722. https://doi.org/10.3390/biom13050722





