Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model
Abstract
:1. Introduction
2. Materials and Methods
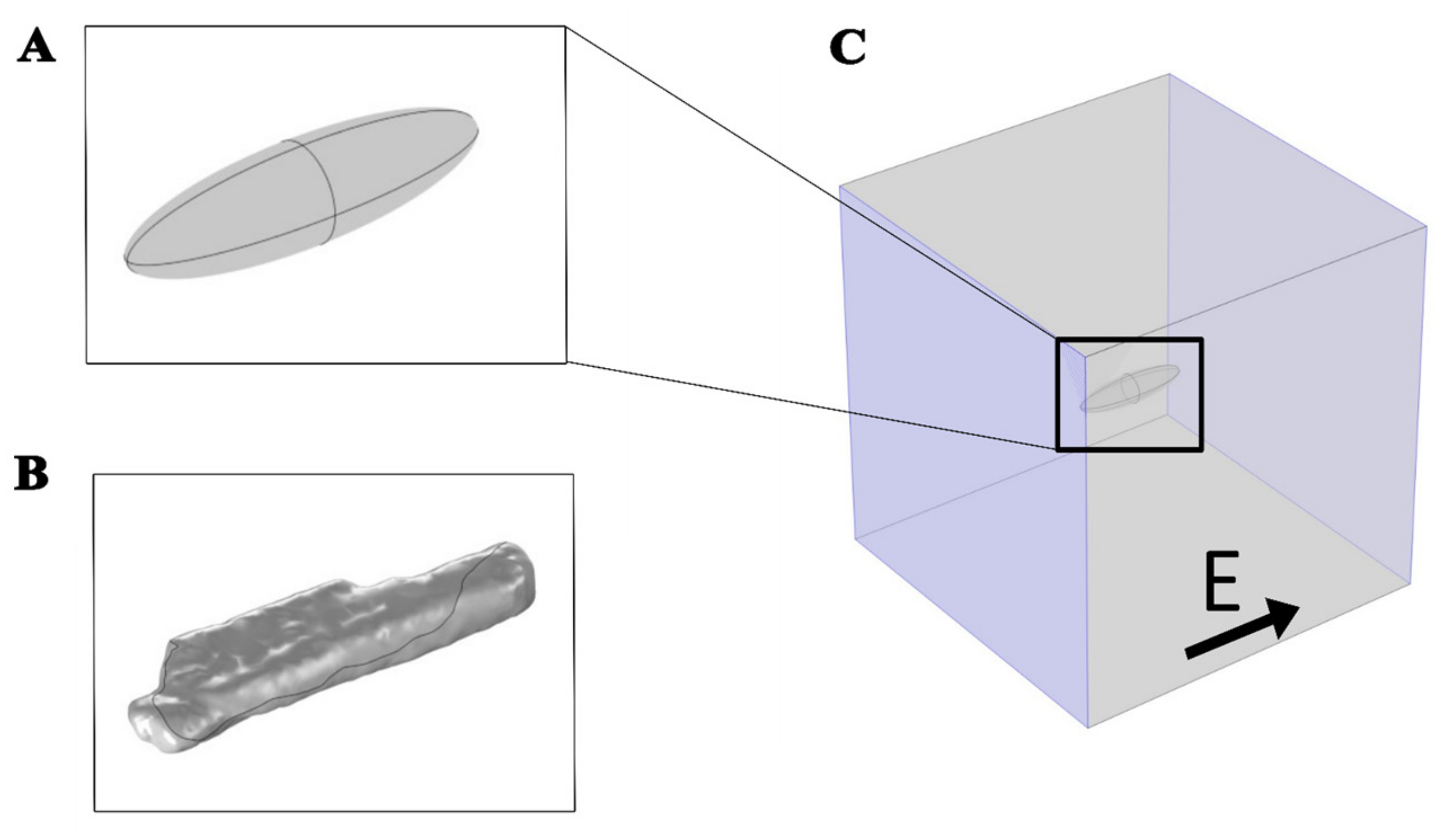
2.1. The Time-Dependent Numerical Model with Electroporation
2.2. In Vitro Experiments: Calcium Transients in Cardiomyocyte-Derived Cell Lines
2.3. In Vitro Experiments: Electric Field Effect on Diastolic Calcium Level in Primary Cardiomyocytes
3. Results and Discussion
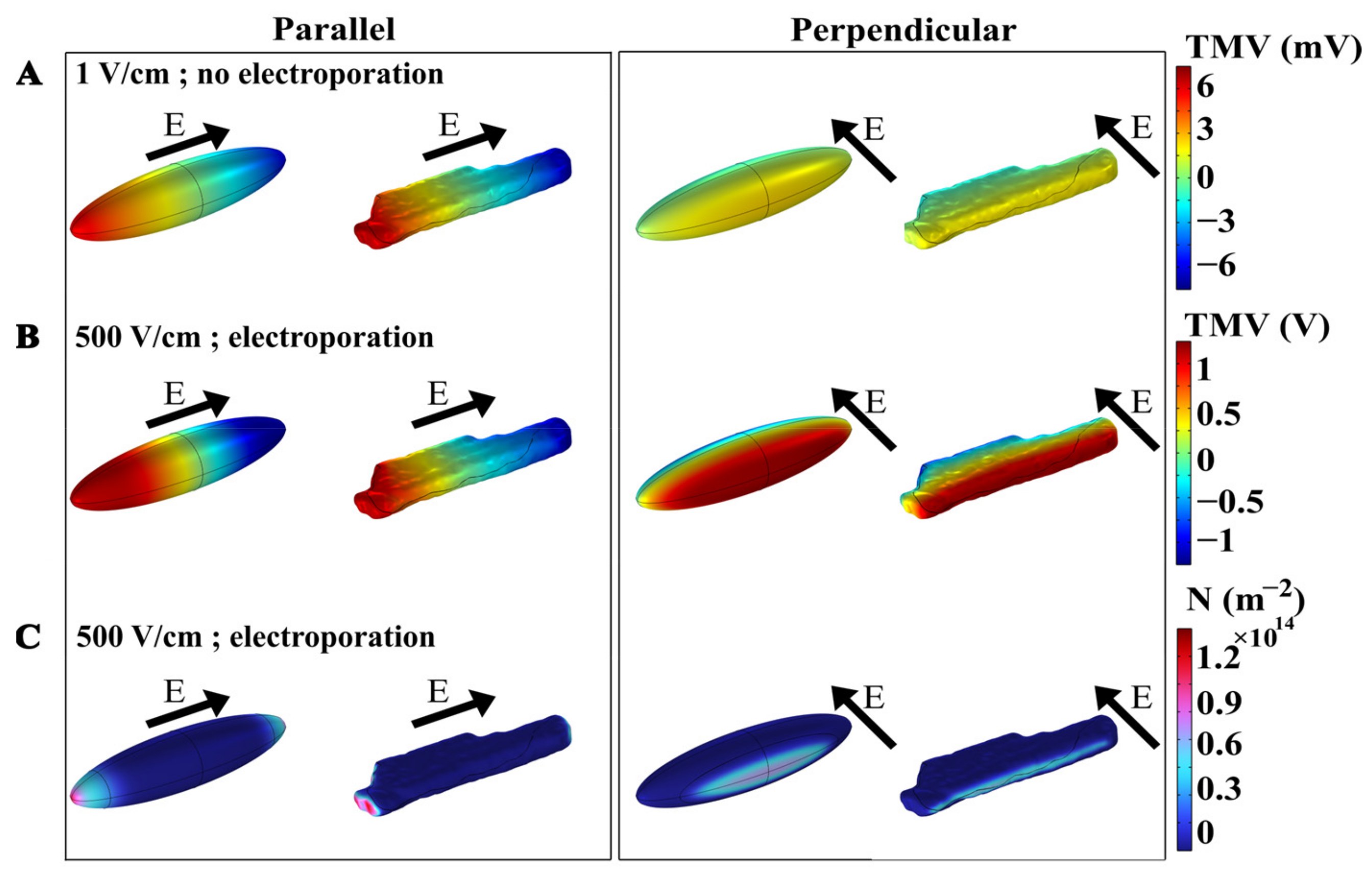
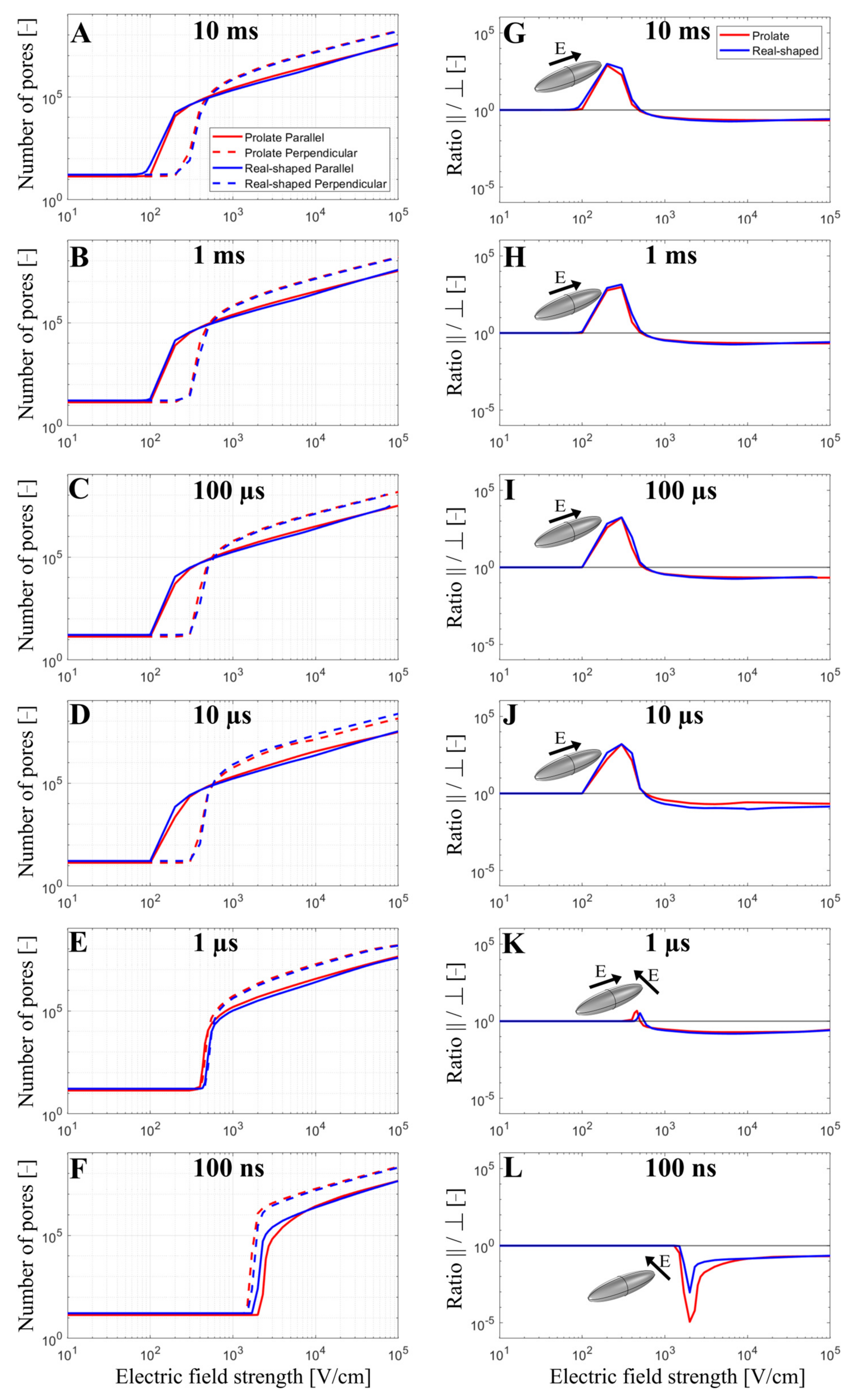
3.1. The Time-Dependent Numerical Model with Included Electroporation
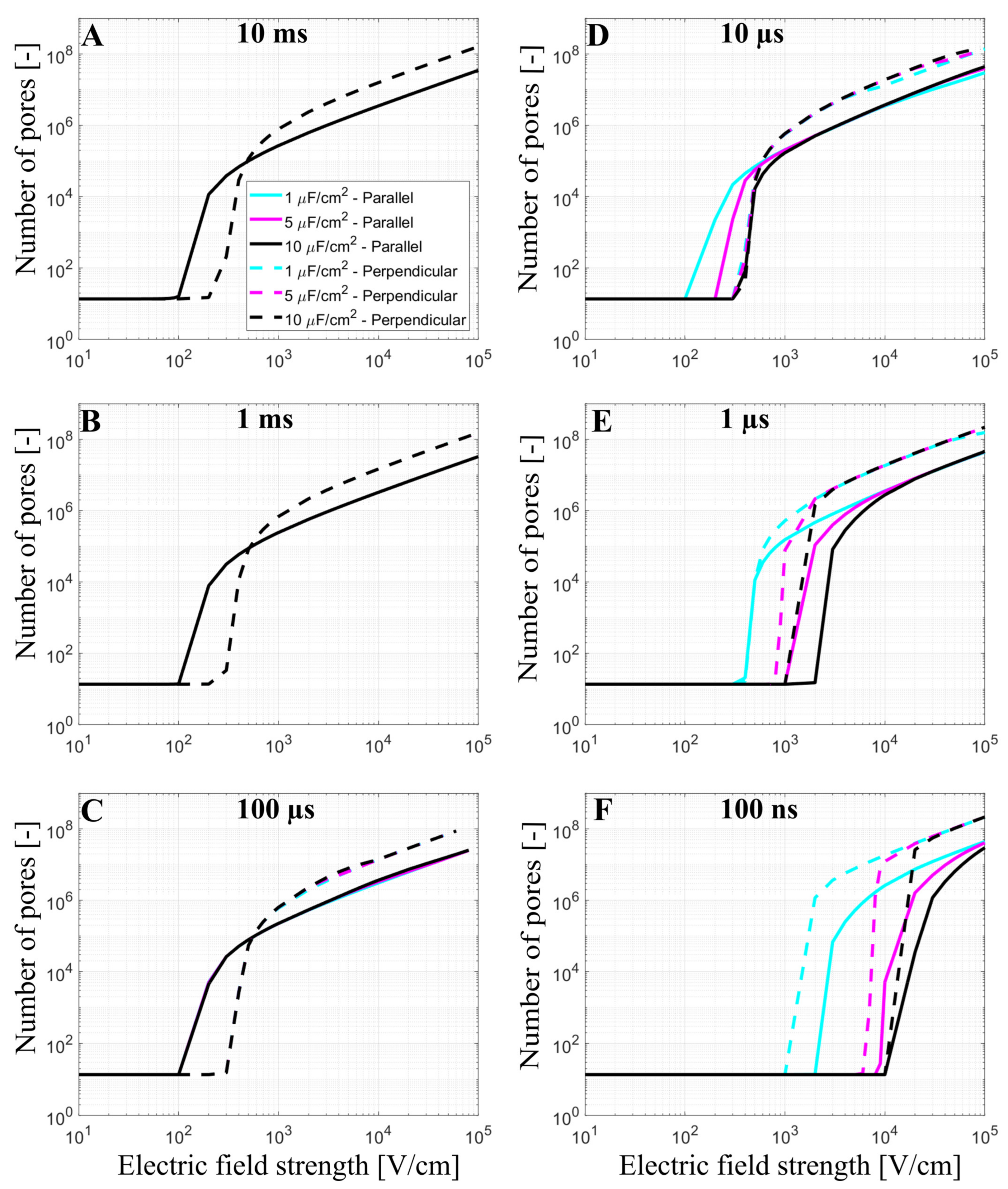
3.2. The Effect of T-Tubules
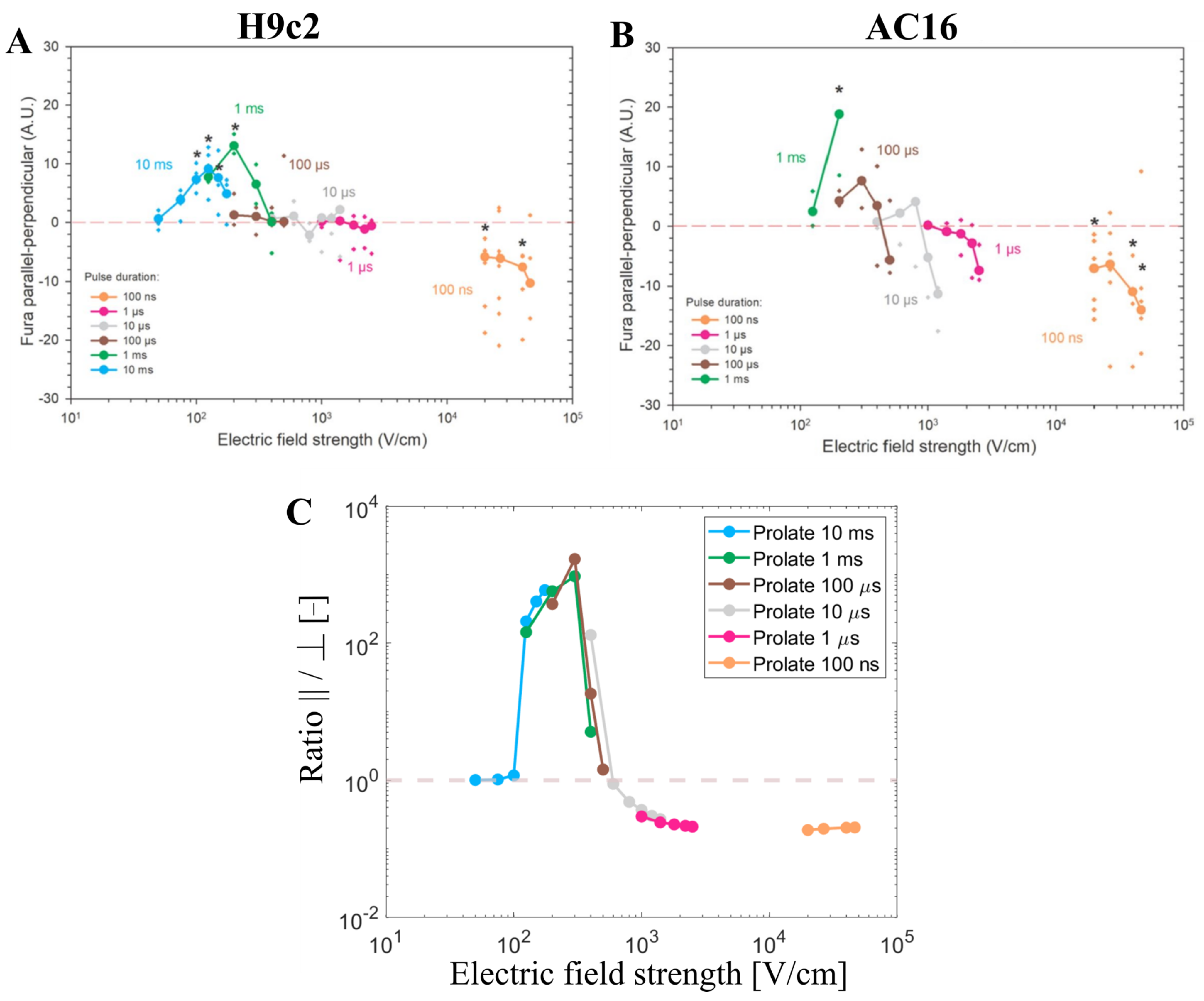
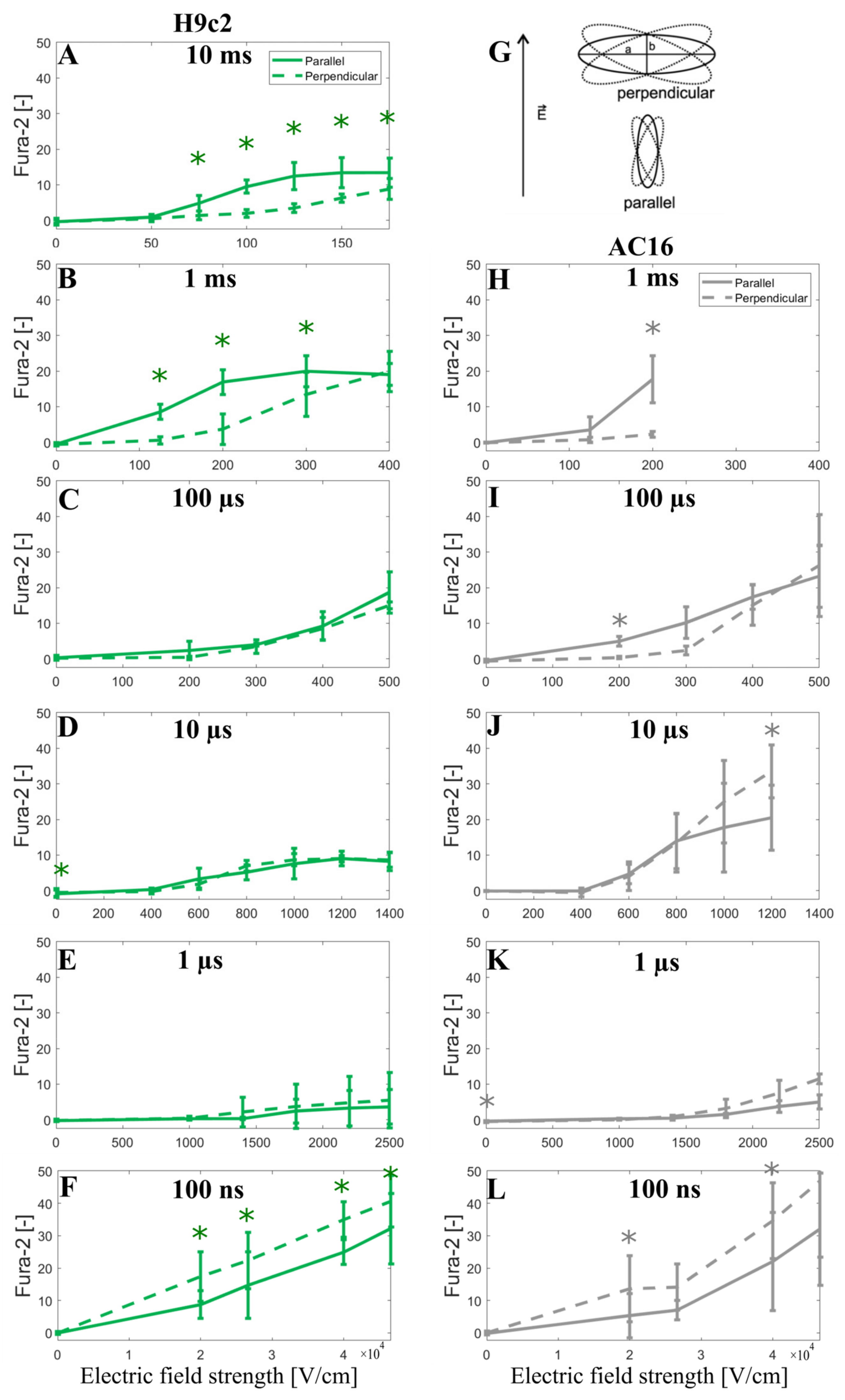
3.3. Experimental Results and Model Predictions: Calcium Transients in Cardiomyocyte-Derived Cell Lines
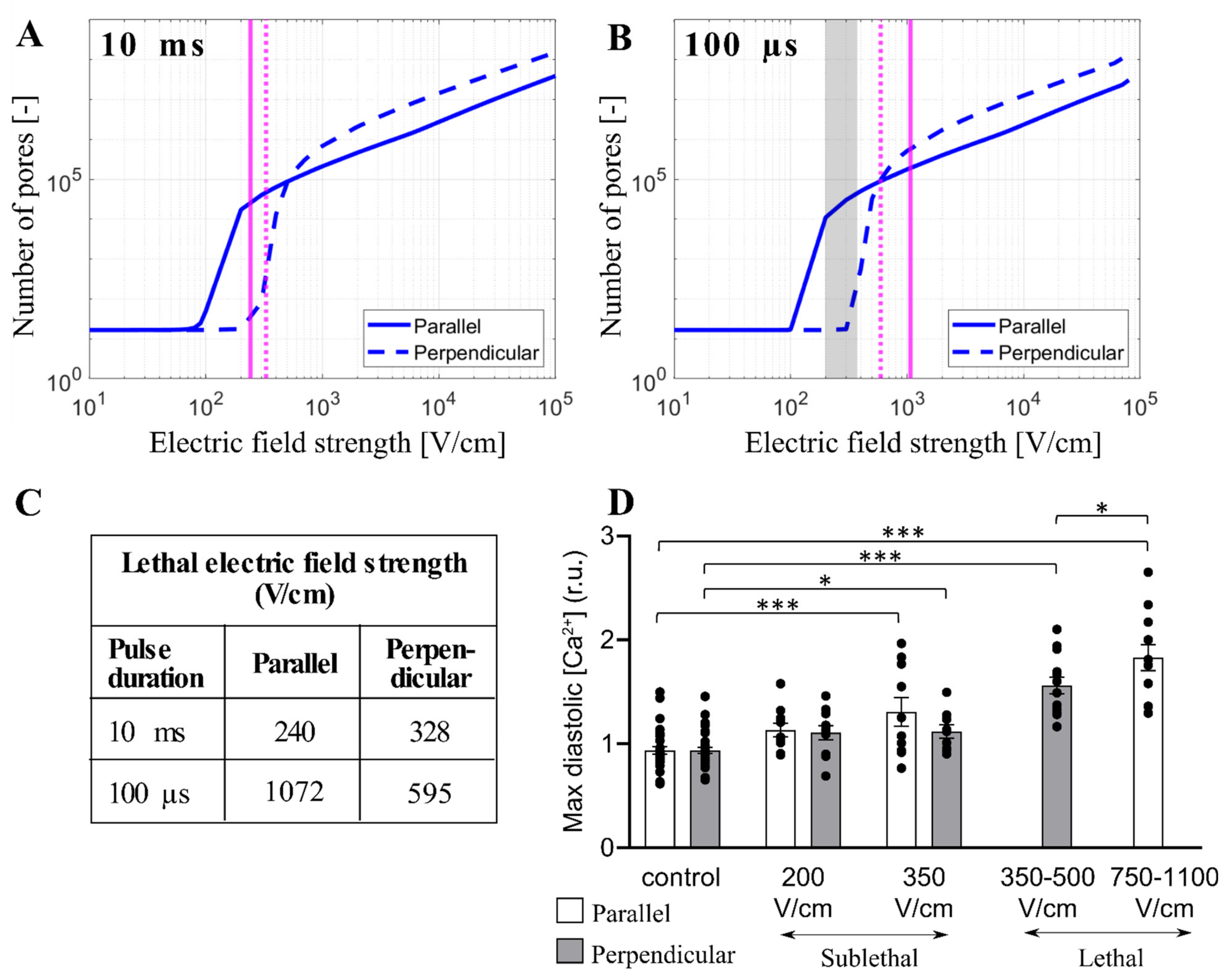
3.4. Experimental Results and Model Predictions: Lethal Electric Field Strengths in Primary Cardiomyocytes
3.5. Limitations of the Model
3.6. Clinical Relevance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McBride, S.; Avazzadeh, S.; Wheatley, A.M.; O’Brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Quinlan, L.R. Ablation Modalities for Therapeutic Intervention in Arrhythmia-Related Cardiovascular Disease: Focus on Electroporation. J. Clin. Med. 2021, 10, 2657. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.J.; Haines, D.E. Pulsed Field Ablation for Pulmonary Vein Isolation in the Treatment of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.-H. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023. [Google Scholar] [CrossRef] [PubMed]
- Nikolski, V.P.; Efimov, I.R. Electroporation of the Heart. EP Eur. 2005, 7, S146–S154. [Google Scholar] [CrossRef]
- Ayuni, E.L.; Gazdhar, A.; Giraud, M.N.; Kadner, A.; Gugger, M.; Cecchini, M.; Caus, T.; Carrel, T.P.; Schmid, R.A.; Tevaearai, H.T. In Vivo Electroporation Mediated Gene Delivery to the Beating Heart. PLoS ONE 2010, 5, e14467. [Google Scholar] [CrossRef]
- Bulysheva, A.A.; Hargrave, B.; Burcus, N.; Lundberg, C.G.; Murray, L.; Heller, R. Vascular Endothelial Growth Factor-A Gene Electrotransfer Promotes Angiogenesis in a Porcine Model of Cardiac Ischemia. Gene Ther. 2016, 23, 649–656. [Google Scholar] [CrossRef]
- Vižintin, A.; Miklavčič, D. The Electropermeome: Cellular Response to Electroporation. Slov. Med. J. 2022, 91, 483–495. [Google Scholar]
- Tsong, T.Y. Electroporation of Cell Membranes. In Electroporation and Electrofusion in Cell Biology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 149–163. [Google Scholar]
- Kotnik, T.; Pucihar, G.; Miklavčič, D. Induced Transmembrane Voltage and Its Correlation with Electroporation-Mediated Molecular Transport. J. Membr. Biol. 2010, 236, 3–13. [Google Scholar] [CrossRef]
- Kinosita, K.; Tsong, T.T. Hemolysis of Human Erythrocytes by Transient Electric Field. Proc. Natl. Acad. Sci. USA 1977, 74, 1923–1927. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Kotnik, T.; Miklavčič, D. Analytical Description of Transmembrane Voltage Induced by Electric Fields on Spheroidal Cells. Biophys. J. 2000, 79, 670–679. [Google Scholar] [CrossRef]
- Pucihar, G.; Kotnik, T.; Valič, B.; Miklavčič, D. Numerical Determination of Transmembrane Voltage Induced on Irregularly Shaped Cells. Ann. Biomed. Eng. 2006, 34, 642. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Shaw, R.M. Cardiac T-Tubule Microanatomy and Function. Physiol. Rev. 2017, 97, 227–252. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Krishnamoorthy, V.K.; Korinek, J.; Narula, J.; Vannan, M.A.; Lester, S.J.; Tajik, J.A.; Seward, J.B.; Khandheria, B.K.; Belohlavek, M. Left Ventricular Form and Function Revisited: Applied Translational Science to Cardiovascular Ultrasound Imaging. J. Am. Soc. Echocardiogr. 2007, 20, 539–551. [Google Scholar] [CrossRef]
- Clayton, R.H.; Bernus, O.; Cherry, E.M.; Dierckx, H.; Fenton, F.H.; Mirabella, L.; Panfilov, A.V.; Sachse, F.B.; Seemann, G.; Zhang, H. Models of Cardiac Tissue Electrophysiology: Progress, Challenges and Open Questions. Prog. Biophys. Mol. Biol. 2011, 104, 22–48. [Google Scholar] [CrossRef] [PubMed]
- Trifunović-Zamaklar, D.; Jovanović, I.; Vratonjić, J.; Petrović, O.; Paunović, I.; Tešić, M.; Boričić-Kostić, M.; Ivanović, B. The Basic Heart Anatomy and Physiology from the Cardiologist’s Perspective: Toward a Better Understanding of Left Ventricular Mechanics, Systolic, and Diastolic Function. J. Clin. Ultrasound 2022, 50, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Jiao, P.; Ye, X.; Xia, L. An Image-Based Model of the Whole Human Heart with Detailed Anatomical Structure and Fiber Orientation. Comput. Math. Methods Med. 2012, 2012, 891070. [Google Scholar] [CrossRef]
- Pashakhanloo, F.; Herzka, D.A.; Ashikaga, H.; Mori, S.; Gai, N.; Bluemke, D.A.; Trayanova, N.A.; McVeigh, E.R. Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging. Circ. Arrhythm. Electrophysiol. 2016, 9, e004133. [Google Scholar] [CrossRef]
- Roney, C.H.; Bendikas, R.; Pashakhanloo, F.; Corrado, C.; Vigmond, E.J.; McVeigh, E.R.; Trayanova, N.A.; Niederer, S.A. Constructing a Human Atrial Fibre Atlas. Ann. Biomed. Eng. 2021, 49, 233–250. [Google Scholar] [CrossRef]
- Ye, X.; Liu, S.; Yin, H.; He, Q.; Xue, Z.; Lu, C.; Su, S. Study on Optimal Parameter and Target for Pulsed-Field Ablation of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 690092. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.N.; Goulart, J.T.; Zoccoler, M.; Oliveira, P.X. Ventricular Myocyte Injury by High-Intensity Electric Field: Effect of Pulse Duration. Gen. Physiol. Biophys. 2016, 35, 121–130. [Google Scholar] [CrossRef]
- de Oliveira, P.X.; Bassani, R.A.; Bassani, J.W.M. Lethal Effect of Electric Fields on Isolated Ventricular Myocytes. IEEE Trans. Biomed. Eng. 2008, 55, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Wittkampf, F.H.; Van Driel, V.J.; Van Wessel, H.; Vink, A.; Hof, I.E.; Gründeman, P.F.; Hauer, R.N.; Loh, P. Feasibility of Electroporation for the Creation of Pulmonary Vein Ostial Lesions. J. Cardiovasc. Electrophysiol. 2011, 22, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Neuber, J.U.; Varghese, F.; Pakhomov, A.G.; Zemlin, C.W. Using Nanosecond Shocks for Cardiac Defibrillation. Bioelectricity 2019, 1, 240–246. [Google Scholar] [CrossRef]
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and Injury of Adult Ventricular Cardiomyocytes by Nano-to Millisecond Electric Shocks. Sci. Rep. 2018, 8, 8233. [Google Scholar] [CrossRef]
- Hartl, S.; Reinsch, N.; Füting, A.; Neven, K. Pearls and Pitfalls of Pulsed Field Ablation. Korean Circ. J. 2022, 53, e26. [Google Scholar] [CrossRef]
- Maan, A.; Koruth, J. Pulsed Field Ablation: A New Paradigm for Catheter Ablation of Arrhythmias. Curr. Cardiol. Rep. 2022, 24, 103–108. [Google Scholar] [CrossRef]
- Milan, H.F.; Bassani, R.A.; Santos, L.E.; Almeida, A.C.; Bassani, J.W. Accuracy of Electromagnetic Models to Estimate Cardiomyocyte Membrane Polarization. Med. Biol. Eng. Comput. 2019, 57, 2617–2627. [Google Scholar] [CrossRef]
- Dermol-Černe, J.; Batista Napotnik, T.; Reberšek, M.; Miklavčič, D. Short Microsecond Pulses Achieve Homogeneous Electroporation of Elongated Biological Cells Irrespective of Their Orientation in Electric Field. Sci. Rep. 2020, 10, 9149. [Google Scholar] [CrossRef]
- Henslee, B.E.; Morss, A.; Hu, X.; Lafyatis, G.P.; Lee, L.J. Electroporation Dependence on Cell Size: Optical Tweezers Study. Anal. Chem. 2011, 83, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, S.; Sigg, D.C.; Stewart, M.T.; Hocini, M.; Batista Napotnik, T.; Miklavčič, D.; Bernus, O.; Benoist, D. Reversible and Irreversible Effects of Electroporation on Contractility and Calcium Homeostasis in Isolated Cardiac Ventricular Myocytes. Circ. Arrhythm. Electrophysiol. 2022, 15, e011131. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, B.; Vernier, P.; Ivorra, A. Dependence of Electroporation Detection Threshold on Cell Radius: An Explanation to Observations Non Compatible with Schwan’s Equation Model. J. Membr. Biol. 2016, 249, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C.; Weaver, J.C. Active Mechanisms Are Needed to Describe Cell Responses to Submicrosecond, Megavolt-per-Meter Pulses: Cell Models for Ultrashort Pulses. Biophys. J. 2008, 95, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Rems, L.; Ušaj, M.; Kandušer, M.; Reberšek, M.; Miklavčič, D.; Pucihar, G. Cell Electrofusion Using Nanosecond Electric Pulses. Sci. Rep. 2013, 3, 3382. [Google Scholar] [CrossRef] [PubMed]
- DeBruin, K.A.; Krassowska, W. Modeling Electroporation in a Single Cell. I. Effects of Field Strength and Rest Potential. Biophys. J. 1999, 77, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Krassowska, W.; Filev, P.D. Modeling Electroporation in a Single Cell. Biophys. J. 2007, 92, 404–417. [Google Scholar] [CrossRef]
- Kotnik, T.; Miklavčič, D.; Slivnik, T. Time Course of Transmembrane Voltage Induced by Time-Varying Electric Fields—A Method for Theoretical Analysis and Its Application. Bioelectrochem. Bioenerg. 1998, 45, 3–16. [Google Scholar] [CrossRef]
- Fozzard, H.A. Membrane Capacity of the Cardiac Purkinje Fibre. J. Physiol. 1966, 182, 255. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Miklavčič, D. In Vitro Electroporation Detection Methods–An Overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef]
- Fishler, M.G.; Sobie, E.A.; Tung, L.; Thakor, N.V. Cardiac Responses to Premature Monophasic and Biphasic Field Stimuli: Results from Cell and Tissue Modeling Studies. J. Electrocardiol. 1995, 28, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.A.P.; Bassani, R.A.; Bassani, J.W.M. Electric Field Stimulation of Cardiac Myocytes during Postnatal Development. IEEE Trans. Biomed. Eng. 2001, 48, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Itoh, H.; Kinosita, K., Jr. Time Courses of Cell Electroporation as Revealed by Submicrosecond Imaging of Transmembrane Potential. Biophys. J. 1993, 64, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Boyden, P.A.; Hirose, M.; Dun, W. Cardiac Purkinje Cells. Heart Rhythm 2010, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pucihar, G.; Krmelj, J.; Reberšek, M.; Napotnik, T.B.; Miklavčič, D. Equivalent Pulse Parameters for Electroporation. IEEE Trans. Biomed. Eng. 2011, 58, 3279–3288. [Google Scholar] [CrossRef]
- Scuderi, M.; Dermol-Černe, J.; da Silva, C.A.; Muralidharan, A.; Boukany, P.E.; Rems, L. Models of Electroporation and the Associated Transmembrane Molecular Transport Should Be Revisited. Bioelectrochemistry 2022, 147, 108216. [Google Scholar] [CrossRef]
- Rems, L.; Viano, M.; Kasimova, M.A.; Miklavčič, D.; Tarek, M. The Contribution of Lipid Peroxidation to Membrane Permeability in Electropermeabilization: A Molecular Dynamics Study. Bioelectrochemistry 2019, 125, 46–57. [Google Scholar] [CrossRef]
- Wiczew, D.; Szulc, N.; Tarek, M. Molecular Dynamics Simulations of the Effects of Lipid Oxidation on the Permeability of Cell Membranes. Bioelectrochemistry 2021, 141, 107869. [Google Scholar] [CrossRef]
- Breton, M.; Mir, L.M. Investigation of the Chemical Mechanisms Involved in the Electropulsation of Membranes at the Molecular Level. Bioelectrochemistry 2018, 119, 76–83. [Google Scholar] [CrossRef]
- Rems, L.; Kasimova, M.A.; Testa, I.; Delemotte, L. Pulsed Electric Fields Can Create Pores in the Voltage Sensors of Voltage-Gated Ion Channels. Biophys. J. 2020, 119, 190–205. [Google Scholar] [CrossRef]
- Rols, M.-P.; Teissie, J. Electropermeabilization of Mammalian Cells. Quantitative Analysis of the Phenomenon. Biophys. J. 1990, 58, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Sözer, E.B.; Pocetti, C.F.; Vernier, P.T. Asymmetric Patterns of Small Molecule Transport after Nanosecond and Microsecond Electropermeabilization. J. Membr. Biol. 2018, 251, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Polajzer, T.; Jarm, T.; Miklavcic, D. Analysis of Damage-Associated Molecular Pattern Molecules Due to Electroporation of Cells in Vitro. Radiol. Oncol. 2020, 54, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, F.; Golzio, M.; Kovacs, E.; Teissié, J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018, 251, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.; Roth, D.; Ward, D.M.; Kaplan, J.; Andrews, N.W. Defective Lysosomal Exocytosis and Plasma Membrane Repair in Chediak–Higashi/Beige Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16795–16800. [Google Scholar] [CrossRef]
- Shamoon, D.; Dermol-Černe, J.; Rems, L.; Reberšek, M.; Kotnik, T.; Lasquellec, S.; Brosseau, C.; Miklavčič, D. Assessing the Electro-Deformation and Electro-Poration of Biological Cells Using a Three-Dimensional Finite Element Model. Appl. Phys. Lett. 2019, 114, 063701. [Google Scholar] [CrossRef]
- Graybill, P.M.; Davalos, R.V. Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers 2020, 12, 1132. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell Death Due to Electroporation–A Review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Son, R.S.; Gowrishankar, T.R.; Smith, K.C.; Weaver, J.C. Modeling a Conventional Electroporation Pulse Train: Decreased Pore Number, Cumulative Calcium Transport and an Example of Electrosensitization. IEEE Trans. Biomed. Eng. 2015, 63, 571–580. [Google Scholar] [CrossRef]
- Im, S.I.; Higuchi, S.; Lee, A.; Stillson, C.; Buck, E.; Morrow, B.; Schenider, K.; Speltz, M.; Gerstenfeld, E.P. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. Clin. Electrophysiol. 2022, 8, 722–731. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Iwasawa, J.; Viswanathan, R.; Brose, R.; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial Ventricular Pulsed Field Ablation: A Proof-of-Concept Preclinical Evaluation. EP Eur. 2020, 22, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Castellvi, Q.; Neal, R.; Girouard, S.; Ikeda, A.; Kuroda, S.; Hussein, A.A.; Saliba, W.I.; Wazni, O.M. B-PO03-131 Effects of Contact Force on Lesion Size during Pulsed Field Ablation. Heart Rhythm 2021, 18, S242–S243. [Google Scholar] [CrossRef]
- Yavin, H.D.; Higuchi, K.; Sroubek, J.; Younis, A.; Zilberman, I.; Anter, E. Pulsed-Field Ablation in Ventricular Myocardium Using a Focal Catheter: The Impact of Application Repetition on Lesion Dimensions. Circ. Arrhythm. Electrophysiol. 2021, 14, e010375. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Verheule, S.; Wilson, E.E.; Arora, R.; Engle, S.K.; Scott, L.R.; Olgin, J.E. Tissue Structure and Connexin Expression of Canine Pulmonary Veins. Cardiovasc. Res. 2002, 55, 727–738. [Google Scholar] [CrossRef]
- Mary-Rabine, L.; Albert, A.; Pham, T.D.; Hordof, A.; Fenoglio, J.J., Jr.; Malm, J.R.; Rosen, M.R. The Relationship of Human Atrial Cellular Electrophysiology to Clinical Function and Ultrastructure. Circ. Res. 1983, 52, 188–199. [Google Scholar] [CrossRef]
- Nygren, A.; Fiset, C.; Firek, L.; Clark, J.W.; Lindblad, D.S.; Clark, R.B.; Giles, W.R. Mathematical Model of an Adult Human Atrial Cell: The Role of K+ Currents in Repolarization. Circ. Res. 1998, 82, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, B.; Downey, H.; Strange, R.; Murray, L.; Cinnamond, C.; Lundberg, C.; Israel, A.; Chen, Y.-J.; Marshall, W.; Heller, R. Electroporation-Mediated Gene Transfer Directly to the Swine Heart. Gene Ther. 2013, 20, 151–157. [Google Scholar] [CrossRef]
| Parameter | Symbol | Value | Ref. |
|---|---|---|---|
| Intracellular permittivity | εi | 80 | [39] |
| Extracellular permittivity | εe | 80 | [39] |
| Intracellular conductivity | σi | 0.8 S/m | [30] |
| Extracellular conductivity | σe | 1.4 S/m | [30] |
| Membrane conductivity | σm | 1.4925 × 10−8 S/m | [30] |
| Membrane thickness | dm | 5 nm | [37] |
| Membrane capacitance | Cm | 0 μF/cm2 5–10 μF/cm2 * | [37] [40] |
| Block length | L | 400 µm | Arbitrary |
| Electroporation constant | q | 1.46 | [31] |
| Electroporation parameter | A | 109 1/(m2s) | [37] |
| Characteristic voltage of electroporation | Vep | 0.258 V, | [37] |
| Equilibrium pore density | N0 | 1.5∙109 1/m2 | [37] |
| Pore radius | rp | 0.76 nm | [37] |
| Pore Conductivity (cell membrane) | σp | (σe – σi)/ln(σe – σi) | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuderi, M.; Dermol-Černe, J.; Batista Napotnik, T.; Chaigne, S.; Bernus, O.; Benoist, D.; Sigg, D.C.; Rems, L.; Miklavčič, D. Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model. Biomolecules 2023, 13, 727. https://doi.org/10.3390/biom13050727
Scuderi M, Dermol-Černe J, Batista Napotnik T, Chaigne S, Bernus O, Benoist D, Sigg DC, Rems L, Miklavčič D. Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model. Biomolecules. 2023; 13(5):727. https://doi.org/10.3390/biom13050727
Chicago/Turabian StyleScuderi, Maria, Janja Dermol-Černe, Tina Batista Napotnik, Sebastien Chaigne, Olivier Bernus, David Benoist, Daniel C. Sigg, Lea Rems, and Damijan Miklavčič. 2023. "Characterization of Experimentally Observed Complex Interplay between Pulse Duration, Electrical Field Strength, and Cell Orientation on Electroporation Outcome Using a Time-Dependent Nonlinear Numerical Model" Biomolecules 13, no. 5: 727. https://doi.org/10.3390/biom13050727





