Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review
Abstract
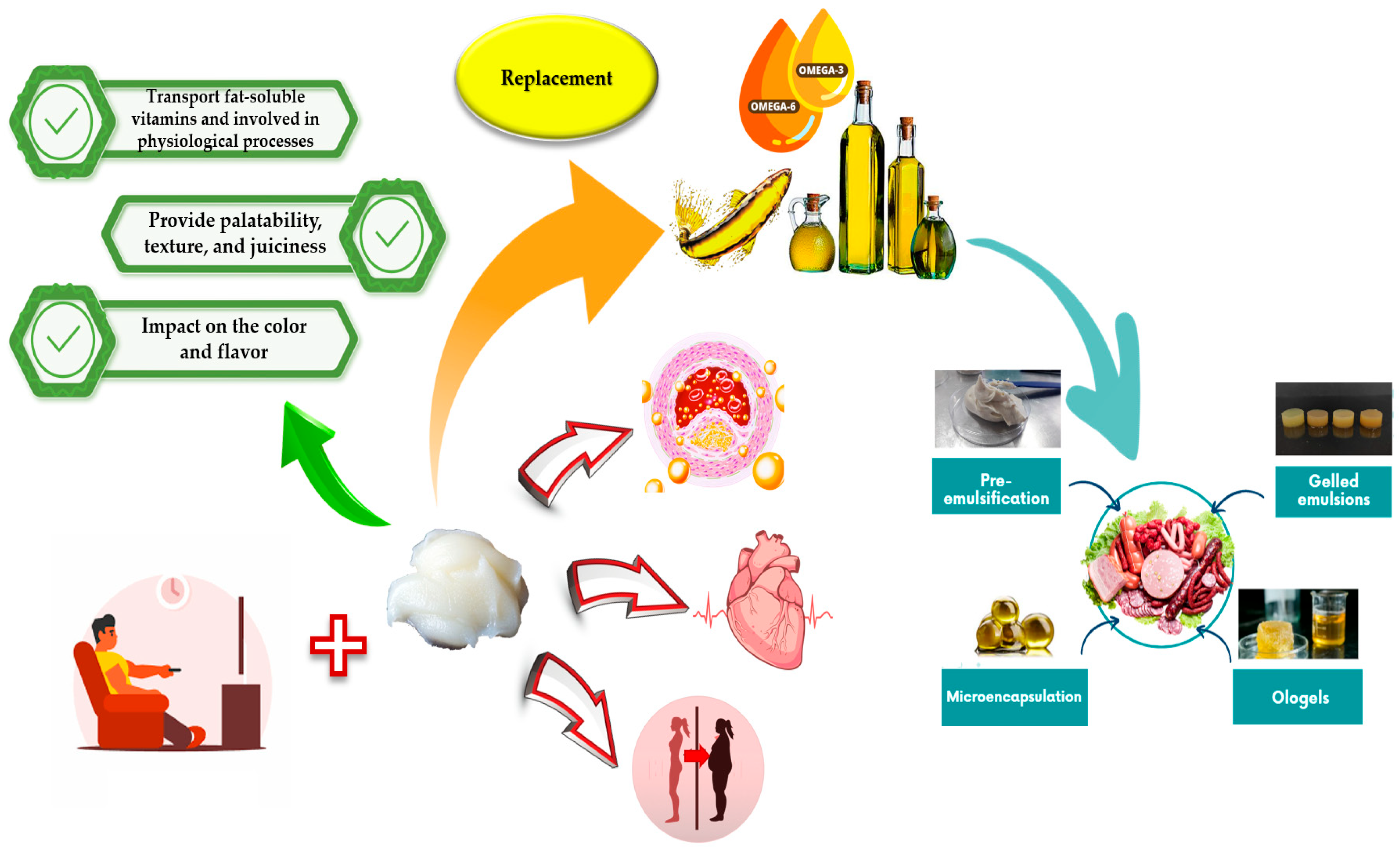
:1. Introduction
2. Healthier Oils
2.1. Vegetable Oils
2.1.1. Oils from Oilseeds
Soybean Oil
Canola Oil
Sunflower Seed Oil
Cottonseed Oil
Corn Oil
Peanut Oil
Walnut Oil
Chia Oil
Oil from Non-Traditional Oilseeds
2.1.2. Oils from Fruits
Olive Oil
Coconut Oil
Avocado Oil
2.2. Oils from Marine Origin
2.2.1. Seaweed Oils
2.2.2. Fish Oils
2.3. Insect Oils
3. Strategies for Structuring Oils
3.1. Pre-Emulsification
3.2. Encapsulation
3.3. Gelled Emulsions
3.4. Oleogels
4. Food Application
4.1. Incorporation of Structured Healthier Oils in Meat Products
4.1.1. Fresh Meat Products
4.1.2. Emulsified Meat Products
4.1.3. Traditional Fermented Meat Products
4.2. Incorporation of Structured Healthier Oils in Dairy Products
4.2.1. Cheese
- Analogue or imitation cheese, made from dairy and/or nondairy ingredients, formulated with specific nutritional and/or functional properties, according to consumer needs [184,189]. In imitation cheese, the fat source can come from soybean oil, rapeseed oil, palm oil, canola oil, sunflower oil, and corn oil [165,186].
- Functional processed cheese, made from dairy and/or nondairy ingredients and fortified with some bioactive compounds with functional properties.
- Plant-based processed cheese, made from nondairy ingredients [189].
4.2.2. Yoghurt
4.2.3. Ice Cream
4.2.4. Butter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022; pp. 1–131. [Google Scholar]
- Bennett, J.; Stevens, G.; Bonita, R.; Rehm, J.; Kruk, M.; Riley, L.; Dain, K.; Kengne, A.; Chalkidou, K.; Beagley, J.; et al. NCD countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet North Am. Ed. 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Cardiovascular Disease. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 February 2023).
- Meng, H.; Ruan, J.; Yan, Z.; Chen, Y.; Liu, J.; Li, X.; Meng, F. New Progress in Early Diagnosis of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 8939. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 11881204. [Google Scholar] [CrossRef] [PubMed]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling atherosclerosis via selected nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Drake, I.; Engström, G.; Acosta, S. Modifiable and non-modifiable risk factors for atherothrombotic ischemic stroke among subjects in the Malmö diet and cancer study. Nutrients 2021, 13, 1952. [Google Scholar] [CrossRef]
- Astrup, A.; Teicholz, N.; Magkos, F.; Bier, D.M.; Brenna, J.T.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; Yusuf, S.; et al. Dietary saturated fats and health: Are the U.S. guidelines evidence-based? Nutrients 2021, 13, 3305. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 6 February 2023).
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [Green Version]
- Gershuni, V.M. Saturated Fat: Part of a Healthy Diet. Curr. Nutr. Rep. 2018, 7, 85–96. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Choi, M.J.; Cho, Y.J. Improved physicochemical properties of pork patty supplemented with oil-in-water nanoemulsion. Food Sci. Anim. Resour. 2020, 40, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C. Novel lipid materials based on gelling procedures as fat analogues in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 39, 1–6. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pérez-Álvarez, J.A.; Pateiro, M.; Viuda-Matos, M.; Fernández-López, J.; Lorenzo, J.M. Satiety from healthier and functional foods. Trends Food Sci. Technol. 2021, 113, 397–410. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L.; Lombardo, M. Promising sources of plant-derived polyunsaturated fatty acids: A narrative review. Int. J. Environ. Res. Public Health 2023, 20, 1683. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.L.S.; Freire da Costa, G.; Do Nascimento Alves, R.; Leal de Araújo, C.D.; Gonçalves da Silva, G.F.; Ribeiro, N.L.; Vieira de Figueiredo, C.F.; de Andrade, R.O. Vegetable oils in emulsified meat products: A new strategy to replace animal fat. Food Sci. Technol. 2022, 42, e103621. [Google Scholar] [CrossRef]
- Utama, D.T.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, S.K. Fatty acid composition and quality properties of chicken sausage formulated with pre-emulsified perilla-canola oil as an animal fat replacer. Poult. Sci. 2019, 98, 3059–3066. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci. 2021, 173, 108396. [Google Scholar] [CrossRef] [PubMed]
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Gea-Quesada, A.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Improving the lipid profile of beef burgers added with chia oil (Salvia hispanica L.) or hemp oil (Cannabis sativa L.) gelled emulsions as partial animal fat replacers. LWT-Food Sci. Technol. 2022, 161, 113416. [Google Scholar] [CrossRef]
- Airoldi, R.; da Silva, T.L.T.; Neves Rodrigues Ract, J.; Foguel, A.; Colleran, H.L.; Ibrahim, S.A.; Claro da Silva, R. Potential use of carnauba wax oleogel to replace saturated fat in ice cream. J. Am. Oil Chem Soc. 2022, 99, 1085–1099. [Google Scholar] [CrossRef]
- Martínez, E.; Pardo, J.E.; Álvarez-Ortí, M.; Rabadán, A.; Pardo-Giménez, A.; Alvarruiz, A. Substitution of Pork Fat by Emulsified Seed Oils in Fresh Deer Sausage (‘Chorizo’) and Its Impact on the Physical, Nutritional, and Sensory Properties. Foods 2023, 12, 828. [Google Scholar] [CrossRef]
- Wolfer, T.L.; Acevedo, N.C.; Prusa, K.J.; Sebranek, J.G.; Tarté, R. Replacement of pork fat in frankfurter-type sausages by soybean oil oleogels structured with rice bran wax. Meat Sci. 2018, 145, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards replacing pork backfat with a linseed oleogel in frankfurter sausages and its evaluation on physicochemical, nutritional, and sensory characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbut, S.; Marangoni, A. Organogels use in meat processing–Effects of fat/oil type and heating rate. Meat Sci. 2019, 149, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Widmar, D. Two Charts: Global Vegetable Oil Trends. 2022. Available online: https://aei.ag/2022/07/18/vegetable-oil-trends-production-oilseeds/ (accessed on 4 December 2022).
- Lv, M.; Zhang, X.; Ren, H.; Liu, L.; Zhao, Y.M.; Wang, Z.; Wu, Z.L.; Liu, L.M.; Xu, H.J. A rapid method to authenticate vegetable oils through surface-enhanced Raman scattering. Sci. Rep. 2016, 6, 23405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Cao, Y.; Zhao, L.; Kong, X.; Hua, Y. Macronutrients and micronutrients of soybean oil bodies extracted at different pH. J. Food Sci. 2014, 79, C1285–C1291. [Google Scholar] [CrossRef]
- Zaaboul, F.; Zhao, Q.; Xu, Y.J.; Liu, Y.F. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Vingering, N.; Oseredczuk, M.; Du Chaffaut, L.; Ireland, J.; Ledoux, M. Fatty acid composition of commercial vegetable oils from the French market analysed using a long highly polar column. OCL 2010, 17, 185–192. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Healthy fats and oil. In Encyclopedia of Food Grain, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 157–167. [Google Scholar] [CrossRef]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef]
- NSA (National Sunflower Assocciation). Four Types of Sunflower Oil. 2018. Available online: https://www.sunflowernsa.com/oil/Four-Types-of-Sunflower-Oil/ (accessed on 19 November 2022).
- Sharif, I.; Farooq, J.; Chohan, S.M.; Saleem, S.; Kainth, R.A.; Mahmood, A.; Sarwar, G. Strategies to enhance cottonseed oil contents and reshape fatty acid profile employing different breeding and genetic engineering approaches. J. Integr. Agric. 2019, 18, 2205–2218. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. Br. Mol. Biol. J. 2016, 355, i5796. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, W.; Carpio, C.; Morales, D.; Vilcacundo, E.; Álvarez, M.; Silva, M. Content of fatty acids in corn (Zea mays L.) oil from Ecuador. Asian J. Pharm. Clin. Res. 2017, 10, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Moreau, R.A.; Liu, K.; Winkler-Moser, J.K.; Singh, V. Changes in lipid composition during dry grind ethanol processing of corn. J. Am. Oil Chem. Soc. 2011, 88, 435–442. [Google Scholar] [CrossRef]
- Carrin, M.E.; Carelli, A.A. Peanut oil: Compositional data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Idrissi, Z.L.E.; El Moudden, H.; Mghazli, N.; Bouyahya, A.; Guezzane, C.E.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Harhar, H.; et al. Effects of extraction methods on the bioactivities and nutritional value of virginia and valencia-type peanut oil. Molecules 2022, 27, 7709. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.C.; Huang, Y.Z.; Pegg, R.B.; Phillips, R.D.; Eitenmiller, R.R. Commercial runner peanut cultivars in the United States: Tocopherol composition. J. Agric. Food Chem. 2009, 57, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro, C.; Haros, C.M.; Pérez-Alvarez, J.A. Chia (Salvia hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Sci. 2019, 156, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Oteri, M.; Bartolomeo, G.; Rigano, F.; Aspromonte, J.; Trovato, E.; Purcaro, G.; Dugo, P.; Mondello, L.; Beccaria, M. Comprehensive chemical characterization of chia (Salvia hispanica L.) seed oil with a focus on minor lipid components. Foods 2023, 12, 23. [Google Scholar] [CrossRef]
- Marzocchi, S.; Caboni, M.F. Effect of harvesting time on hemp (Cannabis sativa L.) seed oil lipid composition. Ital. J. Food Sci. 2020, 32, 1018–1029. [Google Scholar] [CrossRef]
- Siudem, P.; Wawer, I.; Paradowska, K. Rapid evaluation of edible hemp oil quality using NMR and FT-IR spectroscopy. J. Mol. Struct. 2019, 1177, 204–208. [Google Scholar] [CrossRef]
- Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date seeds: A promising source of oil with functional properties. Foods 2020, 9, 787. [Google Scholar] [CrossRef]
- Espínola, F.; Vidal, A.M.; Espínola, J.M.; Moya, M. Processing effect and characterization of olive oils from Spanish wild olive trees (Olea europaea var. sylvestris). Molecules 2021, 26, 1304. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Suryani, S.; Sariani, S.; Earnestly, F.; Marganof, M.; Rahmawati, R.; Sevindrajuta, S.; Mahlia, T.M.I.; Fudholi, A. A Comparative study of virgin coconut oil, coconut oil and palm oil in terms of their active ingredients. Processes 2020, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Davenport, A. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Caf, F.; Şen Özdemir, N.; Yılmaz, Ö.; Durucan, F.; Ak, İ. Fatty acid and lipophilic vitamin composition of seaweeds from Antalya and Çanakkale (Turkey). Grasas Aceites 2019, 70, e312. Available online: https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/1783 (accessed on 25 February 2023). [CrossRef]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbo, R.; Lock, E.-J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.K. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Nitesh, C.; Himanshu, V.; Roshan, D. Fish Oil Market by Species (Anchovy, Mackerel, Sardines, Cod, Herring, Menhaden and Others), and (Aquaculture, Animal Nutrition & Pet Food, Pharmaceuticals, Supplements & Functional Food and Others): Global Opportunity Analysis and Industry Forecast, 2021-2027. 2020. Available online: https://www.alliedmarketresearch.com/fish-oil-market (accessed on 2 December 2022).
- Cardona, L.; Martínez-Iñigo, L.; Mateo, R.; González-Solís, J. The role of sardine as prey for pelagic predators in the western Mediterranean Sea assessed using stable isotopes and fatty acids. Mar. Ecol. Prog. Ser. 2015, 531, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lorrette, B.; Sánchez, L. New lipid sources in the insect industry, regulatory aspects and applications. OCL 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Caparros Megido, R. Insect fatty acids: A comparison of lipids from three orthopterans and Tenebrio molitor L. larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flav. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and protein recovery from corn germ: Extraction yield, composition and protein functionality. Food Bioprod. Proces. 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Li, Q.; Tang, X.; Lu, S.; Wu, J. Composition and tocopherol, fatty acid, and phytosterol contents in micro-endosperm ultra-high oil corn. Grasas Aceites 2019, 70, e311. Available online: https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/1782 (accessed on 16 February 2023). [CrossRef] [Green Version]
- Bilal, M.; Shabbir, M.A.; Xiaobo, Z.; Arslan, M.; Usman, M.; Azam, M.; Aadil, R.M.; Ahmad, N. Characterization of peanut seed oil of selected varieties and its application in the cereal-based product. J. Food Sci. Technol. 2020, 57, 4044–4053. [Google Scholar] [CrossRef]
- Bonku, R.; Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Well. 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Toomer, O.T. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Masoodi, L.; Gull, A.; Masoodi, F.A.; Gani, A.; Nissar, J.; Ahad, T.; Nayik, G.A.; Mukarram, S.A.; Kovács, B.; Prokisch, J.; et al. An Overview on traditional vs. green technology of extraction methods for producing high quality walnut oil. Agronomy 2022, 12, 2258. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Martín, D.; Gallo, S.; Fuente, G.; Bodoira, R.; Marcela Martínez, M.; Maestri, D. Evaluation of hazelnut and walnut oil chemical traits from conventional cultivars and native genetic resources in a non-traditional crop environment from Argentina. Eur. Food Res. Technol. 2020, 246, 833–843. [Google Scholar] [CrossRef]
- Elouafy, Y.; El Yadini, A.; El Moudden, H.; Harhar, H.; Alshahrani, M.M.; Awadh, A.A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Tabyaoui, M. Influence of the extraction method on the quality and chemical composition of walnut (Juglans regia L.) Oil. Molecules 2022, 27, 7681. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Key chemical composition of walnut (Juglans regia. L) Oils generated with different processing methods and their cholesterol-lowering effects in HepG2 cells. Food Biosci. 2022, 45, 101436. [Google Scholar] [CrossRef]
- Mitsikaris, P.D.; Kokokiris, L.; Pritsa, A.; Papadopoulos, A.N.; Kalogiouri, N.P. Investigating the tocopherol contents of walnut seed oils produced in different European countries analyzed by HPLC-UV: A comparative study on the basis of geographical origin. Foods 2022, 11, 3719. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tébar, N.; De la Vara, J.A.; Ortiz de Elguea-Culebras, G.; Cano, E.L.; Berruga, M.I. Enrichment of sheep cheese with chia (Salvia hispanica L.) oil as a source of omega-3. LWT-Food Sci. Technol. 2019, 108, 407–415. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion of the panel on the dietetic products, nutrition and allergies on a request from European Commission related to labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J. 2009, 1176, 1–11. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/1176 (accessed on 12 January 2023).
- Wang, X.; Han, Y.; Li, Y.; Wang, S.; Wang, J. Detection of Qinghai-Tibet Plateau flaxseed oil adulteration based on fatty acid profiles and chemometrics. Food Control 2021, 130, 108332. [Google Scholar] [CrossRef]
- Romanić, R.S.; Lužaić, T.Z.; Radić, B.D. Enriched sunflower oil with omega 3 fatty acids from flaxseed oil: Prediction of the nutritive characteristics. LWT-Food Sci. Technol. 2021, 150, 112064. [Google Scholar] [CrossRef]
- Laghouiter, O.K.; Benalia, M.; Gourine, N.; Djeridane, A.; Bombarda, I.; Yousfi, M. Chemical characterization and in vitro antioxidant capacity of nine Algerian date palm cultivars (Phoenix dactylifera L.) seed oil. Mediterr. J. Nutr. Metab. 2018, 11, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado oil: Characteristics, properties, and applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef] [Green Version]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Durmus, M. Fish oil for human health: Omega-3 fatty acid profiles of marine seafood species. Food Sci. Technol. 2019, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Heck, R.T.; Lorenzo, J.M.; dos Santos, B.A.; Cichoski, A.J.; de Menezes, C.R.; Campagnol, P.C.B. Microencapsulation of Healthier Oils: An Efficient Strategy to Improve the Lipid Profile of Meat Products. Curr. Opin. Food Sci. 2021, 40, 6–12. [Google Scholar] [CrossRef]
- Bolger, Z.; Brunton, N.P.; Monahan, F.J. Impact of inclusion of flaxseed oil (pre-emulsified or encapsulated) on the physical characteristics of chicken sausages. J. Food Eng. 2018, 230, 39–48. [Google Scholar] [CrossRef]
- Heck, R.T.; Saldaña, E.; Lorenzo, J.M.; Pereira Correa, L.; Bittencourt Fagundes, M.; Cichoski, A.J.; Ragagnin de Menezes, C.; Wagner, R.; Campagnol, P.C.B. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Urgu-Öztürk, M.; Öztürk-Kerimoğlu, B.; Serdaroğlu, M. Design of healthier beef sausage formulations by hazelnut-based pre-emulsion systems as fat substitutes. Meat Sci. 2020, 167, 108162. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Viuda-Martos, M.; Fernández-López, J.A.; Pérez-Alvarez, J.A.; Fernández-López, J. Development of plant-based burgers using gelled emulsions as fat source and beetroot juice as colorant: Effects on chemical, physicochemical, appearance and sensory characteristics. LWT-Food Sci. Technol. 2022, 172, 114193. [Google Scholar] [CrossRef]
- Dent, T.; Hallinan, R.; Chitchumroonchokchai, C.; Maleky, F. Rice Bran Wax Structured Oleogels and in Vitro Bioaccessibility of Curcumin. J. Am. Oil Chem. Soc. 2022, 99, 299–311. [Google Scholar] [CrossRef]
- Khan, M.A.; Bao, H.; Cheng, H.; Feng, S.; Wang, Y.; Liang, L. Fabrication of Whey-Protein-Stabilized G/O/W Emulsion for the Encapsulation and Retention of -Ascorbic Acid and α-Tocopherol. J. Food Eng. 2023, 341, 111335. [Google Scholar] [CrossRef]
- Espert, M.; Salvador, A.; Sanz, T.; Hernández, M.J. Cellulose ether emulsions as fat source in cocoa creams: Thermorheological properties (flow and viscoelasticity). LWT-Food Sci. Technol. 2020, 117, 108640. [Google Scholar] [CrossRef]
- Asuming-Bediako, N.; Jaspal, M.H.; Hallett, K.; Bayntun, J.; Baker, A.; Sheard, P.R. Effects of replacing pork backfat with emulsified vegetable oil on fatty acid composition and quality of UK-style sausages. Meat Sci. 2014, 96, 187–194. [Google Scholar] [CrossRef]
- Yıldız-Turp, G.; Serdaroğlu, M. Effect of replacing beef fat with hazelnut oil on quality characteristics of sucuk–A Turkish fermented sausage. Meat Sci. 2008, 78, 447–454. [Google Scholar] [CrossRef]
- Cheetangdee, N. Effect of partial replacement of porcine fat with pre-emulsified soybean oil using fish protein isolate as emulsifier on characteristic of sausage. J. Food Sci. Technol. 2017, 54, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pâté. LWT-Food Sci. Technol. 2020, 125, 109223. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Agregán, R.; Lorenzo, J.M. Effect of the partial replacement of pork backfat by microencapsulated fish oil or mixed fish and olive oil on the quality of frankfurter type sausage. J. Food Sci. Technol. 2017, 54, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heck, R.T.; Lucas, B.N.; Dos Santos, D.J.P.; Pinton, M.B.; Fagundes, M.B.; Etchepare, M.A.; Cichoski, A.J.; Menezes, C.R.; Barin, J.S.; Wagner, R.; et al. Oxidative stability of burgers containing chia oil microparticles enriched with rosemary by green–extraction techniques. Meat Sci. 2018, 146, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez–Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese burgers with omega-3 fatty acids. Effect of type of addition and storage conditions on quality characteristics. Grasas Aceites 2018, 69, 235. Available online: https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/1702 (accessed on 12 January 2023). [CrossRef] [Green Version]
- Alejandre, M.; Poyato, C.; Ansorena, D.; Astiasarán, I. Linseed oil gelled emulsion: A successful fat replacer in dry fermented sausages. Meat Sci. 2016, 121, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Munekata, P.E.S.; Gagaoua, M.; Franco, D.; Campagnol, P.C.B.; Pateiro, M.; da Barretto, A.C.S.; Domínguez, R.; Lorenzo, J.M. Inclusion of Healthy Oils for Improving the Nutritional Characteristics of Dry-Fermented Deer Sausage. Foods 2020, 9, 1487. [Google Scholar] [CrossRef]
- Hanula, M.; Szpicer, A.; Górska-Horczyczak, E.; Khachatryan, G.; Pogorzelska-Nowicka, E.; Poltorak, A. Quality of Beef Burgers Formulated with Fat Substitute in a Form of Freeze-Dried Hydrogel Enriched with Açai Oil. Molecules 2022, 27, 3700. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Zulim Botega, D.C.; Marangoni, A.G.; Smith, A.K.; Go, H.D. The potential application of rice bran wax oleogel to replace solid fat and enhance unsaturated fat content in ice cream. J. Food Sci. 2013, 78, C1334–C1339. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Vicente, A.A.; Cunha, R.L.; Pastrana, L.M.; Quiñones, J.; Cerqueira, M.A. Omega-3 and Polyunsaturated Fatty Acids-Enriched Hamburgers Using Sterol-Based Oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1900111. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.S.; Lee, S. Utilization of oleogels as a replacement for solid fat in aerated baked goods: Physicochemical, rheological, and tomographic characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.A.L.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of replacing backfat with vegetable oils during the shelf-life of cooked lamb sausages. LWT-Food Sci. Technol. 2020, 122, 109052. [Google Scholar] [CrossRef]
- Li, K.; Zhang, M.; Bhandari, B.; Li, L.; Yang, C. Effect of pre-emulsified soybean oil as a fat replacer on the physical and sensory attributes of reduced-fat filling in steamed buns. J. Food Eng. 2020, 43, e13306. [Google Scholar] [CrossRef]
- Santos Lima, T.L.; Freire da Costa, G.; da Silva Araújo, I.B.; Beltrão da Cruz, G.R.; Ribeiro, N.L.; Beltrão Filho, E.M.; Domínguez, R.; Lorenzo, J.M. Pre-emulsioned linseed oil as animal fat replacement in sheep meat sausages: Microstructure and physicochemical properties. J. Food Process. Preserv. 2021, 45, e15051. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Imran, M.; Khan, M.K.; Mushtaq, Z.; Asif, M.; Din, A. Effect of Microcapsules of Chia Oil on Ω-3 Fatty Acids, Antioxidant Characteristics and Oxidative Stability of Butter. Lipids Health Dis. 2020, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, G.; Franco, P.; de Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Alemzadeh, I.; Hajiabbas, M.; Pakzad, H.; Dehkordi, S.S.; Vossoughi, A. Encapsulation of food components and bioactive ingredients and targeted release. Int. J. Eng. 2020, 33, 1. [Google Scholar] [CrossRef] [Green Version]
- Ojagh, S.M.; Hasani, S. Characteristics and Oxidative Stability of Fish Oil Nano-Liposomes and Its Application in Functional Bread. J. Food Meas. Charact. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Evaluating the use of fish oil microcapsules as omega-3 vehicle in cooked and dry-cured sausages as affected by their processing, storage and cooking. Meat Sci. 2020, 162, 108031. [Google Scholar] [CrossRef] [PubMed]
- Venturini, L.H.; Moreira, T.F.M.; da Silva, T.B.V.; de Almeida, M.M.C.; Francisco, C.R.L.; de Oliveira, A.; de Campos, S.S.; Bilck, A.P.; de Souza Leone, R.; Tanamati, A.A.C.; et al. Partial Substitution of Margarine by Microencapsulated Chia Seeds Oil in the Formulation of Cookies. Food Bioproc. Tech. 2019, 12, 77–87. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Heydari, S.; Raeisi, S. encapsulation of fish oil by carrageenan and gum tragacanth as wall materials and its application to the enrichment of chicken nuggets. LWT 2021, 137, 110334. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Guo, J.; Cui, L.; Meng, Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: A review. Food Hidrocoll. 2023, 137, 108313. [Google Scholar] [CrossRef]
- Nasirpour-Tabrizi, P.; Azadmard-Damirchi, S.; Hesari, J.; Khakbaz Heshmati, M.; Savage, G.P. Rheological and Physicochemical Properties of Novel Low-Fat Emulgels Containing Flaxseed Oil as a Rich Source of ω-3 Fatty Acids. LWT-Food Sci. Technol. 2020, 133, 110107. [Google Scholar] [CrossRef]
- Domínguez, R.; Bohrer, B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Recent discoveries in the field of lipid bio-based ingredients for meat processing. Molecules 2021, 26, 190. [Google Scholar] [CrossRef]
- De Souza Paglarini, C.; Vidal, V.A.; Ribeiro, W.; Badan Ribeiro, A.P.; Bernardinelli, O.D.; Herrero, A.M.; Ruiz-Capillas, C.; Sabadini, E.; Pollonio, M.A. Using Inulin-based Emulsion Gels as Fat Substitute in Salt Reduced Bologna Sausage. J. Sci. Food Agric. 2021, 101, 505–517. [Google Scholar] [CrossRef]
- Öztürk-Kerimoğlu, B.; Kavuşan, H.S.; Benzer Gürel, D.; Çağındı, Ö.; Serdaroğlu, M. Cold-Set or Hot-Set Emulsion Gels Consisted of a Healthy Oil Blend to Replace Beef Fat in Heat-Treated Fermented Sausages. Meat Sci. 2021, 176, 108461. [Google Scholar] [CrossRef]
- Lee, J.; Wi, G.; Choi, M.-J. The Rheological Properties and Stability of Gelled Emulsions Applying to κ-Carrageenan and Methyl Cellulose as an Animal Fat Replacement. Food Hydrocoll. 2023, 136, 108243. [Google Scholar] [CrossRef]
- Okuro, P.K.; Martins, A.J.; Vicente, A.A.; Cunha, R.L. Perspective on oleogelator mixtures, structure design and behaviour towards digestibility of oleogels. Cur. Op. Food Sci. 2020, 35, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.; Wright, A.J.; Marangoni, A.G. Oil organogels: The fat of the future? Soft Matter 2009, 5, 1594–1596. [Google Scholar] [CrossRef]
- Malvano, F.; Laudisio, M.; Albanese, D.; d’Amore, M.; Marra, F. Olive oil-based oleogel as fat replacer in a sponge cake: A comparative study and optimization. Foods 2022, 11, 2643. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Anderson, J.; Byars, J.A.; Singh, M.; Hwang, H.S. Evaluation of beeswax, candelilla wax, rice bran wax, and sunflower wax as alternative stabilizers for peanut butter. J. Am. Oil Chem. Soc. 2019, 96, 1235–1248. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.D.; Sacchetti, G.; Flamminii, F.; Gravelle, A.J.; Baylis, B.; Dutcher, J.R.; Marangoni, A.G.; Pittia, P. Ethylcellulose oleogels with extra virgin olive oil: The role of oil minor components on microstructure and mechanical strength. Food Hydrocoll. 2018, 84, 508–514. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A review of current and potential applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Sowiński, M.; Szymańska, I.; Żbikowska, K.; Marciniak-Łukasiak, K.; Werpachowski, W. Analysis of stability, rheological and structural properties of oleogels obtained from peanut oil structured with yellow beeswax. Gels 2022, 8, 448. [Google Scholar] [CrossRef]
- Pintado, T.; Cofrades, S. Quality Characteristics of Healthy Dry Fermented Sausages Formulated with a Mixture of Olive and Chia Oil Structured in Oleogel or Emulsion Gel as Animal Fat Replacer. Foods 2020, 9, 830. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Martins, A.J.; Fuciños, P.; Cerqueira, M.A.; Pastrana, L.M. Food additive manufacturing with lipid-based inks: Evaluation of phytosterol-lecithin oleogels. J. Food Eng. 2023, 341, 111317. [Google Scholar] [CrossRef]
- Wang, W.; Sun, R.; Dong, Z.; Ji, S.; Xia, Q. Preparation of a stable gel-in-crystallized oil-in-gel type structured W 1 /O/W 2 double emulsions: Effect of internal aqueous phase gelation on the system stability. J. Dispers. Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Gullón, B.; Campagnol, P.C.B.; Franco, D. Novel strategy for developing healthy meat products replacing saturated fat with oleogels. Curr. Opin. Food Sci. 2021, 40, 40–45. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Pérez-Álvarez, J.Á.; Sayas-Barberá, E.; Fernández-López, J.; Viuda-Martos, M. Assessment of chemical, physicochemical, and lipid stability properties of gelled emulsions elaborated with different oils chia (Salvia hispanica L.) or hemp (Cannabis sativa L.) and pseudocereals. Foods 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Momchilova, M.; Gradinarska-Ivanova, D.; Petrova, T.; Yordanov, D. Influence of emulsions of vegetable oils as fat substitutes on the colour and sensory quality of cooked sausages during storage. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1052. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive compounds in functional meat products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thøgersen, R.; Bertram, H.C. Reformulation of processed meat to attenuate potential harmful effects in the gastrointestinal tract–A review of current knowledge and evidence of health prospects. Trends Food Sci. Technol. 2021, 108, 111–118. [Google Scholar] [CrossRef]
- De Souza Paglarini, C.; Vidal, V.A.; Martini, S.; Cunha, R.L.; Pollonio, M.A.R. Protein-based hydrogelled emulsions and their application as fat replacers in meat products: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 640–655. [Google Scholar] [CrossRef]
- Botez, E.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D.; Ghinea, I.O. Meat product reformulation: Nutritional benefits and effects on human health. In Functional Food-Improve Health through Adequate Food; Chavarri Hueda, M., Ed.; TechOpen: London, UK, 2017; pp. 167–184. [Google Scholar]
- Botella-Martínez, C.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Fernández-López, J. Total and partial fat replacement by gelled emulsion (hemp oil and buckwheat flour) and its impact on the chemical, technological and sensory properties of Frankfurters. Foods 2021, 10, 1681. [Google Scholar] [CrossRef]
- Nieto, G.; Lorenzo, J.M. Use of olive oil as fat replacer in meat emulsions. Curr. Opin. Food Sci. 2021, 40, 179–186. [Google Scholar] [CrossRef]
- Ferro, A.C.; de Souza Paglarini, C.; Rodrigues Pollonio, M.A.; Lopes Cunha, R. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef]
- Lucas-González, R.; Roldán-Verdu, A.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of emulsion gels formulated with chestnut (Castanea sativa M.) flour and chia (Salvia hispanica L.) oil as partial fat replacers in pork burger formulation. J. Sci. Food Agric. 2020, 100, 1265–1273. [Google Scholar] [CrossRef]
- Carvalho-Barros, J.; Munekata, P.E.S.; de Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100–105. [Google Scholar] [CrossRef]
- Heck, R.T.; Vendruscolo, R.G.; de Araújo Etchepare, M.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Is it possible to produce a low-fat burger with a healthy n-6/n-3 PUFA ratio without affecting the technological and sensory properties? Meat Sci. 2017, 130, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.T.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Volatile compounds and sensory profile of burgers with 50% fat replacement by microparticles of chia oil enriched with rosemary. Meat Sci. 2019, 148, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Cavalheiro, C.P.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef]
- De Souza Paglarini, C.; Martini, S.; Pollonio, M.A.R. Using emulsion gels made with sonicated soy protein isolate dispersions to replace fat in frankfurters. LWT Food Sci. Technol. 2019, 99, 453–459. [Google Scholar] [CrossRef]
- Nacak, B.; Öztürk-Kerimoglu, B.; Yildiz, D.; Çagindi, Ö.; Serdaroglu, M. Peanut and linseed oil emulsion gels as potential fat replacer in emulsified sausages. Meat Sci. 2021, 176, 108464. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Cerqueira, M.A.; Purriños, L.; Pastrana, L.M.; Vicente, A.A.; Lorenzo, J.M. Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages. J. Sci. Food Agric. 2020, 100, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Glisic, M.; Baltic, M.; Glisic, M.; Trbovic, D.; Jokanovic, M.; Parunovic, N.; Dimitrijevic, M.; Suvajdzic, B.; Boskovic, M.; Vasilev, D. Inulin-based emulsion-filled gel as a fat replacer in prebiotic-and PUFA-enriched dry fermented sausages. Int. J. Food Sci. Technol. 2019, 54, 787–797. [Google Scholar] [CrossRef]
- Stajić, S.; Živković, D.; Tomović, V.; Nedović, V.; Perunović, M.; Kovjanić, N.; Lević, S.; Stanišić, N. The utilisation of grapeseed oil in improving the quality of dry fermented sausages. Int. J. Food Sci. Technol. 2014, 49, 2356–2363. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Herrero, A.M. New lipid materials based on chia emulsion gels: Application in meat products. J. Biomed. Sci. 2019, 18, 13215–13218. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Sayas-Barberá, E.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Chia and hemp oils-based gelled emulsions as replacers of pork backfat in burgers: Effect on lipid profile, technological attributes and oxidation stability during frozen storage. Int. J. Food Sci. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica, L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–78. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Physicochemical effects of the lipid phase and protein level on meat emulsion stability, texture, and microstructure. J. Food Sci. 2010, 75, S108–S114. [Google Scholar] [CrossRef]
- Öztürk-Kerimoğlu, B.; Urgu-Öztürk, M.; Serdaroglu, M. A new inverse olive oil emulsion plus carrot powder to replace animal fat in model meat batters. LWT-Food Sci. Technol. 2021, 135, 110044. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R. Healthy Spanish salchichón enriched with encapsulated n− 3 long chain fatty acids in konjac glucomannan matrix. Food Res. Int. 2016, 89, 289–295. [Google Scholar] [CrossRef]
- Fonseca, S.; Gómez, M.; Domínguez, R.; Lorenzo, J.M. Physicochemical and sensory properties of Celta dry-ripened “salchichón” as affected by fat content. Grasas Aceites 2015, 66, e059. Available online: https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/1524 (accessed on 18 February 2023).
- Munekata, P.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Triki, M.; Herrero, A.M.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Healthy oil combination stabilized in a konjac matrix as pork fat replacement in low-fat, PUFA-enriched, dry fermented sausages. LWT Food Sci. Technol. 2013, 51, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Navarro-Rodríguez de Vera, C.; Fernández-López, M.; Viuda-Martos, M.; Fernández-López, J. Sustainability and gender perspective in food innovation: Foods and food processing coproducts as source of macro- and micro-nutrients for woman-fortified foods. Foods 2022, 11, 3661. [Google Scholar] [CrossRef] [PubMed]
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients. Food Res Int. 2022, 157, 111212. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, K.; Ansorena, D.; Astiasarán, I. Use of hydrocolloids and vegetable oils for the formulation of a butter replacer: Optimization and oxidative stability. LWT-Food Sci. Technol. 2022, 153, 112538. [Google Scholar] [CrossRef]
- Kamath, R.; Basak, S.; Gokhale, J. Recent trends in the development of healthy and functional cheese analogues—A review. LWT-Food Sci. Technol. 2022, 155, 112991. [Google Scholar] [CrossRef]
- Varela, C.; Aghababaei, F.; Cano-Sarabia, M.; Turitich, L.; Trujillo, A.J.; Ferragut, V. Characterization and oxidation stability of spray-dried emulsions with omega-3 oil and buttermilk processed by ultra-high-pressure homogenization (UHPH). LWT-Food Sci. Technol. 2022, 162, 113493. [Google Scholar] [CrossRef]
- Hamed, A.M.; Aborass, M.; El-Kafrawy, I.; Safwat, G. Comparative study for the detection of Egyptian buffalo butter adulteration with vegetable oils using conventional and advanced methods. J. Food Safe. 2019, 39, e12655. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Shirdeli, M.; Hosseini, S.S. Detection of vegetable oils in dairy products using the polymerase chain reaction technique. J. Com. Health Res. 2019, 8, 1–2. [Google Scholar] [CrossRef]
- Pirsa, S.; Banafshechin, E.; Amiri, S.; Rahimirad, A.; Ghafarzadeh, J. Detection of fraud of palm, sunflower (Helianthus L.), and corn oil in butter using HPLC profile of tocopherols and tocotrienols by response surface method. J. Iran. Chem. Soc. 2021, 18, 1167–1177. [Google Scholar] [CrossRef]
- Garbin Cardoso, L.; Bordignon, I.J., Jr.; Vieira da Silva, R.; Mossmann, J.; Oliveira Reinehr, C.; Barbosa Brião, V.; Colla, L.M. Processed cheese with inulin and microencapsulated chia (Salvia hispanica) oil. Food Biosci. 2020, 37, 100731. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Imran, M.; Taj Khan, I.; Shahbaz, M.; Mahmud, A.; Tayyab Kavak, D.D.; Karabiyik, H. Omega fatty acids, phenolic compounds, and lipolysis of cheddar cheese supplemented with Chia (Salvia hispanica L.) oil. J. Food Process. Preserv. 2018, 42, e13566. [Google Scholar] [CrossRef]
- Gurdian, C.; Reyes, V.; Kyereh, E.; Bonilla, F.; Galindo, C.; Chouljenko, A.; Mis Solval, K.; Boeneke, C.; King, J.M.; Sathivel, S. Incorporating flaxseed (Linum usitatissimum) oil into queso blanco at different stages of the cheese manufacturing process. J. Food. Process. Preserv. 2017, 41, e13279. [Google Scholar] [CrossRef]
- Izadi, Z.; Nasirpour, A.; Garoosi, G.A.; Tamjidi, F. Rheological and physical properties of yoghurt enriched with phytosterol during storage. J. Food Sci. Technol. 2015, 52, 5341–5346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, H.S.; Youssef, K.; Hashim, A.F. Stirred yoghurt as a delivery matrix for freeze-dried microcapsules of synbiotic evoo nanoemulsion and nanocomposite. Front. Microbiol. 2022, 13, 893053. [Google Scholar] [CrossRef] [PubMed]
- Carvalho da Silva, L.; Menezes Castelo, R.; Rodrigues Magalhães, H.C.; Ferro Furtado, R.; Cheng, N.; Biswas, A.; Alves, C.R. Characterization and controlled release of pequi oil microcapsules for yoghurt application. LWT-Food Sci. Technol. 2022, 157, 113105. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Jia, Y.; Yuan, Y.; Li, H.; Cui, W.; Yu, J. Application of whey protein emulsion gel microparticles as fat replacers in low-fat yoghurt: Applicability of vegetable oil as the oil phase. J. Dairy Sci. 2022, 105, 9404–9416. [Google Scholar] [CrossRef]
- Almasi, K.; Esnaashari, S.S.; Khosravani, M.; Adabi, M. Yoghurt fortified with omega-3 using nanoemulsion containing flaxseed oil: Investigation of physicochemical properties. Food Sci. Nutr. 2021, 9, 6186–6193. [Google Scholar] [CrossRef]
- Genovese, A.; Balivo, A.; Salvati, A.; Sacchi, R. Functional ice cream health benefits and sensory implications. Food Res Int. 2022, 161, 111858. [Google Scholar] [CrossRef]
- Akca, S.; Akpinar, A. The effects of grape, pomegranate, sesame seed powder and their oils on probiotic ice cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Paul, V.; Kumar, A.; Rai, D.C.; Pandhi, S.; Seth, A. Development of functional ice cream using basil oil microcapsules. Indian J. Dairy Sci. 2020, 73, 542–548. [Google Scholar] [CrossRef]
- Sacchi, R.; Caporaso, N.; Squadrilli, G.A.; Paduano, A.; Ambrosino, M.L.; Cavella, S.; Genovese, A. Sensory profile, biophenolic and volatile compounds of an artisanal ice cream (‘gelato’) functionalised using extra virgin olive oil. Int. J. Gastro Food Sci. 2019, 18, 100173. [Google Scholar] [CrossRef]
- Güven, M.; Kalender, M.; Taşpinar, T. Effect of using different kinds and ratios of vegetable oils on ice cream quality characteristics. Foods 2018, 7, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achachlouei, B.F.; Hesari, J.; Damirchi, S.A.; Peighambardoust, S.; Esmaiili, M.; Alijani, S. Production and characterization of a functional Iranian white brined cheese by replacement of dairy fat with vegetable oils. Food Sci. Technol. Int. 2013, 19, 389–398. [Google Scholar] [CrossRef]
- Giha, V.; Ordoñez, M.J.; Villamil, R.A. How does milk fat replacement influence cheese analogue microstructure, rheology, and texture profile? J. Food Sci. 2021, 86, 2802–2815. [Google Scholar] [CrossRef]
- Villamil, R.A.; Guzmán, M.P.; Ojeda-Arredondo, M.; Cortés, L.Y.; Gil Archila, E.; Giraldo, A.; Mondragón, A.I. Cheese fortification through the incorporation of UFA-rich sources: A review of recent (2010–2020) evidence. Heliyon 2021, 7, e05785. [Google Scholar] [CrossRef]
- Yu, L.; Hammond, E.G. The modification and analysis of vegetable oil for cheese making. J. Am. Oil Chem. Soc. 2000, 77, 911–916. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Quality of selected cheeses fortified with vegetable and animal sources of omega-3. LWT-Food Sci. Technol. 2011, 44, 1577–1584. [Google Scholar] [CrossRef]
- Sulejmani, E.; Beqiri, L.; Popeski-Dimovski, R. Effect of vegetable fat on the texture, colour and sensory properties of Macedonian white brined cheese. Mljekarstvo Dairy 2020, 71, 25–34. [Google Scholar] [CrossRef]
- Cumhur, O.; Kilic-Akyilmaz, M. Special processed cheeses, cheese spreads, and analogue cheeses. In Processed Cheese Science and Technology; El-Bakry, M., Mehta, B.M., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 269–295. [Google Scholar] [CrossRef]
- Valencia, E.; García-Pérez, M.; Garnica Romo, M.G.; Figueroa, J.; Paciulli, M.; Martinez-Flores, H. Chemical composition, physicochemical evaluation and sensory analysis of yoghurt added with extract of polyphenolic compounds from Quercus crassifolia oak bark. Funct. Foods Health Dis. 2022, 12, 502. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, P.; Wu, F.; He, W.; Mao, L.; Jia, W.; Zhang, Y.; Chen, X.; Jiao, J. Cooking oil/fat consumption and deaths from cardiometabolic diseases and other causes: Prospective analysis of 521,120 individuals. BMC Med. 2021, 19, 92. [Google Scholar] [CrossRef] [PubMed]

| Oils from Oilseeds | |||||||
|---|---|---|---|---|---|---|---|
| Saturated Fatty Acids | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | Ref. | ||||
| Oleic Acid | Other | Linoleic Acid (w-6) | Linolenic Acid (w-3) | Others | |||
| Soybean | 15.6–16.2 | 21.4–22.6 | 0.2–1.0 | 52.0–54.5 | 10.0–12.8 | 0.8–1.2 | [29,30] |
| Canola | 7.4–8.2 | 61.8–63.5 | 1.5–2.4 | 18.6–20.2 | 9.1–10.4 | 0.4–0.7 | [31,32] |
| Sunflower | 15.5–17.0 | 22.4–23.3 | 0.2–0.8 | 55.0–57.8 | 3.6–4.2 | 0.9–1.4 | [33,34] |
| Cottonseed | 25.9–26.8 | 17.8–18.6 | 1.2–1.8 | 52.1–54.0 | 0.6–1.0 | 0.1–0.5 | [35,36] |
| Corn | 15.6–17.0 | 25.3–26.7 | 0.3–0.5 | 52.0–53.4 | 1.0–1.2 | 4.4–4.9 | [37,38] |
| Peanut | 13.5–15.4 | 53.0–54.2 | 0.3–0.6 | 29.8–32.0 | 0.2–0.4 | 0.8–1.0 | [39,40,41] |
| Chia | 8.0–8.5 | 4.0–4.8 | --- | 20.0–22.7 | 68.0–69.5 | --- | [42,43] |
| Hemp | 7.0–7.8 | 8.3–9.0 | --- | 52.6–54.0 | 21.7–22.0 | 8.0–8.6 | [44,45] |
| Date | 44.5–47.0 | 42.6–45.0 | --- | 8.0–8.6 | n.d. | n.d. | [46] |
| Oils from fruits | |||||||
| Olive | 11.1–12.7 | 72.3–78.6 | 1.0–1.5 | 8.8–9.1 | 0.7–0.9 | 5.1–5.5 | [31,47,48] |
| Coconut | 80.0–82.5 | 6.0–7.8 | 5.0–6.3 | --- | --- | 1.7–2.1 | [49,50] |
| Avocado | 9.60–12.5 | 58.6–61.5 | 8.7–10.5 | 9.0–12.5 | 1.5–2.4 | 1.5–1.9 | [31,51] |
| Oils from marine origin | |||||||
| Seaweed | 50 | 11.2 | 11.2–12.0 | 5.0–5.8 | 1.5–1.8 | 26.5–28.0 | [52,53,54] |
| Fish (Sardina pilchardus) | 32 | 25 | 7.2–7.8 | 3.5–4.2 | 1.0–1.2 | 29.5–31.3 | [55,56] |
| Oils from Insects | |||||||
| Tenebrio mollitor | 33.4 | 35.8 | 2.1 | 22.8 | 0.1 | 5.8 | [57] |
| Acheta domesticus | 31.2 | 20.2 | 0.8 | 41.4 | 1.1 | 5.3 | [58] |
| Structure Components | Structuring Strategy | Procedure | Application | Ref. |
|---|---|---|---|---|
| Pre-emulsification | First, the cellulose ether was dispersed in the oil and then the water was gradually added. Finally, the mixture was homogenized until the emulsion was obtained. | Cocoa creams | [85] |
| Pre-emulsification | Initially, soy protein was added into the water and mixed. Then, the oil was added gradually in a bowl chopper fitted with three blades and operating at two speeds. | UK-style sausages | [86] |
| Pre-emulsification | The whey protein was mixed with hazelnut oil with a hand blender until the emulsion was obtained. | Sucuk Turkish fermented sausages | [87] |
| Pre-emulsification | Fish protein isolate was dissolved in phosphate buffer (10 mM, pH 7). The solution was mixed with soybean oil, in equal amounts (oil volume fraction 0.5), using the homogenizer at 19,000 rpm for 5 min. | Pork sausages | [88] |
| Pre-emulsification | Oil and maltodextrin were mixed using a spoon and kept at 4 °C for 24 h. | Fresh chorizo | [23] |
| Encapsulation | Dry spryer conditions were: feed rate 30%, inlet temperature 145 °C, and the aspirator at 80%. | Deer pâté | [89] |
| Encapsulation | Dry spryer conditions were: feed rate 75 L/h, inlet temperature 180 °C, and outlet temperature 80 °C. Drying process lasted 3 h. | Frankfurters | [90] |
| Encapsulation | External ionic gelation technique. | Pork burgers | [91] |
| Encapsulation | Dry spryer conditions were: feed rate 1 L/h, inlet temperature 180 °C, and outlet temperature ranged 85–90 °C. | Pork burgers | [92] |
| Gelled emulsion | The oil phase with the surfactant was added to the aqueous phase (water + carrageenan) and homogenized. | Dry fermented sausages | [93] |
| Gelled emulsion | Water and olive oil were mixed for 1 min. Then, gelling agent was addedand homogenized during 3 min and then left to rest for 2 h. After that the mixture was cooled at 4 °C. | Deer fermented sausages | [94] |
| Gelled emulsion | Sodium alginate and konjac flour were dissolved in water at 60 °C with constant stirring. The emulsion (2:1 oil:water) was homogenized in the biocomposite obtained. | Beef burgers | [95] |
| Gelled emulsion | Carrageenans and locust bean gum were dissolved in water at 80 °C with constant stirring. Chia or hemp were added to aqueous solution and homogenized. | Plant-based burgers | [82] |
| Direct oleogel | Oil and beeswax mixture were heated at 70 °C with continuous stirring until complete dissolution of beeswax.After that the blend was cooled at room Ta. | Beef burgers | [96] |
| Direct oleogel | Sunflower oil was combined with rice brand wax and the mixture was heated at 80 °C and stirred (5 min). After that the blend was cooled at room Ta. | Ice cream | [97] |
| Direct oleogel | Water, high oleic sunflower oil, and pork skin (cooked at 80 °C) were mixed in a blender. | Bologna sausages | [98] |
| Direct oleogel | Oryzanol and β-sitosterol were dispersed under stirring until solubilization in linseed oil at 80 °C for 30 min. | Pork burgers | [99] |
| Direct oleogel | Carnauba wax and canola oil were heated at 90 °C with continuous agitation. Then the sample was cooled at room Ta. | Cakes | [100] |
| Meat Products | Vegetable Oil | Incorporation Strategy | Replacement (%) | Response on Meat Product | Ref. |
|---|---|---|---|---|---|
| Burger | Chia and linseed oils | Microencapsulation (external ionic gelation) | Replacement at 50% | Improved important technological properties (cooking loss and fat retention). Low fat, higher content of healthier polyunsaturated fatty acids/saturated fatty acids, and ω-6/ω-3 ratio ratios. Acceptable sensory properties. | [142] |
| Burger | Chia and linseed oils | Hydrogelled emulsion | Replacement at 20, 40, 60, 80, and 100% | Increased protein:lipid ratio. Improved the fatty acid profile of raw burgers. Increased TBARs. Non-affected technological properties. | [80] |
| Burger | Chia oil | Microencapsulation (enriched with rosemary) | Replacement at 50% | Non-impact on the volatiles profile. Decreased in volatiles from lipid and protein oxidation. Decreased sensory descriptors related to lipid oxidation. | [143] |
| Burger | Chia and hemp oil | Gelled emulsions (amaranth flour, gellan gum, and gelatin) | Replacement at 25 and 50% | Improved nutritional characteristics of burgers. Non-affected technological or sensory properties. More susceptible to lipid oxidation. | [21] |
| Fresh chorizo | Melon and pumpkin seed oils | Oil emulsions | Replacement at 50%, 75%, and 100% | Softer texture. Better fatty acid profile, decreased in saturated fatty acids, and increased linoleic and linolenic fatty acids. | [23] |
| Fresh sausages | Olive oil | Gelled emulsions prepared with chia and oats | Replacement at 90% | Improved fat, minerals, and amino acid contents. Cooking loss was lower. Higher Kramer shear force values. Affected sensory properties, but were judged acceptable. | [144] |
| Emulsified Meat Products | |||||
| Frankfurter | Soybean oil | Emulsion gels (EG) prepared, sonicated and non-sonicated soy protein isolate dispersions, carrageenan, and inulin | Replacement at 100% The pork backfat (10 and 20%) was replaced by the gelled emulsion | Good source of fiber. A reduction of 19–29% in energy value. A reduction of 35, 72, and 63% in as ω-6/ω-3 ratio, atherogenic index (AI), and thrombogenic index (TI), respectively. | [145] |
| Frankfurter | Soybean oil | Oleogels structured with rice bran wax | Replacement at 100% | Less dark and less red. Higher in the essential polyunsaturated fatty acids linoleic (18:2n6) and α-linolenic (18:3n3). Non-negatively influence the technological quality. | [24] |
| Frankfurter | Linseed oil | Oleogel gelled with beeswax | Replacement at 0% 25% and 50% | The fatty acid profile was substantially improved and saturated fatty acid content, as ω-6/ω-3 ratio and cholesterol were reduced. Increased the yellowness with linseed oleogel. Increased cohesiveness, gumminess, and chewiness. | [25] |
| Frankfurter | Canola/soy/ flaxseed oil | Oleogels | Replacement of 100% | Higher hardness values. Springiness was lower. Flaxseed oil provided the highest b*. Reduced cooking loss. | [26] |
| Emulsified sausages | Peanut and linseed oil | Gelled emulsion | Replacement of up to 40% | Healthier lipid composition and improved nutritional ratios: Decreased saturated fatty acids and cholesterol and increased mono and polyunsaturated fatty acids. Improved emulsion stability and cooking behaviors. Alterations in color and texture: higher yellowness and increased the hardness. Decreased oxidative stability. | [146] |
| Bologna sausage | Soybean oil | Emulsion gels prepared with chia flour and/or soy protein isolate, inulin, carrageenan, sodium caseinate, and sodium tripolyphosphate | Replacement at 50 and 100% | Improved lipid profile of the sausage. Lower fat content. Affected the color of sausages: increased L* and reduced a*. More homogeneous batter and a compact structure. Greater hardness, chewiness, and shear force. | [145] |
| Deer pâté | Tigernut, chia, or linseed oils | Microencapsulated | Replacement at 50% | Decreased fat and cholesterol contents. Decreased the total amount of saturated fatty acids and increased polyunsaturated fatty acids (chia and linseed pâtés) or monosaturated fatty acids contents (tigernut pâtés). Modification of color parameters. Softer textures. | [89] |
| Bologna | High oleic sunflower oil | Oleogel prepared with pork skin, water, and high oleic sunflower oil | Replacement at 25, 50, 75, and 100% | Healthier lipid profile: reduction of approximately 10% cholesterol levels.Increased the proportion of oleic acid and decreased the proportion of linoleic acid. Non-changes in the oxidative stability. The acceptance and the sensory profile of the samples were not affected by the substitution of up to 50%. Decrease in cooking loss. | [98] |
| Pâtés | Mixture of olive, linseed, and fish oil | Oleogels produced with ethyl cellulose and beeswax oleogel | Replacement at 15% | Optimal fatty acid profile from a health standpoint (high polyunsaturated fatty acids/saturated fatty acids ratio and low as ω-6/ω-3 ratio). Emulsion stability, texture, and color of pâtés not affected. Increased lipid oxidation. Sensory attributes similar. | [147] |
| Traditional Fermented Meat Products | |||||
| Dry fermented sausages | Linseed oil | Gelled emulsion | Replacement at 26.3%, 32.8%, and 39.5% | Increased polyunsaturated fatty acids supply (up to 10.3%) and reductions in ω-6/ω-3 ratio (75, 82, and 84%, respectively). Non-affected peroxides and Thiobarbituric reactive substances (TBARs) values. | [93] |
| Dry fermented sausage (Salchichón) | Linseed oil | Oleogels produced with 8% ɣ-oryzanol, β-sitosterol, and beeswax | Replacement at 20 and 40% | Improvement of the fatty acid profile. Color and sensory parameters were strongly affected. Quality parameters such as pH and color also changed with the inclusion of oleogels. Changed in the sensory quality. | [148] |
| Dry fermented sausage | Linseed oil | Gelled emulsion | Replacement at 65% | Lower saturated fatty acids and monounsaturated fatty acids and higher polyunsaturated fatty acids content with an improved as ω-6/ω-3 ratio α- and linolenic acid increment. Decreased in springiness, chewiness, and hardness and increase in adhesiveness. Lower L* and higher a*. Higher susceptibility to oxidation and lipolysis. | [149] |
| Dry fermented sausage | Grapeseed oil | Liquid, encapsulated, and pre-emulsified | Replacement at 20% | Higher weight loss. Lower hardness, chewiness, cohesiveness. | [150] |
| Dry fermented meat product (Fuet) | Olive and chia oil | Oleogels and gelled emulsion | Replacement at 80% | Improved fatty acid profile. Decrease of ω-6/ω-3 ratio. Emulsion gel as animal fat replacer had similar hardness to the control whereas those with oleogel were softer. | [126] |
| Dairy Product | Vegetable Oil | Incorporation Strategy | Effect on Dairy Product | Ref. |
|---|---|---|---|---|
| Processed cheese | Chia oil | Microcapsules versus free oil | Microencapsulation process masked the flavor of the oil. | [170] |
| Cheddar cheese | Chia oil | Incorporated in mixture of ingredients | No effect on sensory attributes; antioxidant properties improved. | [171] |
| Queso blanco cheese | Flaxseed oil | Incorporated during homogenization, coagulation, or salting | The best scores are obtained when oil is incorporated during homogenization. | [172] |
| Yoghurt | Soybean oil | Emulsion O/W for dissolving phytosterols as functional ingredient | No significant difference in texture, appearance, flavor, and overall acceptance;lower syneresis, higher firmness, and lower apparent viscosity. | [173] |
| Yoghurt | Extra virgin olive oil (EVOO) | Microcapsules of EVOO and synbiotic bacteria | Increased antioxidant activity. | [174] |
| Yoghurt | Pequi oil | Microcapsules of pequi oil | Increase in the percentage of oleic acid; delayed oxidation. | [175] |
| Yoghurt | Corn oil | Emulsion gel microparticles from corn oil | Improvement in textural and rheological properties, water holding capacity, and storage stability; sensory defects were reduced. | [176] |
| Yoghurt | Flaxseed | Nanoemulsion | Increase solubility, bioavailability, and protection of ω-3. | [177] |
| Ice cream | Vegetal oil | Oleogels | Greater overrun, which implies an improvement in texture and appearance. | [178] |
| Ice cream | Grape seed oil | Incorporated in mixture of ingredients | High nutritional antioxidant activity. | [179] |
| Ice cream | Basil oil | Encapsulated by Spray-drying | High antioxidant and phenolic content; sensorial attributes were not affected. | [180] |
| Ice cream | Extra virgin olive | Incorporated in mixture of ingredients | Matrix masked EVOO bitterness; higher content of biophenols. | [181] |
| Ice cream | Hazelnut and olive oil | Incorporated in mixture of ingredients | Similar or even better quality characteristics. | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botella-Martínez, C.; Pérez-Álvarez, J.Á.; Sayas-Barberá, E.; Navarro Rodríguez de Vera, C.; Fernández-López, J.; Viuda-Martos, M. Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review. Biomolecules 2023, 13, 778. https://doi.org/10.3390/biom13050778
Botella-Martínez C, Pérez-Álvarez JÁ, Sayas-Barberá E, Navarro Rodríguez de Vera C, Fernández-López J, Viuda-Martos M. Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review. Biomolecules. 2023; 13(5):778. https://doi.org/10.3390/biom13050778
Chicago/Turabian StyleBotella-Martínez, Carmen, José Ángel Pérez-Álvarez, Estrella Sayas-Barberá, Casilda Navarro Rodríguez de Vera, Juana Fernández-López, and Manuel Viuda-Martos. 2023. "Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review" Biomolecules 13, no. 5: 778. https://doi.org/10.3390/biom13050778
APA StyleBotella-Martínez, C., Pérez-Álvarez, J. Á., Sayas-Barberá, E., Navarro Rodríguez de Vera, C., Fernández-López, J., & Viuda-Martos, M. (2023). Healthier Oils: A New Scope in the Development of Functional Meat and Dairy Products: A Review. Biomolecules, 13(5), 778. https://doi.org/10.3390/biom13050778








