Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Analysis
2.2. Composition Analysis
3. Materials and Methods
3.1. Quality Indices
3.2. Fatty Acid Profile
3.3. Phytosterol Analysis
3.4. Tocopherol and Tocotrienol Analysis
3.5. Carotenoid Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrillo Bautista, M.P.; Cardona Jaramillo, J.E.C.; Diaz Salcedo, R.O. Los Ingredientes Naturales de La Amazonia Colombiana: Sus Aplicaciones y Especificaciones Técnicas/Natural Ingredients from Amazonian Plant Species, Applications and Technical Specifications; Instituto Amazónico de Investigaciones Científicas–Sinchi: Bogota, Colombia, 2017; ISBN 9789585951389. [Google Scholar]
- Ramos-Escudero, F.; Muñoz, A.M.; Ramos Escudero, M.; Viñas-Ospino, A.; Morales, M.T.; Asuero, A.G. Characterization of Commercial Sacha Inchi Oil According to Its Composition: Tocopherols, Fatty Acids, Sterols, Triterpene and Aliphatic Alcohols. J. Food Sci. Technol. 2019, 56, 4503–4515. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and Nutrient Composition of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de Colombia. Colombia’s National Bioeconomy Strategy: Bioeconomía para una Colombia potencia viva y diversa: Hacia una sociedad impulsada por el conocimiento; Gobierno de Colombia: Bogota, Colombia, 2020. [Google Scholar]
- Papell, M.; Medina, F.; Domínguez, J.D. Resumen Ejecutivo 2020. Construcción de un Plan de Acción para el cierre de brechas de competitividad de la cadena de valor de ingredientes naturales para cosméticos. 2020. Available online: https://www.colombiamascompetitiva.com/wp-content/uploads/2021/03/Resumen-Ejecutivo-Plan-De-accio%CC%81n-Ingredientes-Naturales.pdf (accessed on 20 December 2022).
- Cardona Jaramillo, J.E.C.; Carrillo Bautista, M.P.; Alvarez Solano, O.A.; Achenie, L.E.K.; González Barrios, A.F. Impact of the Mode of Extraction on the Lipidomic Profile of Oils Obtained from Selected Amazonian Fruits. Biomolecules 2019, 9, 329. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Cárdenas-L, D.; Ramírez-A, J.G. Plantas Útiles y Su Incorporación a Los Sistemas Productivos Del Departamento Del Guaviare (Amazonia colombiana). Caldasia 2004, 26, 95–110. [Google Scholar]
- Carrillo, M.P.; Cardona, J.E.C.; Peña, L.F.; Díaz, R.O.; Mosquera, L.E.; Hernández, M.S. El Rol de La Ingeniería En El Aprovechamiento Sostenible de La Biodiversidad de La Amazonia. Rev. Ing. 2020, 42, 67–70. [Google Scholar] [CrossRef]
- Departamento Nacional de Planeación (DNP). Oleaginosas, Aceites y Grasas. Rev. VirtualPRO; DNP: Bogota, Colombia, 2010. [Google Scholar]
- Macía, M.J.; Armesilla, P.J.; Cámara-Leret, R.; Paniagua-Zambrana, N.; Villalba, S.; Balslev, H.; Pardo-de-Santayana, M. Palm Uses in Northwestern South America: A Quantitative Review. Bot. Rev. 2011, 77, 462–570. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Fernandes, A.S.; Gomes, E.M.; Oliveira, L.L.; Macedo, A.F.; Antoniassi, R.; Wilhelm, A.E.; Aiub, C.A.F.; Felzenszwalb, I. Antioxidant Activity and Genotoxic Assessment of Crabwood (Andiroba, Carapa guianensis Aublet) Seed Oils. Oxid. Med. Cell. Longev. 2018, 2018, 3246719. [Google Scholar] [CrossRef]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.T.; Oliveira, J.A.R.; do Nascimento, L.A.S.; Costa, C.E.F.; Filho, G.N.R.; Concha, V.O.C.; Passos, M.F. PCL/Andiroba Oil (Carapa guianensis Aubl.) Hybrid Film for Wound Healing Applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- Feitosa, J.M.; Silva, T.S.d.A.; de Xavier, A.E.F.; Rodrigues, W.C.S.; da Silva, A.C.E.; Corrêa, C.V.P.; de Aguiar, F.S.; Mourão, R.H.V.; de Oliveira, E.G.; Nunes, K.M. Evaluation of the Quality of Amazonian Butters as Sustainable Raw Materials for Applications in Bioproducts. Rev. Cienc. Farm. Basica Apl. 2021, 42, 1–11. [Google Scholar] [CrossRef]
- Pereira Lima, R.; Souza da Luz, P.T.; Braga, M.; dos Santos Batista, P.R.; Ferreira da Costa, C.E.; Zamian, J.R.; Santos do Nascimento, L.A.; da Rocha Filho, G.N. Murumuru (Astrocaryum murumuru Mart.) Butter and Oils of Buriti (Mauritia flexuosa Mart.) and Pracaxi (Pentaclethra macroloba (Willd.) Kuntze) Can Be Used for Biodiesel Production: Physico-Chemical Properties and Thermal and Kinetic Studies. Ind. Crops Prod. 2017, 97, 536–544. [Google Scholar] [CrossRef]
- Aquino, J.d.S.; de Pontes Pessoa, D.C.N.; Araújo, K.d.L.G.V.; Epaminondas, P.S.; Schuler, A.G.d.S.; Stamfordb, T.L.M. Refining of Buriti Oil (Mauritia flexuosa) Originated from the Brazilian Cerrado: Physicochemical, Thermal-Oxidative and Nutritional Implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Vegetable Oils: Composition and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 357–364. ISBN 978-0-12-384953-3. [Google Scholar]
- Kroumova, A.B.; Xie, Z.; Wagner, G.J. A Pathway for the Biosynthesis of Straight and Branched, Odd- and Even-Length, Medium-Chain Fatty Acids in Plants. Proc. Natl. Acad. Sci. USA 1994, 91, 11437–11441. [Google Scholar] [CrossRef]
- Siger, A.; Górnaś, P. Free Tocopherols and Tocotrienols in 82 Plant Species’ Oil: Chemotaxonomic Relation as Demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Sigler, K. Odd-Numbered Very-Long-Chain Fatty Acids from the Microbial, Animal and Plant Kingdoms. Prog. Lipid Res. 2009, 48, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K. The Features of the Fatty Acid Composition of Pyrus L. Total Lipids Are Determined by Mountain Ecosystem Conditions. Plant Physiol. Biochem. 2022, 170, 350–363. [Google Scholar] [CrossRef]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycom. Lipidom. 2014, 4, 4–11. [Google Scholar] [CrossRef]
- Baškirovs, G.; Soliven, A.; Górnaś, P. Analytical Scale Supercritical Fluid Chromatography for the Analysis of Nine Tocochromanols in 24 Different Cold-Pressed Plant Oils: Method Development, Validation, and Isolation of Tocotrienols and Plastochromanol-8. J. Food Compos. Anal. 2022, 110, 104586. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and Esterified Tocopherols, Tocotrienols and Other Extractable and Non-Extractable Tocochromanol-Related Molecules: Compendium of Knowledge, Future Perspectives and Recommendations for Chromatographic Techniques, Tools, and Approaches Used for Tococh. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef]
- Freitas, M.L.F.; Chisté, R.C.; Polachini, T.C.; Sardella, L.A.C.; Aranha, C.P.M.; Ribeiro, A.P.B.; Nicoletti, V.R. Quality Characteristics and Thermal Behavior of Buriti (Mauritia flexuosa L.) Oil. Grasas Y Aceites 2017, 68, e220. [Google Scholar] [CrossRef]
- Bataglion, G.A.; Paz, W.H.P.; Adrião, A.A.X.; da Rocha Albuquerque, J.M.; da Silva, F.M.A.; Numa, I.A.N.; Angolini, C.F.F.; Pastore, G.M.; Koolen, H.H.F. Bioactive Compounds of Buriti Fruit (Mauritia flexuosa L.F.). In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 411–436. ISBN 978-3-030-30182-8. [Google Scholar]
- da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, Non-Anthocyanin Phenolics, Tocopherols and Antioxidant Capacity of Açaí Juice (Euterpe oleracea) as Affected by High Pressure Processing and Thermal Pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Coutinho, D.J.G.; Santos, J.; Cordeiro, R.P.; Muniz, L.R.; Alves, R.C.; Bessa, C.M.A.S.; da Silva, M.V.; Oliveira, M.B.P.P.; de Oliveira, A.F.M. Composition of Fatty Acids, Tocopherols, Tocotrienols and β-Carotene Content in Oils of Seeds of Brazilian Sapindaceae and Meliaceae Species. J. Food Sci. Technol. 2019, 56, 3164–3169. [Google Scholar] [CrossRef]
- de Souza, A.H.P.; Gohara, A.K.; Rodrigues, Â.C.; de Souza, N.E.; Visentainer, J.V.; Matsushita, M. Sacha Inchi Como Fonte Potencial de Ácidos Graxos Essenciais e Tocoferóis: Estudo Multivariado Da Castanha e Casca. Acta Sci. Technol. 2013, 35, 757–763. [Google Scholar] [CrossRef]
- Wojdyło, A.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Fruit Tree Leaves as Valuable New Source of Tocopherol and Tocotrienol Compounds. J. Sci. Food Agric. 2022, 102, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R. Tocotrienols: Vitamin E beyond Tocopherols; Taylor & Francis: Boca Raton, FL, USA, 2011; Volume 44, ISBN 9788578110796. [Google Scholar]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Nasir, H.; Mohd-Setapar, S.H. Natural Ingredients in Cosmetics from Malaysian Plants: A Review. Sains Malays. 2018, 47, 951–959. [Google Scholar] [CrossRef]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and Recovery Methods. Bioresour. Technol. 2007, 98, 2335–2350. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Novello, Z.; Scapinello, J.; Magro, J.D.; Zin, G.; Di Luccio, M.; Tres, M.V.; Oliveira, J.V. Extraction, Chemical Characterization and Antioxidant Activity of Andiroba Seeds Oil Obtained from Pressurized n-Butane. Ind. Crops Prod. 2015, 76, 697–701. [Google Scholar] [CrossRef]
- United States Pharmacopeia and National Formulary (USP38—NF 33) 〈401〉 Fats and Fixed Oils; United States Pharmacopeia: Rockville, MD, USA, 2011; pp. 276–289.
- Asociación Española de Normalización y Certificación. UNE-EN ISO 661:2006/AC: Aceites y Grasas de Origen Animal y Vegetal: Preparación de La Muestra Para Análisis: (ISO 661:2003); AENOR: Madrid, Spain, 2007. [Google Scholar]
- Winkler-Moser, J. Gas Chromatographic Analysis of Plant Sterols. AOCS Lipid Libr. 2011. [Google Scholar] [CrossRef]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of Phytosterols in Foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.N.; Thompson, L.D.; Galyean, M.L.; Brooks, J.C.; Patterson, K.Y.; Boylan, L.M. Cholesterol Content and Methods for Cholesterol Determination in Meat and Poultry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 269–289. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Improved Normal-Phase High-Performance Liquid Chromatography Procedure for the Determination of Carotenoids in Cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.H.; Tan, E.H.P.; Ooi, I.H. Fast Gradient Normal-Phase High Pressure Liquid Chromatography for Rapid Baseline Separation of Vitamin E Forms in Palm-Derived Tocotrienol Rich Fractions (TRFs). Anal. Methods 2017, 9, 5211–5218. [Google Scholar] [CrossRef]
- de Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total Carotenoid Content, α-Carotene and β-Carotene, of Landrace Pumpkins (Cucurbita moschata Duch): A Preliminary Study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Alfahmawi, R.K.H. Gas Chromatography-Mass Spectrometry Analyses of Fatty Acid Methyl Esters from Marine Algae. Master’s Thesis, The University of Bergen, Bergen, Norway, 2019. [Google Scholar]
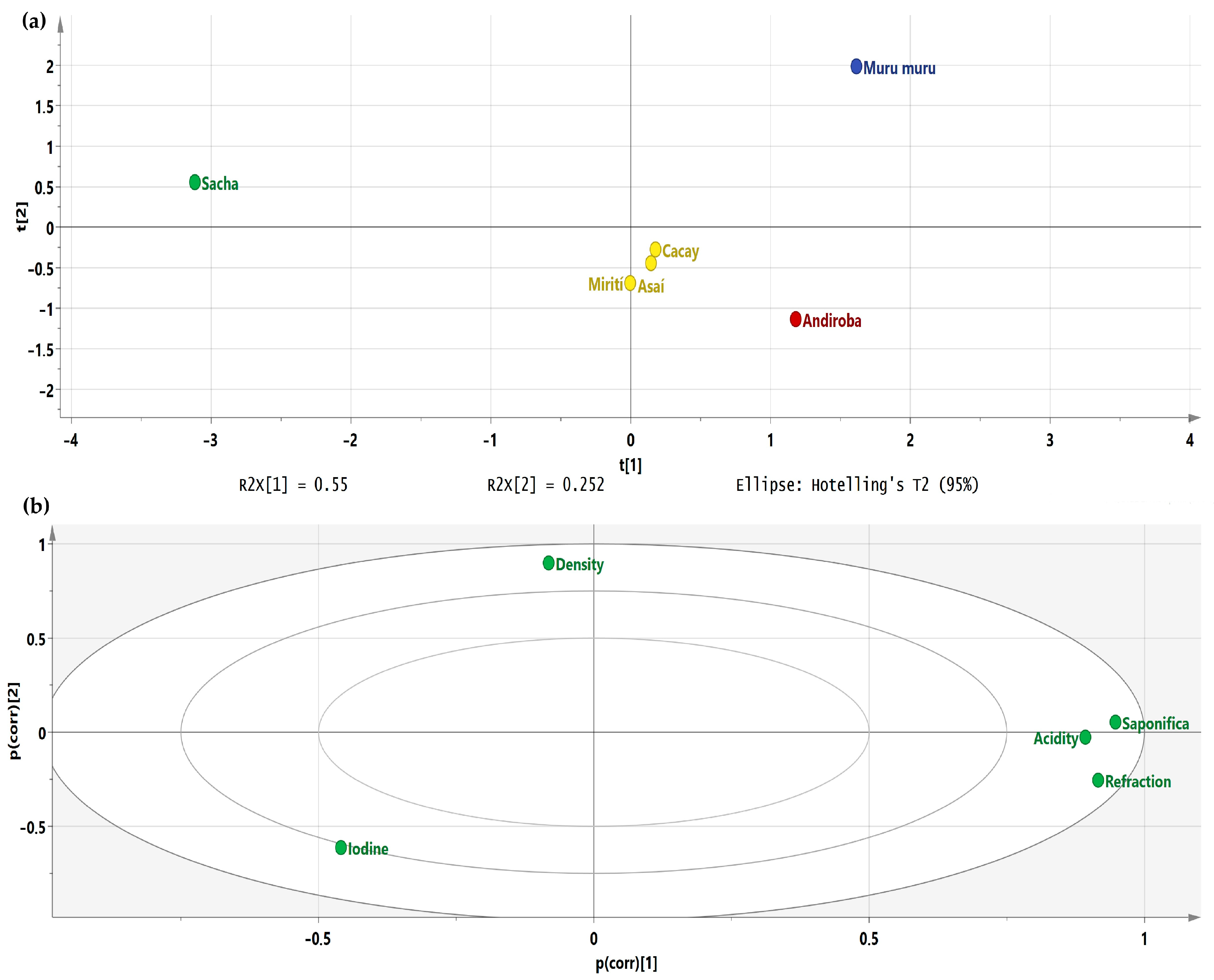
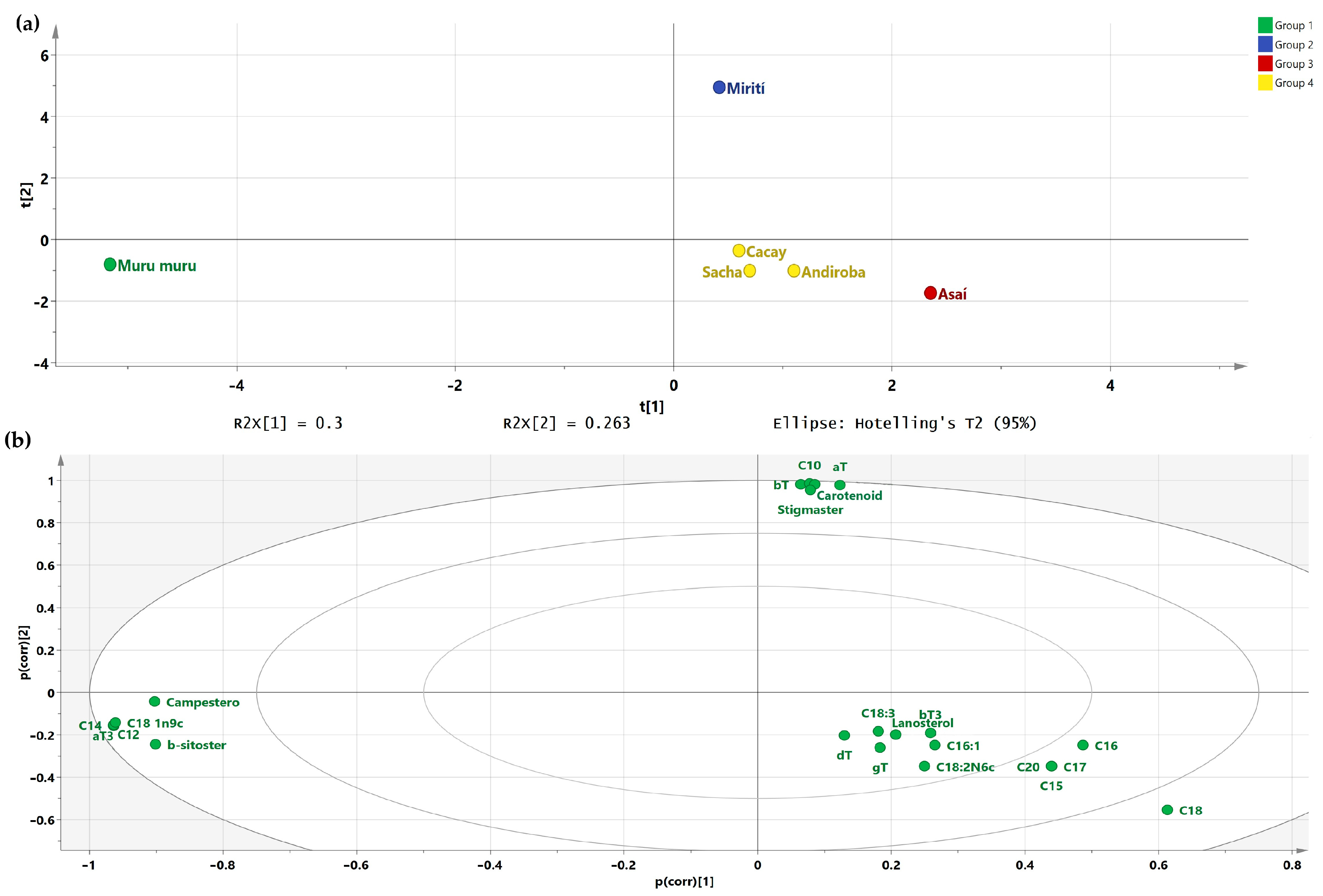
| Oil Source | Oil Yield/Content (% w/w) | Saponification (mg KOH/g Sample) | Iodine (g I2/100 g Sample) | Acidity (mg KOH/g Sample) | Density (g/mL) | Refractive Index |
|---|---|---|---|---|---|---|
| Miriti | 66.63 | 189.21 ± 0,70 | 76.38 ± 0.70 | 3.68 ± 0.70 | 0.9133 ± 0.0002 | 1.4656 ± 0.0003 |
| Cacay | 69.57 | 166.42 ± 1.69 | 253.63 ± 5.88 | 3.61 ± 0.36 | 0.9061 ± 0.0004 | 1.4711 ± 0.0003 |
| Murumuru | 71.33 | 205.1 ± 4.70 | 19.10 ± 0.47 | 7.28 ± 0.11 | 0.9264 ± 0.0001 | 1.4504 ± 0.0001 |
| Andiroba | 73.68 | 189.36 ± 3.56 | 59.2 ± 2.98 | 8.31 ± 0.11 | 0.9054 ± 0.0001 | 1.4663 ± 0.0002 |
| Sacha Inchi | 76.23 | 123.10 ± 1.86 | 65.20 ± 0.41 | 1.00 ± 0.02 | 0.9184 ± 0.0002 | ND 1 |
| Asai | 55.55 | 170.40 ± 9.01 | 63.40 ± 0.43 | 5.51 ± 0.01 | 0.9133 ± 0.0002 | 1.4687 ± 0.0003 |
| Lipidic Profile (Relative %) | |||||||
|---|---|---|---|---|---|---|---|
| Fatty Acid | Miriti | Cacay | Murumuru | Andiroba | Sacha Inchi | Asai | |
| Capric | C:10 | 0.01 ± 0.00 | - | - | - | - | - |
| Lauric | C:12 | 0.08 ± 0.00 | - | 64.26 ± 1.29 | - | - | - |
| Myristic | C:14 | 0.18 ± 0.00 | - | 26.57 ± 0.53 | - | - | 0.1 ± 0.00 |
| Pentadecylic | C:15 | - | - | - | - | - | 0.1 ± 0.00 |
| Palmitic | C:16 | 6.69 ± 0.13 | 0.33 ± 0.00 | 3.39 ± 0.06 | 20.2 ± 0.40 | 4.96 ± 0.10 | 16.7 ± 0.33 |
| Palmitoleic | C16:1 | - | - | - | 0.8 ± 0.016 | - | 0.1 ± 0.00 |
| Margaric | C:17 | - | - | - | - | - | 0.1 ± 0.00 |
| Stearic | C:18 | - | 0.16 ± 0.00 | - | 5.3 ± 0.11 | 2.99 ± 0.06 | 6.8 ± 0.14 |
| Oleic | C18:1n9c | 93.04 ± 1.86 | - | 3.19 ± 0.06 | 45.5 ± 0.91 | 11.1 ± 0.22 | 70 ± 1.4 |
| Linoleic | C18:2n6c | - | 12.74 ± 0.26 | 1.22 ± 0.02 | 8.3 ± 0.17 | 34.56 ± 0.69 | 3.3 ± 0.07 |
| Arachidic | C:20 | - | - | - | - | - | 0.4 ± 0.01 |
| Linolenic | C18:3 | - | 86.77 ± 1.74 | - | 0.8 ± 0.02 | 46.39 ± 0.93 | 0.8 ± 0.02 |
| Tocopherols, Tocotrienols (µg mg−1 Sample) | Phytosterols (µg mg−1 Sample) | Carotenoids (mg 100 g−1 Sample) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| αT | αT3 | βT | γT | γT3 | δT | Stigmasterol | Campesterol | β-Sitosterol | Lanosterol | ||
| Miriti | 1.168 ± 0.008 | - | 1.608 ± 0.017 | - | - | - | 3.824 ± 0.129 | 0.126 ± 0.029 | 0.500 ± 0.006 | - | 128.19 ± 8.14 |
| Cacay | 0.0413 ± 0.002 | - | <LOQ | 0.4436 ± 0.011 | - | - | - | - | - | - | 0.12 ± 0.00 |
| Murumuru | - | 0.034 ± 0.002 | 0.057 ± 0.002 | - | 0.021 ± 0.005 | - | 0.245 ± 0.005 | 0.636 ± 0.013 | 5.257 ± 0.503 | - | - |
| Andiroba | - | - | 0.161 ± 0.008 | - | <LOQ | - | 0.203 ± 0.019 | 0.248 ± 0.006 | 0.679 ± 0.056 | 2.312 ± 0.123 | - |
| Sacha Inchi | - | - | - | 1.795 ± 0.042 | 0.795 ± 0.046 | 0.937± 0.020 | - | 2.265 ± 0.123 | - | - | |
| Asai | 0.085 ± 0.005 | - | <LOQ | 0.1715 ± 0.001 | 0.222 ± 0.015 | - | 0.141 ± 0.015 | - | 1.458 ± 0.123 | - | 2.32 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Garzón, K.; Orduz-Díaz, L.L.; Guerrero-Perilla, C.; Quintero-Mendoza, W.; Carrillo, M.P.; Cardona-Jaramillo, J.E.C. Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential. Biomolecules 2023, 13, 985. https://doi.org/10.3390/biom13060985
Lozano-Garzón K, Orduz-Díaz LL, Guerrero-Perilla C, Quintero-Mendoza W, Carrillo MP, Cardona-Jaramillo JEC. Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential. Biomolecules. 2023; 13(6):985. https://doi.org/10.3390/biom13060985
Chicago/Turabian StyleLozano-Garzón, Kimberly, Luisa L. Orduz-Díaz, Camilo Guerrero-Perilla, Willian Quintero-Mendoza, Marcela P. Carrillo, and Juliana E. C. Cardona-Jaramillo. 2023. "Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential" Biomolecules 13, no. 6: 985. https://doi.org/10.3390/biom13060985
APA StyleLozano-Garzón, K., Orduz-Díaz, L. L., Guerrero-Perilla, C., Quintero-Mendoza, W., Carrillo, M. P., & Cardona-Jaramillo, J. E. C. (2023). Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential. Biomolecules, 13(6), 985. https://doi.org/10.3390/biom13060985






