Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experiments
2.3. Animal Groups
2.4. Mouse Corneal Wounding and Topical Vit D Administration
2.5. Corneal Whole-Mount Confocal Imaging and Three-Dimensional Reconstruction
2.6. Recruitment of CD45 Cells in Wounded WT and VDR KO Mouse Corneas
2.7. Recruitment of CD45 Cells in Wounded Diabetic Mouse Corneas Treated with Vit D
2.8. Statistical Analysis
3. Results
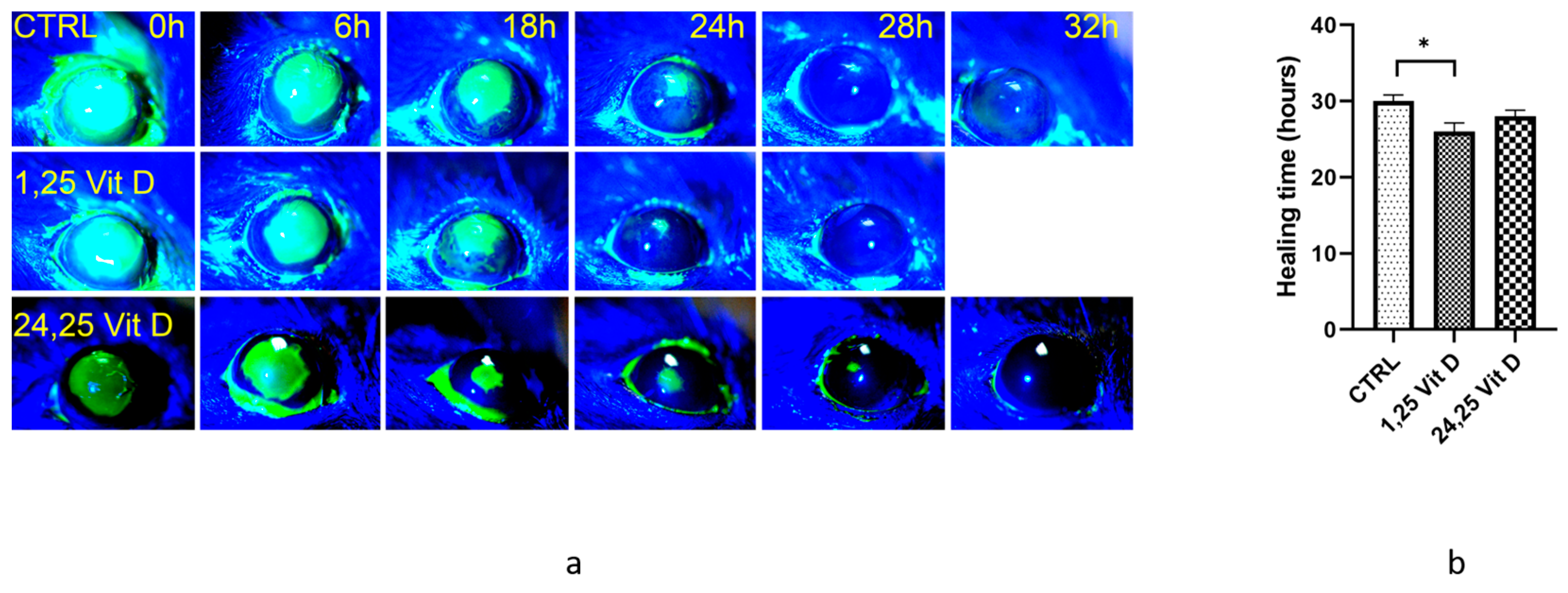
3.1. Normoglycemic Mouse Corneal Wound Healing
3.2. Diabetic Mouse Corneal Wound Healing
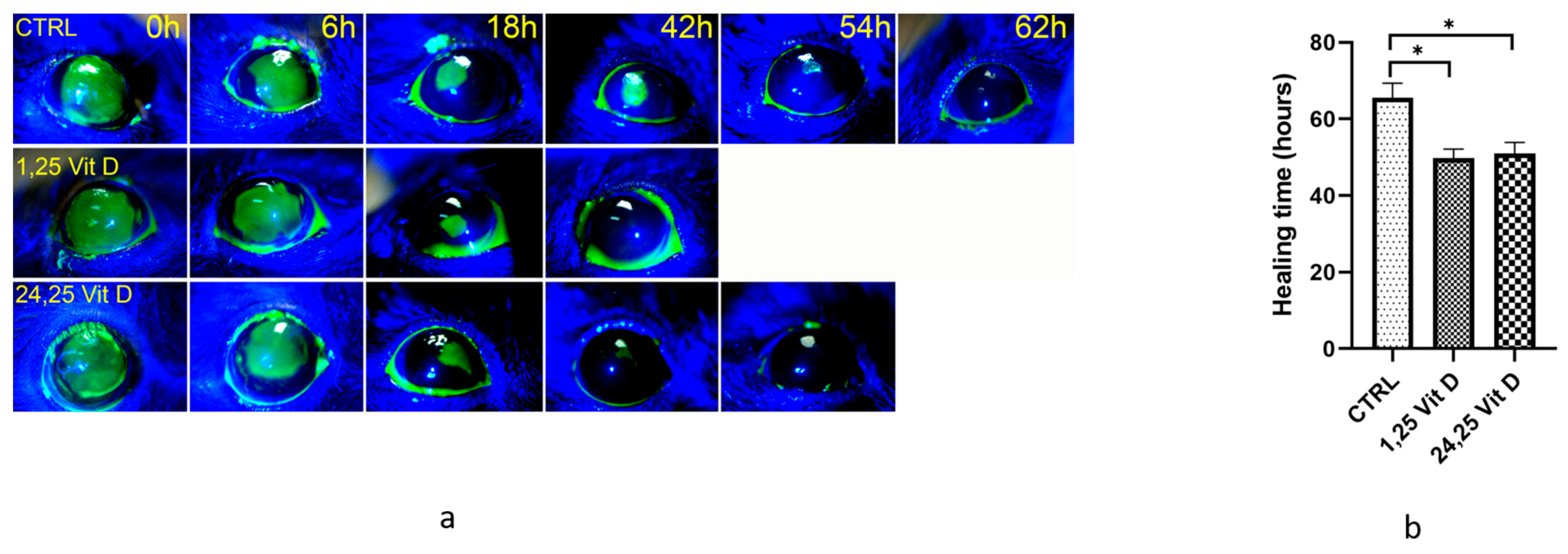
3.3. Diabetic VDD Mouse Corneal Wound Healing
3.4. VDR KO Mouse Corneal Wound Healing
3.5. Distribution of CD45+ Cells in Control and Algerbrush-Wounded Normoglycemic Mouse Corneas
3.6. Distribution of CD45+ Cells in the Wounded Area of Normoglycemic Mice: 3D Reconstruction
3.7. Effects of VDR KO on Recruitment of CD45+ Cells following Wounding
3.8. Effects of 1,25 and 24,25 Vit D on Recruitment of CD45+ Cells following Diabetic Mouse Corneal Wounding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrientez, B.; Nicholas, S.E.; Whelchel, A.; Sharif, R.; Hjortdal, J.; Karamichos, D. Corneal injury: Clinical and molecular aspects. Exp. Eye Res. 2019, 186, 107709. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Shih, K.C.; Kwok, S.S.; Chan, Y.K.; Lo, A.C.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Experimental modeling of cornea wound healing in diabetes: Clinical applications and beyond. BMJ Open Diabetes Res. Care 2019, 7, e000779. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Bikbov, M.; Yamamoto, S. Diabetic corneal neuropathy: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Al Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 59, 263–285. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef]
- Son, Y.J.; Tse, J.W.; Zhou, Y.; Mao, W.; Yim, E.K.F.; Yoo, H.S. Biomaterials and controlled release strategy for epithelial wound healing. Biomater. Sci. 2019, 7, 4444–4471. [Google Scholar] [CrossRef]
- Ziaei, M.; Greene, C.; Green, C.R. Wound healing in the eye: Therapeutic prospects. Adv. Drug Deliv. Rev. 2018, 126, 162–176. [Google Scholar] [CrossRef]
- Naeem, Z. Vitamin d deficiency—An ignored epidemic. Int. J. Health Sci. 2010, 4, V–VI. [Google Scholar]
- Kosmowska-Miskow, A. The role of vitamin D3 in inflammatory bowel diseases. Adv. Clin. Exp. Med. 2014, 23, 497–504. [Google Scholar] [CrossRef]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Sarhan, I.I.; Halawa, M.R.; Afify, E.N.; Hebah, H.A.; Al-Gohary, E.A.; El-Shazly, I.O. Study of the effect of vitamin D supplementation on glycemic control in type 2 diabetic prevalent hemodialysis patients. Hemodial. Int. 2015, 19 (Suppl. S3), S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Brenner, H. Vitamin D as a Resilience Factor, Helpful for Survival of Potentially Fatal Conditions: A Hypothesis Emerging from Recent Findings of the ESTHER Cohort Study and the CHANCES Consortium. Nutrients 2015, 7, 3264–3278. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Gandini, S. Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2007, 167, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 2009, 169, 626–632. [Google Scholar] [CrossRef]
- Cankaya, C.; Cumurcu, T.; Gunduz, A. Corneal endothelial changes in patients with vitamin D deficiency. Indian J. Ophthalmol. 2018, 66, 1256–1261. [Google Scholar] [CrossRef]
- Shetty, R.; Sethu, S.; Deshmukh, R.; Deshpande, K.; Ghosh, A.; Agrawal, A.; Shroff, R. Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. Biomed. Res. Int. 2016, 2016, 4369750. [Google Scholar] [CrossRef]
- Shetty, R.; Deshpande, K.; Deshmukh, R.; Jayadev, C.; Shroff, R. Bowman Break and Subbasal Nerve Plexus Changes in a Patient With Dry Eye Presenting With Chronic Ocular Pain and Vitamin D Deficiency. Cornea 2016, 35, 688–691. [Google Scholar] [CrossRef]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Yazdanpanah, G.; Chen, E.; Afsharkhamseh, N.; Ghassemi, M.; Anwar, K.N.; Fonteh, C.; Djalilian, A.R.; Kang, K.B. Dose-dependent therapeutic effects of topical 1,25 OH-vitamin D3 on corneal wound healing. Mol. Biol. Rep. 2021, 48, 4083–4091. [Google Scholar] [CrossRef]
- Elizondo, R.A.; Yin, Z.; Lu, X.; Watsky, M.A. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5245–5251. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vick, S.; Chen, Z.; Chen, J.; Watsky, M.A. Effects of Vitamin D Receptor Knockout and Vitamin D Deficiency on Corneal Epithelial Wound Healing and Nerve Density in Diabetic Mice. Diabetes 2020, 69, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Vick, S.; Watsky, M.A. Vitamin D receptor and metabolite effects on corneal epithelial cell gap junction proteins. Exp. Eye Res. 2019, 187, 107776. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Watsky, M.A. Influence of Vitamin D on Corneal Epithelial Cell Desmosomes and Hemidesmosomes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4074–4083. [Google Scholar] [CrossRef]
- Suzuki, T.; Sano, Y.; Kinoshita, S. Effects of 1alpha,25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 154–158. [Google Scholar]
- Xue, M.L.; Zhu, H.; Thakur, A.; Willcox, M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol. Cell Biol. 2002, 80, 340–345. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Yazdanpanah, G.; Kanu, L.N.; Anwar, K.N.; Shen, X.; Rabiee, B.; Putra, I.; Eslani, M.; Rosenblatt, M.I.; Hematti, P.; et al. Reproducible Derivation and Expansion of Corneal Mesenchymal Stromal Cells for Therapeutic Applications. Transl. Vis. Sci. Technol. 2020, 9, 26. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Smith, C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: Distinct adhesion molecule requirements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1947–1955. [Google Scholar] [CrossRef]
- Gong, Y.; Koh, D.R. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010, 339, 437–448. [Google Scholar] [CrossRef]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef]
- Reins, R.Y.; Hanlon, S.D.; Magadi, S.; McDermott, A.M. Effects of Topically Applied Vitamin D during Corneal Wound Healing. PLoS ONE 2016, 11, e0152889. [Google Scholar] [CrossRef]
- Yamakawa, I.; Kojima, H.; Terashima, T.; Katagi, M.; Oi, J.; Urabe, H.; Sanada, M.; Kawai, H.; Chan, L.; Yasuda, H.; et al. Inactivation of TNF-alpha ameliorates diabetic neuropathy in mice. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E844–E852. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Gardener, H.; Pouyeh, B.; Feuer, W.; Florez, H. Effect of a Mediterranean dietary pattern and vitamin D levels on Dry Eye syndrome. Cornea 2014, 33, 437–441. [Google Scholar] [CrossRef]
- Yildirim, P.; Garip, Y.; Karci, A.A.; Guler, T. Dry eye in vitamin D deficiency: More than an incidental association. Int. J. Rheum. Dis. 2015, 19, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, B.E.; Ozer, P.A.; Aydinli, M.S. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjogren dry eye. Eye 2015, 29, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Rafie, N.; Miraghajani, M.; Heidari, Z.; Arab, A. Association between vitamin D and dry eye disease: A systematic review and meta-analysis of observational studies. Contact Lens Anterior Eye 2020, 43, 418–425. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, Y.P.; Shin, Y.J. Vitamin D Enhances the Efficacy of Topical Artificial Tears in Patients With Dry Eye Disease. Cornea 2019, 38, 304–310. [Google Scholar] [CrossRef]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, Z.; Li, J.; Qu, M.; Zhou, Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp. Eye Res. 2021, 209, 108668. [Google Scholar] [CrossRef]
- Martineau, C.; Naja, R.P.; Husseini, A.; Hamade, B.; Kaufmann, M.; Akhouayri, O.; Arabian, A.; Jones, G.; St-Arnaud, R. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J. Clin. Investig. 2018, 128, 3546–3557. [Google Scholar] [CrossRef]
- Van Driel, M.; Koedam, M.; Buurman, C.J.; Roelse, M.; Weyts, F.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J. Cell Biochem. 2006, 99, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ubels, J.L.; Schotanus, M.P.; Yin, Z.; Pintea, V.; Hammock, B.D.; Watsky, M.A. Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr. Eye Res. 2012, 37, 871–878. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Mylarapu, N.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Epithelial Proliferation, Migration and Vitamin D Metabolizing and Catabolizing Enzymes. Sci. Rep. 2017, 7, 16951. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Watsky, M.A. Effects of vitamin D receptor knockout on cornea epithelium gap junctions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2975–2982. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Fibroblast VDR and Vitamin D Metabolizing and Catabolizing Enzymes. Curr. Eye Res. 2021, 46, 1271–1282. [Google Scholar] [CrossRef]
- Mayer, D.J.; Daar, A.S.; Casey, T.A.; Fabre, J.W. Localization of Hla-a, Hla-B, Hla-C and Hla-Dr Antigens in the Human Cornea—Practical Significance for Grafting Technique and Hla Typing. Transplant. Proc. 1983, 15, 126–129. [Google Scholar]
- Williams, K.A.; Ash, J.K.; Coster, D.J. Histocompatibility antigen and passenger cell content of normal and diseased human cornea. Transplantation 1985, 39, 265–269. [Google Scholar] [CrossRef]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Reins, R.Y.; Baidouri, H.; McDermott, A.M. Vitamin D Activation and Function in Human Corneal Epithelial Cells during TLR-Induced Inflammation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7715–7727. [Google Scholar] [CrossRef]
- Hanlon, S.D.; Smith, C.W.; Sauter, M.N.; Burns, A.R. Integrin-dependent neutrophil migration in the injured mouse cornea. Exp. Eye Res. 2014, 120, 61–70. [Google Scholar] [CrossRef]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Choi, H.; Lee, R.H.; Roddy, G.W.; Ylostalo, J.H.; Wawrousek, E.; Prockop, D.J. Identification of the HSPB4/TLR2/NF-kappaB axis in macrophage as a therapeutic target for sterile inflammation of the cornea. EMBO Mol. Med. 2012, 4, 435–448. [Google Scholar] [CrossRef]
- Sosnova, M.; Bradl, M.; Forrester, J.V. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells 2005, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Usui, T.; Amano, S.; Ebihara, N. Bone marrow-derived cells in mouse and human cornea. Cornea 2005, 24, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Ruitenberg, M.J.; Plant, G.W.; Pearlman, E.; Jung, S.; McMenamin, P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1568–1574. [Google Scholar] [CrossRef]
- Thill, M.; Schlagner, K.; Altenahr, S.; Ergun, S.; Faragher, R.G.; Kilic, N.; Bednarz, J.; Vohwinkel, G.; Rogiers, X.; Hossfeld, D.K.; et al. A novel population of repair cells identified in the stroma of the human cornea. Stem Cells Dev. 2007, 16, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z. Resident Innate Immune Cells in the Cornea. Front. Immunol. 2021, 12, 620284. [Google Scholar] [CrossRef]
- Wu, M.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 2022, 91, 101105. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Bhalla, A.K.; Amento, E.P.; Clemens, T.L.; Holick, M.F.; Krane, S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983, 57, 1308–1310. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two lineages of immune cells that differentially express the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2023, 228, 106253. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Van Etten, E.; Gysemans, C.; Decallonne, B.; Kato, S.; Laureys, J.; Depovere, J.; Valckx, D.; Verstuyf, A.; Bouillon, R. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J. Bone Miner. Res. 2001, 16, 2057–2065. [Google Scholar] [CrossRef]
- Bruce, D.; Yu, S.; Ooi, J.H.; Cantorna, M.T. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int. Immunol. 2011, 23, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Kamolvit, W.; Scheffschick, A.; Bjorklund, A.; Tovi, J.; Espinosa, A.; Brismar, K.; Nystrom, T.; Schroder, J.M.; Ostenson, C.G.; et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat. Commun. 2022, 13, 4983. [Google Scholar] [CrossRef] [PubMed]
- Insuela, D.B.R.; Ferrero, M.R.; Goncalves-de-Albuquerque, C.F.; Chaves, A.D.S.; da Silva, A.Y.O.; Castro-Faria-Neto, H.C.; Simoes, R.L.; Barja-Fidalgo, T.C.; Silva, P.; Martins, M.A.; et al. Glucagon Reduces Neutrophil Migration and Increases Susceptibility to Sepsis in Diabetic Mice. Front. Immunol. 2021, 12, 633540. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Fattahi, F.; He, K.; Ward, P.A.; Delano, M.J. Diabetes and Sepsis: Risk, Recurrence, and Ruination. Front. Endocrinol. 2017, 8, 271. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Wong, H.L.; Kwok, S.S.; Lo, A.C.; Chan, J.Y.; Ng, A.L.; Chan, T.C.; Jhanji, V.; Tong, L. The association between altered intestinal microbiome, impaired systemic and ocular surface immunity, and impaired wound healing response after corneal alkaline-chemical injury in diabetic mice. Front. Immunol. 2023, 14, 1063069. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef]
- Lu, X.; Elizondo, R.A.; Nielsen, R.; Christensen, E.I.; Yang, J.; Hammock, B.D.; Watsky, M.A. Vitamin D in Tear Fluid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5880–5887. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Chen, Z.; Lu, J.; Watsky, M. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules 2023, 13, 1065. https://doi.org/10.3390/biom13071065
Lu X, Chen Z, Lu J, Watsky M. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules. 2023; 13(7):1065. https://doi.org/10.3390/biom13071065
Chicago/Turabian StyleLu, Xiaowen, Zhong Chen, Jerry Lu, and Mitchell Watsky. 2023. "Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing" Biomolecules 13, no. 7: 1065. https://doi.org/10.3390/biom13071065
APA StyleLu, X., Chen, Z., Lu, J., & Watsky, M. (2023). Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules, 13(7), 1065. https://doi.org/10.3390/biom13071065






