Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome
Abstract
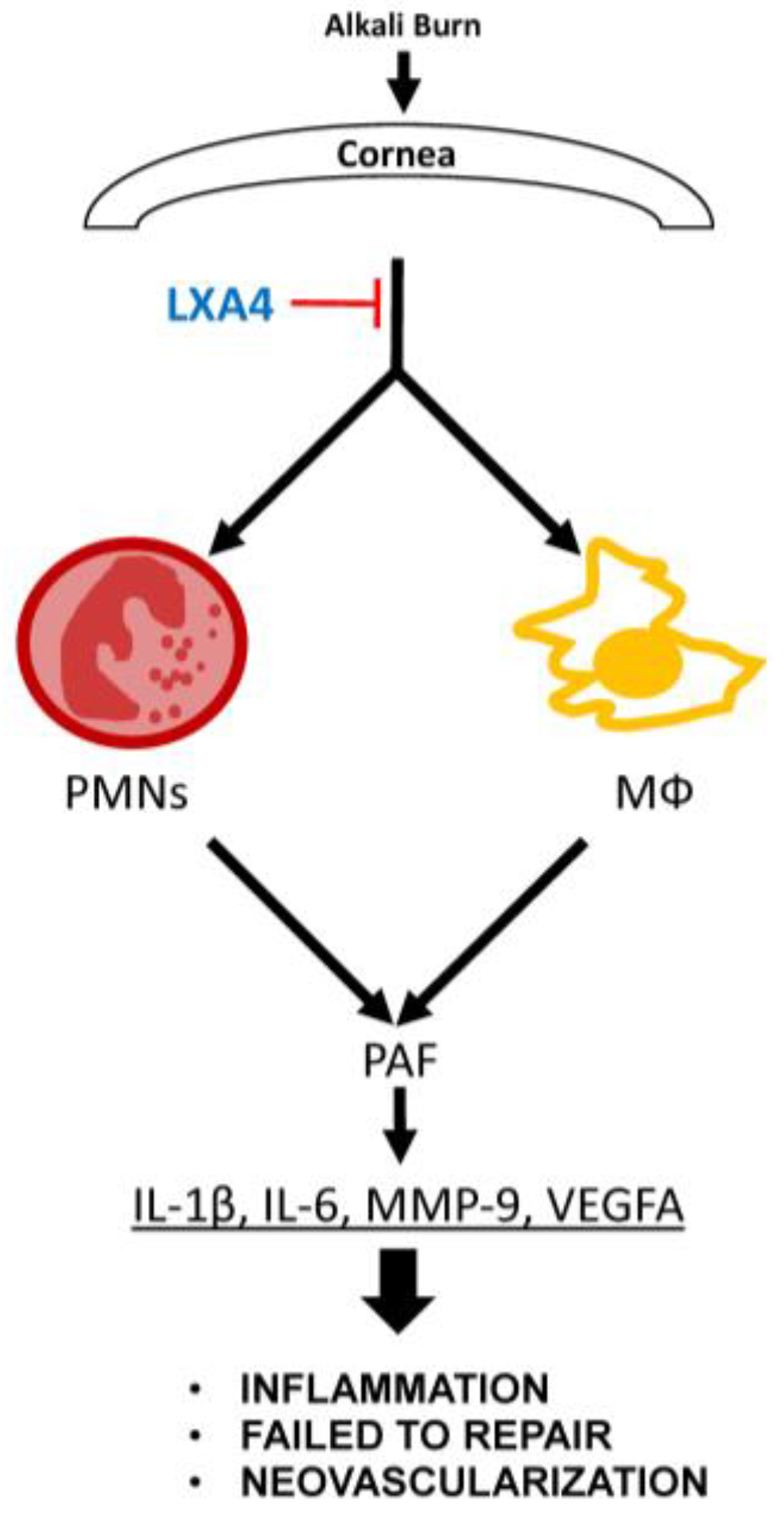
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Corneal Alkali Injury and Clinical Evaluation
2.3. Antibodies
2.4. RNA Sequencing Analysis of Alkali Burnt Corneas Treated with LXA4
2.5. Capillary-Based Western Blot
2.6. Flow Cytometry Analysis
2.7. Immunofluorescence
3. Results
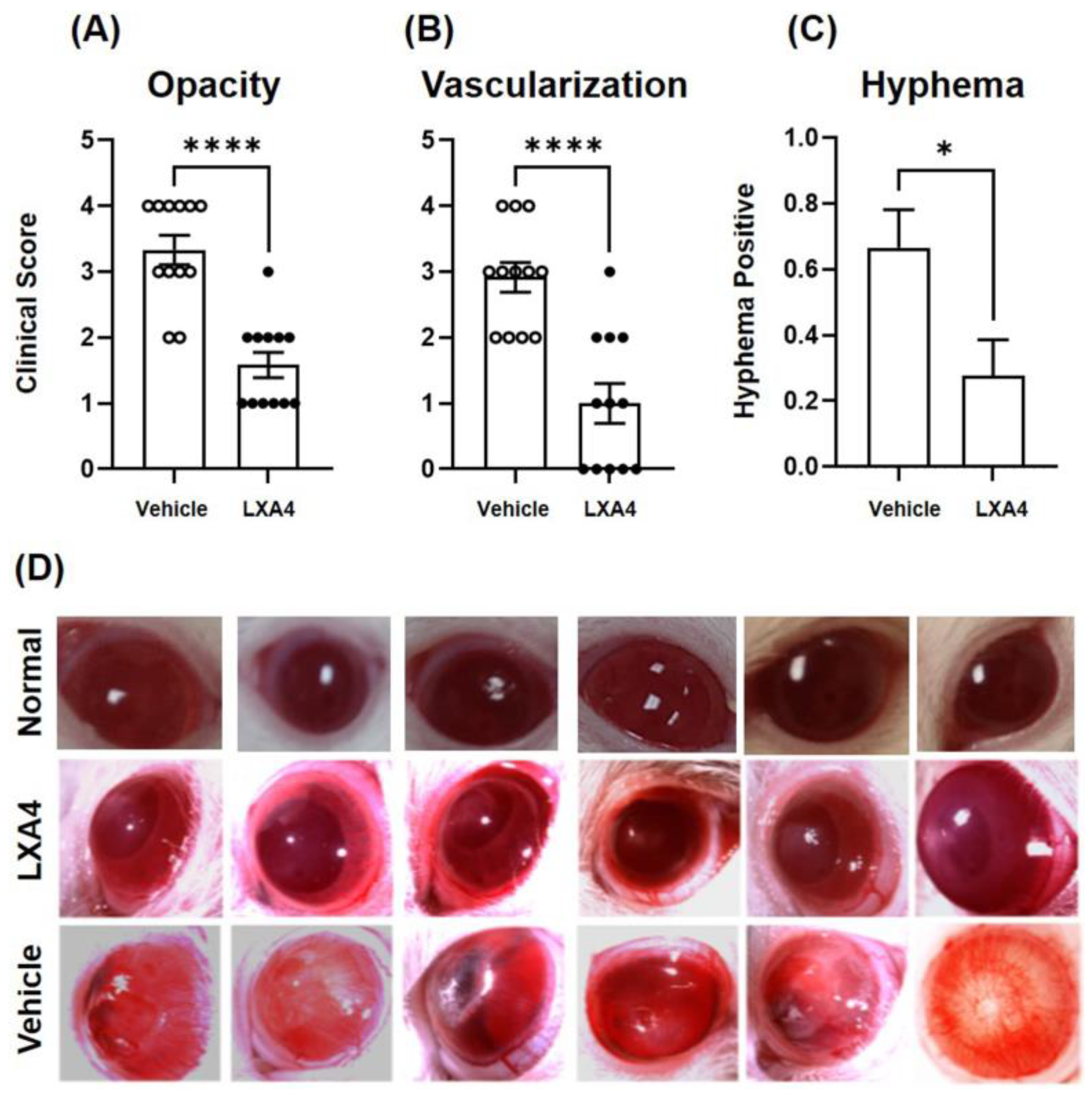
3.1. Clinical Evaluation
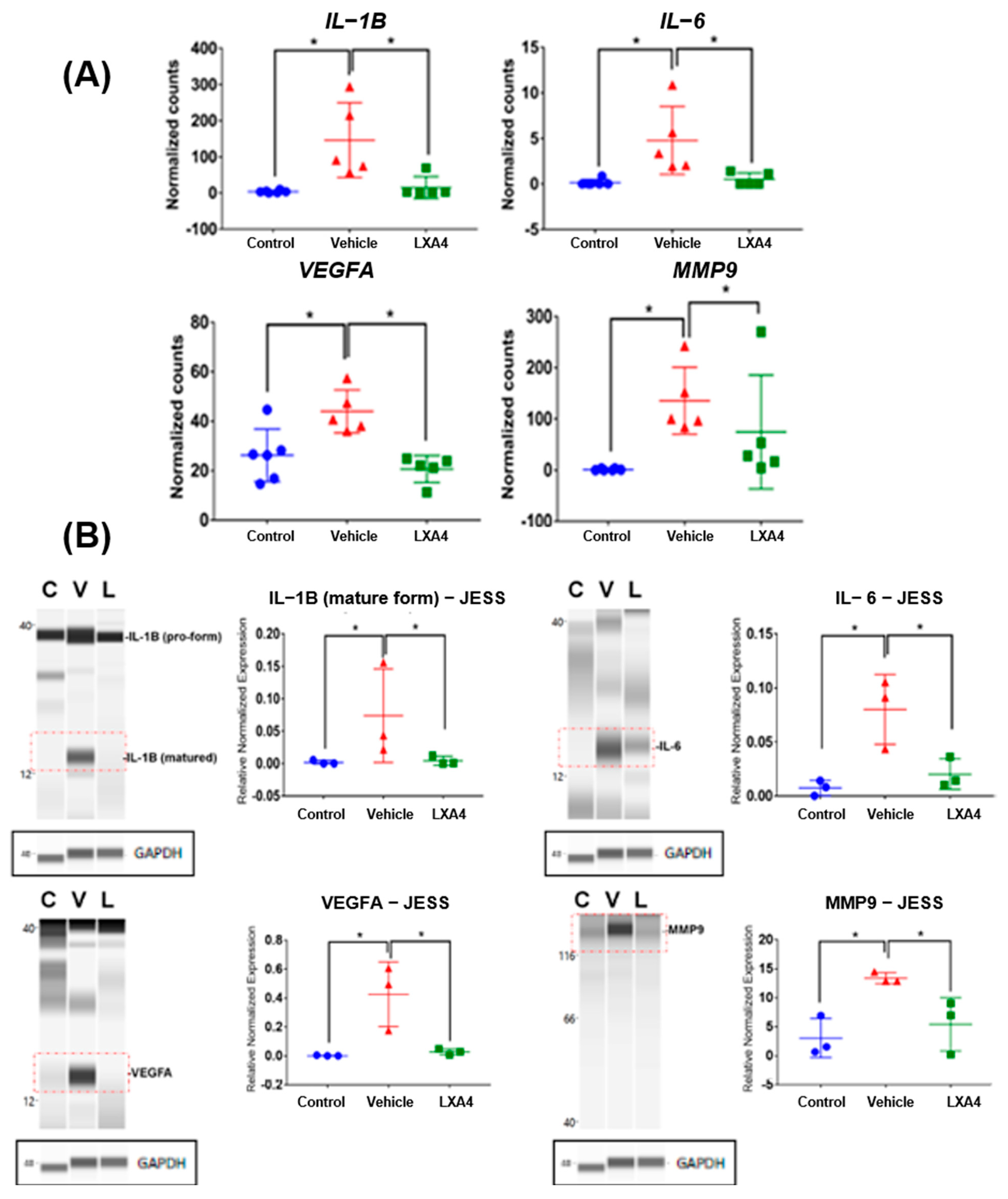
3.2. Expression of Inflammatory Cytokines and Angiogenic Mediators in Alkali-Burned Corneas
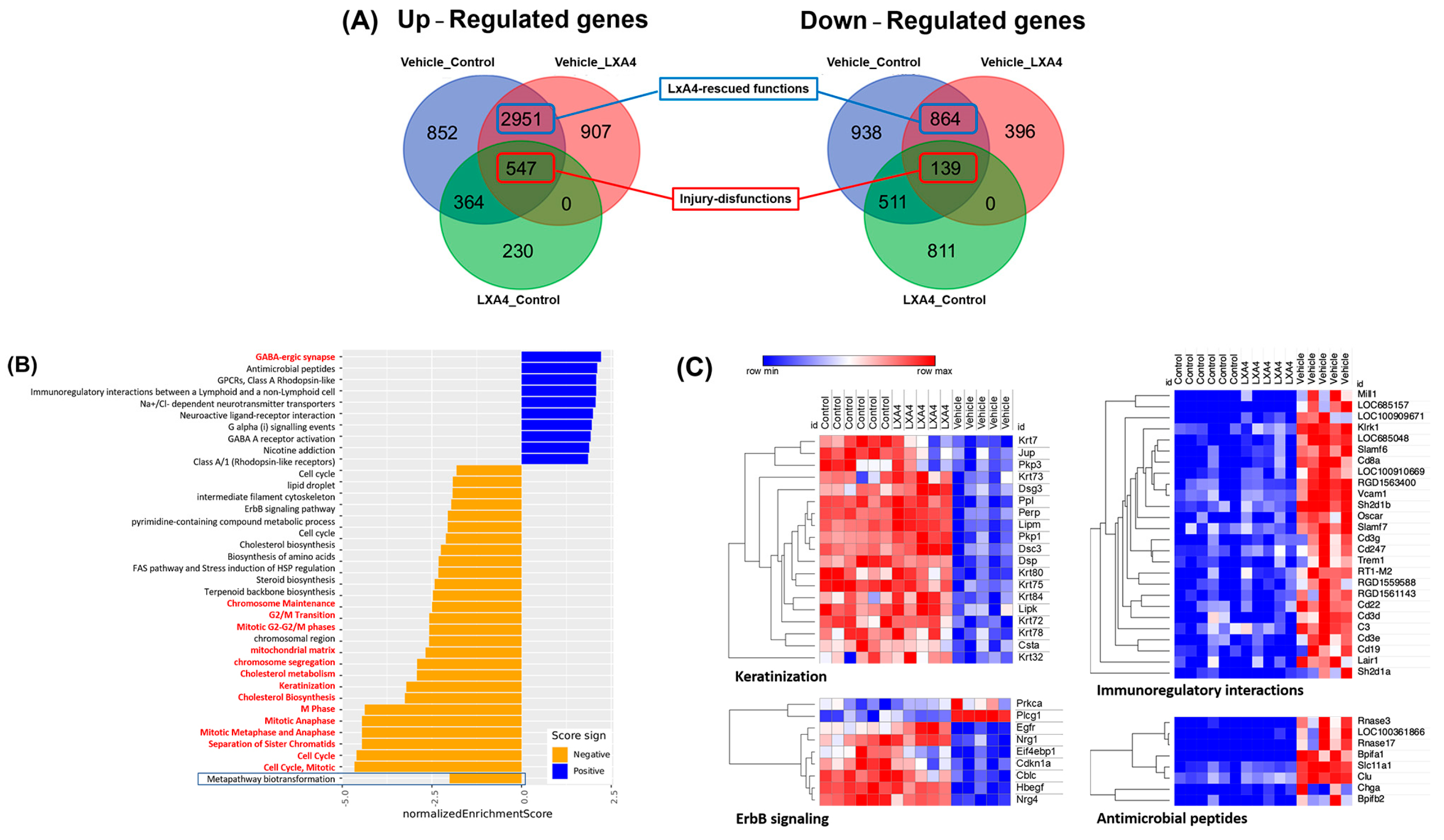
3.3. Molecular Mechanism of LXA4 Enhance Corneal Wound Healing after Alkali-Induced Corneal Damage
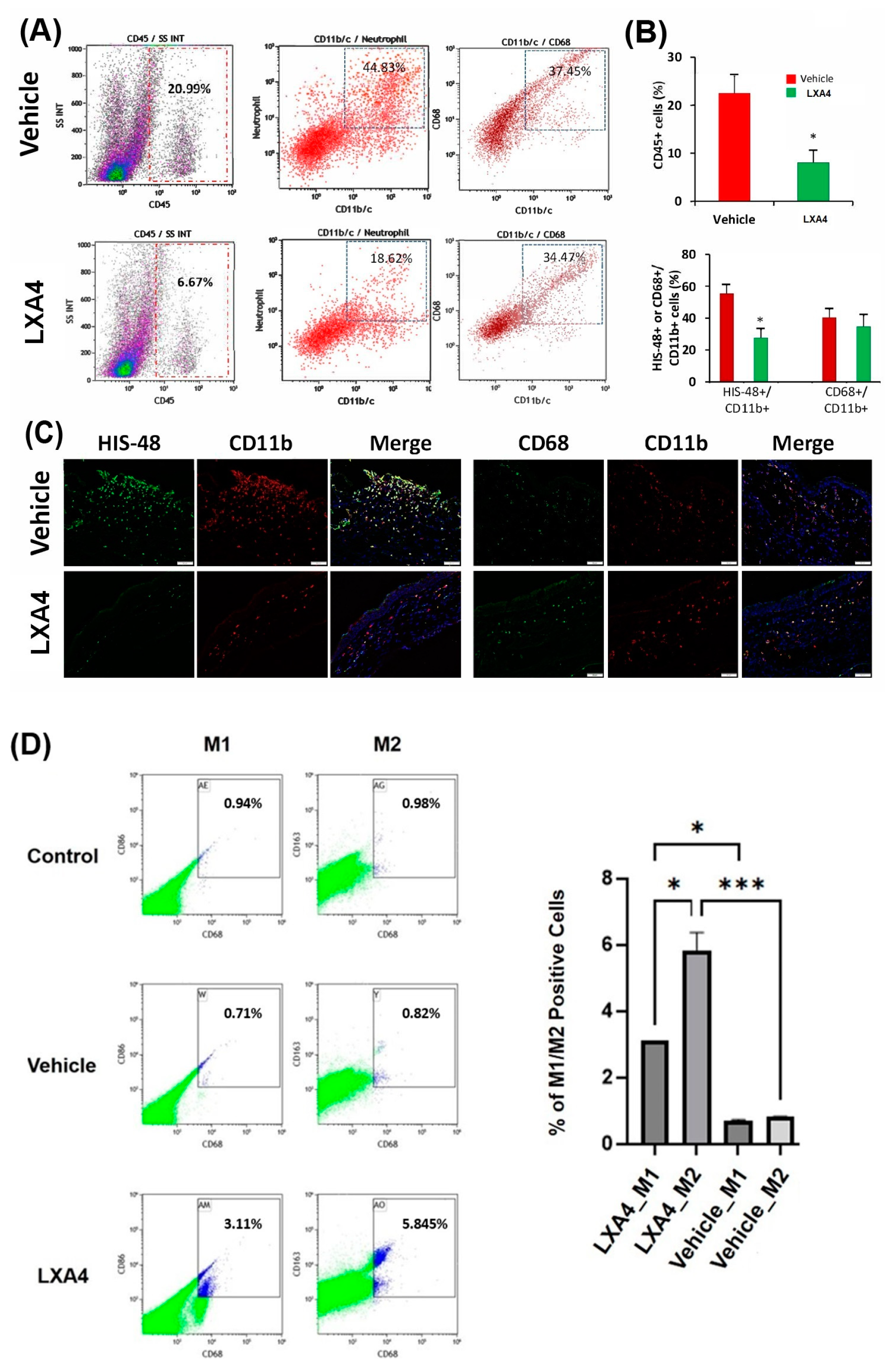
3.4. Effect of LXA4 on Alkali-Induced Inflammatory Cell Infiltration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagoner, M.D. Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv. Ophthalmol. 1997, 41, 275–313. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, B.E.; Klein, R. The prevalence and 5-year incidence of ocular trauma: The beaver dam eye study. Ophthalmology 2000, 107, 2196–2202. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Haq, Z.; Shirzadeh, E.; Huvard, M.J.; Djalilian, A.R. Current and Upcoming Therapies for Ocular Surface Chemical Injuries. Ocul. Surf. 2017, 15, 48–64. [Google Scholar] [CrossRef]
- Pham, T.L.; Bazan, H.E.P. Docosanoids signaling modulates corneal nerve regeneration: Effect on tear secretion, wound healing and neuropathic pain. J. Lipid Res. 2021, 62, 100033. [Google Scholar] [CrossRef]
- Petroutsos, G.; Guimaraes, R.; Giraud, J.P.; Pouliquen, Y. Corticosteroids and corneal epithelial wound healing. Br. J. Ophthalmol. 1982, 66, 705–708. [Google Scholar] [CrossRef]
- Becker, B.; Mills, D.W. Elevated intraocular pressure following corticosteroid eye drops. JAMA 1963, 185, 884–886. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Berman, M.; Rose, J.; Gage, J. Prevention of stromal ulceration in the alkali-burned rabbit cornea by glued-on contact lens. Evidence for the role of polymorphonuclear leukocytes in collagen degradation. Investig. Ophthalmol. Vis. Sci. 1979, 18, 570–587. [Google Scholar]
- Tseng, S.C.; Prabhasawat, P.; Barton, K.; Gray, T.; Meller, D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol. 1998, 116, 431–441. [Google Scholar] [CrossRef]
- Pfister, R.R.; Paterson, C.A.; Spiers, J.W.; Hayes, S.A. The efficacy of ascorbate treatment after severe experimental alkali burns de-pends upon the route of administration. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1526–1529. [Google Scholar]
- Burns, F.R.; Gray, R.D.; Paterson, C.A. Inhibition of alkali-induced corneal ulceration and perforation by a thiol peptide. Investig. Ophthalmol. Vis. Sci. 1990, 31, 107–114. [Google Scholar]
- Pfister, R.R.; Haddox, J.L.; I Sommers, C. Effect of synthetic metalloproteinase inhibitor or citrate on neutrophil chemotaxis and the respiratory burst. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1340–1349. [Google Scholar]
- Sotozono, C.; He, J.; Tei, M.; Honma, Y.; Kinoshita, S. Effect of metalloproteinase inhibitor on corneal cytokine expression after alkali injury. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2430–2434. [Google Scholar]
- Holland, E.J. Management of limbal stem cells deficiency: A historical perspective, past, present and future. Cornea 2015, 34, 9–15. [Google Scholar] [CrossRef]
- Nieto Nicolau, N.; Martinez-Conesa, E.M.; Fuentes-Julian, S.; Arnalich-Montiel, F.; Garcia-Tunon, I.; De Miguel, M.P.; Casaroli-Marano, R.P. Priming human adipose-derived mesenchymal stem cells for corneal surface regeneration. J. Cell. Mol. Med. 2021, 25, 5124–5137. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2018, 206, 18–40. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Yin, J.; Jurkunas, U. Limbal stem cell transplantation and complications. Semin. Ophthalmol. 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Bazan, H.E.P.; Reddy, S.T.K.; Lin, N. Platelet-activating factor (PAF) accumulation correlates with injury in the cornea. Exp. Eye Res. 1991, 52, 481–491. [Google Scholar] [CrossRef]
- Bazan, H.E.P.; Ottino, P. The role of platelet-activating factor in the corneal response to injury. Prog. Retin. Eye Res. 2002, 21, 449–464. [Google Scholar] [CrossRef]
- He, J.; Eastlack, J.P.; Bazan, H.E. The induction of an angiogenic response in corneal myofibroblasts by platelet-activating factor (PAF). Curr. Eye Res. 2010, 35, 1063–1071. [Google Scholar] [CrossRef]
- Sotozono, C.; He, J.; Matsumoto, Y.; Kita, M.; Imanishi, J.; Kinoshita, S. Cytokine expression in the alkali-burned cornea. Curr. Eye Res. 1997, 16, 670–676. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- Flitter, B.A.; Fang, X.; Matthay, M.A.; Gronert, K. The potential of lipid mediator networks as ocular surface therapeutics and biomarkers. Ocul. Surf. 2020, 19, 104–114. [Google Scholar] [CrossRef]
- He, J.; Kakazu, A.H.; Bazan, N.G.; Bazan, H.E. Aspirin-Triggered Lipoxin A4 (15-epi-LXA4) Increases the Endothelial Viability of Human Corneas Storage in Optisol-GS. J. Ocul. Pharmacol. Ther. 2011, 27, 235–241. [Google Scholar] [CrossRef]
- Kakazu, A.; He, J.; Kenchegowda, S.; Bazan, H.E.P. Lipoxin A₄ inhibits platelet-activating factor inflammatory response and stim-ulates corneal wound healing of injuries that compromise the stroma. Exp. Eye Res. 2012, 103, 9–16. [Google Scholar] [CrossRef]
- Kenchegowda, S.; Bazan, N.G.; Bazan, H.E.P. EGF Stimulates Lipoxin A4 Synthesis and Modulates Repair in Corneal Epithelial Cells through ERK and p38 Activation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2240–2249. [Google Scholar] [CrossRef]
- Yamada, J.; Dana, M.R.; Sotozono, C.; Kinoshita, S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp. Eye Res. 2003, 76, 161–167. [Google Scholar] [CrossRef]
- Gupta, N.; Kalaivani, M.; Tandon, R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. Br. J. Ophthalmol. 2010, 95, 194–198. [Google Scholar] [CrossRef]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.-K.; Won, J.-K.; Fulcher, S. Comprehensive Modeling of Corneal Alkali Injury in the Rat Eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general-purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; He, J.; Kakazu, A.H.; Calandria, J.; Do, K.V.; Nishimiyimana, R.; Lam, T.F.; Petasis, N.A.; Bazan, H.E.P.; Bazan, N.G. ELV-N32 and RcD6 isomer decrease proinflammatory cytokines, senescence programming, ACE2 and SARS-CoV-2 spike protein RBD binding in injured cornea. Sci. Rep. 2021, 11, 12787. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Navarro, A.; Guerrero-Hue, M.; Martín-Fernandez, B.; Cortegano, I.; Olivares-Alvaro, E.; de Las Heras, N.; Alía, M.; de Andrés, B.; Gaspar, M.L.; Egido, J.; et al. Phenotypic Characterization of Macrophages from Rat Kidney by Flow Cytometry. J. Vis. Exp. 2016, 116, e54599. [Google Scholar]
- Able, J.R.; Boyles, D.A.; Walters, A.W.; Kujawa, M.R.; McMillen, C.M.; Reed, D.S.; Hartman, A.L. Neutrophil and macrophage influx into the central nervous system are inflammatory components of lethal Rift Valley fever encephalitis in rats. PLoS Pathog. 2019, 15, e1007833. [Google Scholar]
- Tao, Y.; Bazan, H.E.; Bazan, N.G. Platelet-activating factor induces the expression of metalloproteinases-1 and -9, but not -2 or -3, in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 345–354. [Google Scholar]
- Chandrasekher, G.; Ma, X.; Lallier, T.E.; Bazan, H.E.P. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet-activating factor. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1422–1428. [Google Scholar]
- He, J.; Bazan, N.G.; Bazan, H.E. Alkali-induced corneal stromal melting prevention by a novel platelet-activating factor receptor antagonist. Arch. Ophthalmol. 2006, 124, 70–78. [Google Scholar] [CrossRef]
- Sanchez-Crespo, M.; Alonso, F.; Figaro, J. Platelet activating factor in anaphylaxis and phagocytosis. I. Release from human peripheral polymorphonuclear and monocytes during the stimulation by ionophore A23187 and phagocytosis but not from degranulating basophils. Immunology 1980, 40, 645–655. [Google Scholar]
- Arnoux, B.; Duval, D.; Benveniste, J. Release of platelet-activating factor (PAF-acether) from alveolar macrophages by the calcium ionophore A 23187 and phagocytosis. Eur. J. Clin. Investig. 1980, 10, 437–441. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar]
- Clements, J.L.; Dana, R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin. Ophthalmol. 2011, 26, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, T.; Sugaya, S.; Ishimori, A.; Sawa, M. Anti-inflammatory effect of IL-6 receptor blockade in corneal alkali burn. Exp. Eye Res. 2012, 97, 98–104. [Google Scholar] [CrossRef]
- Keating, A.M.; Jacobs, D.S. Anti-VEGF Treatment of Corneal Neovascularization. Ocul. Surf. 2011, 9, 227–238. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; He, Y.; Yao, B.; Zhang, Y. Regulation of matrix metalloproteinases 2 and 9 in corneal neovascularization. Chem. Biol. Drug Des. 2019, 95, 485–492. [Google Scholar] [CrossRef]
- Hollborn, M.; Stathopoulos, C.; Steffen, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Ebrahem, Q.; Chaurasia, S.S.; Vasanji, A.; Qi, J.H.; Klenotic, P.A.; Cutler, A.; Asosingh, K.; Erzurum, S.; Anand-Apte, B. Crosstalk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am. J. Pathol. 2010, 176, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sotozono, C.; Kinoshita, S. The epidermal growth factor receptor (EGFR): Role on corneal wound healing and homeostasis. Exp. Eye Res. 2001, 72, 511–517. [Google Scholar] [CrossRef]
- Conrad, C.; Meller, S.; Gilliet, M. Plasmacytoid dendritic cells in the skin: To sense or not to sense nucleic acids. Semin. Immunol. 2009, 21, 101–109. [Google Scholar] [CrossRef]
- Biteman, B.; Hassan, I.R.; Walker, E.; Leedom, A.J.; Dunn, M.; Seta, F.; Laniado-Schwartzman, M.; Gronert, K. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007, 21, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Gronert, K.; Maheshwari, N.; Khan, N.; Hassan, I.R.; Dunn, M.; Laniado-Schwartzman, M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005, 280, 15267–15278. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Supplier | Cat. Number | Dilution |
|---|---|---|---|
| Mouse anti-HIS48, FITC | Santa Cruz BT | sc-19613 FITC | 1:100 (IF) |
| Mouse anti-CD68 (KP1), FITC | Santa Cruz BT | sc-20060 FITC | 1:100 (IF) |
| Mouse anti-CD11b, (TJL-163), Alexa Fluor 594 | Creative Biolabs | CTMM-0322-JL1586 | 1:200 (IF) |
| Rabbit anti-IL-1β (EPR23851-127) | Abcam | ab254360 | 1:50 (Jess WB) |
| Rabbit anti-IL-6 (EPR23819-11) | Abcam | ab281935 | 1:50 (Jess WB) |
| Rabbit anti-MMP9 (EPR22140-154) | Abcam | ab228402 | 1:50 (Jess WB) |
| Rabbit anti-VEGFA | Abcam | ab231260 | 1:50 (Jess WB) |
| Mouse anti-GAPDH (6C5) | Santa Cruz BT | sc-32233 | 1:50 (Jess WB) |
| Mouse anti-CD45, Pacific Blue | Biolegend | 202226 | 1:100 (FC) |
| Mouse anti-CD11b/c, PE/Cyanine7 | Biolegend | 201818 | 1:100 (FC) |
| Mouse anti-CD86, Alexa Fluor 647 | Biolegend | 200314 | 1:100 (FC) |
| Mouse anti-CD68, Alexa Fluor 700 | Bio-Rad | MCA341A700 | 1:100 (FC |
| Mouse anti-CD163, FITC | Bio-Rad | MCA342F | 1:100 (FC) |
| Mouse anti-HIS48, PE | Santa Cruz BT | sc-19613 PE | 1:50 (FC) |
| Mouse anti-rat CD32 (for blocking) | BD Biosciences | 550270 | 1:50 (FC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Pham, T.L.; Kakazu, A.H.; Ponnath, A.; Do, K.V.; Bazan, H.E.P. Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome. Biomolecules 2023, 13, 831. https://doi.org/10.3390/biom13050831
He J, Pham TL, Kakazu AH, Ponnath A, Do KV, Bazan HEP. Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome. Biomolecules. 2023; 13(5):831. https://doi.org/10.3390/biom13050831
Chicago/Turabian StyleHe, Jiucheng, Thang L. Pham, Azucena H. Kakazu, Abhilash Ponnath, Khanh V. Do, and Haydee E. P. Bazan. 2023. "Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome" Biomolecules 13, no. 5: 831. https://doi.org/10.3390/biom13050831
APA StyleHe, J., Pham, T. L., Kakazu, A. H., Ponnath, A., Do, K. V., & Bazan, H. E. P. (2023). Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome. Biomolecules, 13(5), 831. https://doi.org/10.3390/biom13050831






