Preclinical Characterization of a Stabilized Gastrin-Releasing Peptide Receptor Antagonist for Targeted Cancer Theranostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Radiolabeling
2.3. Radiometal Complex Stability
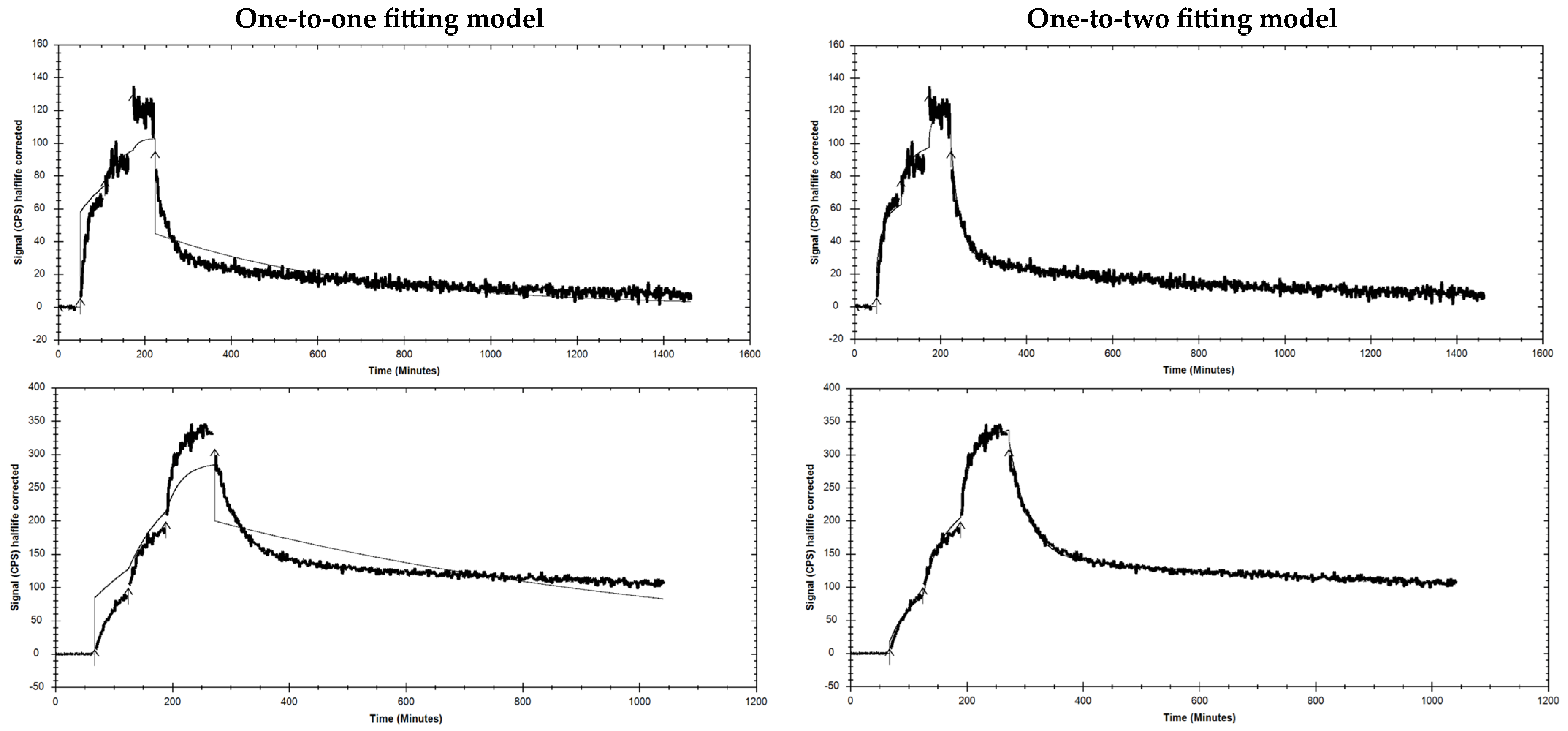
2.4. In Vivo Metabolic Stability
2.5. Cell Binding and Internalization
2.6. Affinity Measurements
2.7. In Vivo Experiments
2.8. SPECT/CT Imaging
3. Results
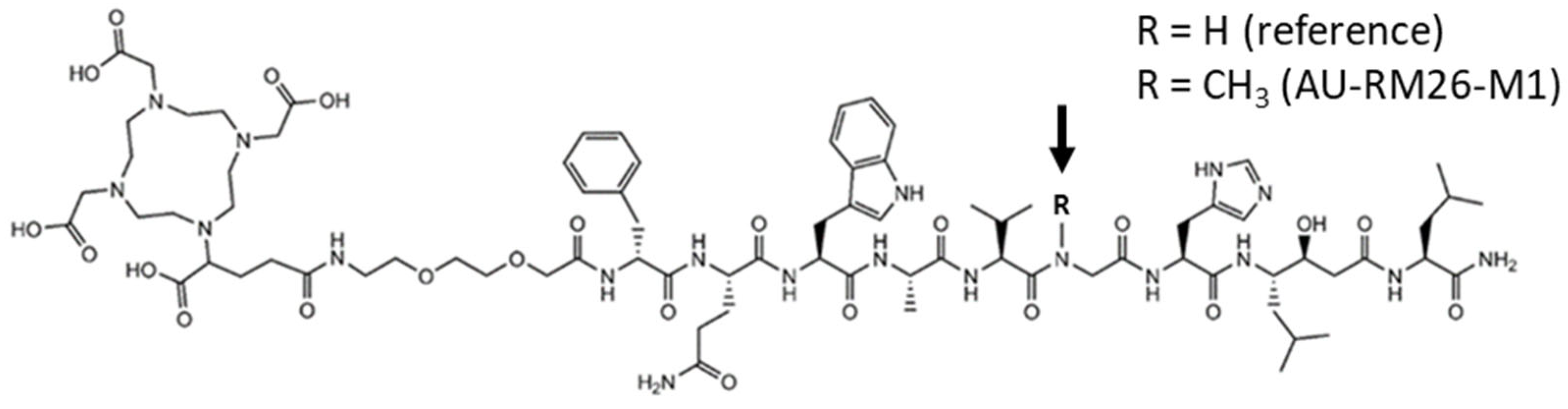
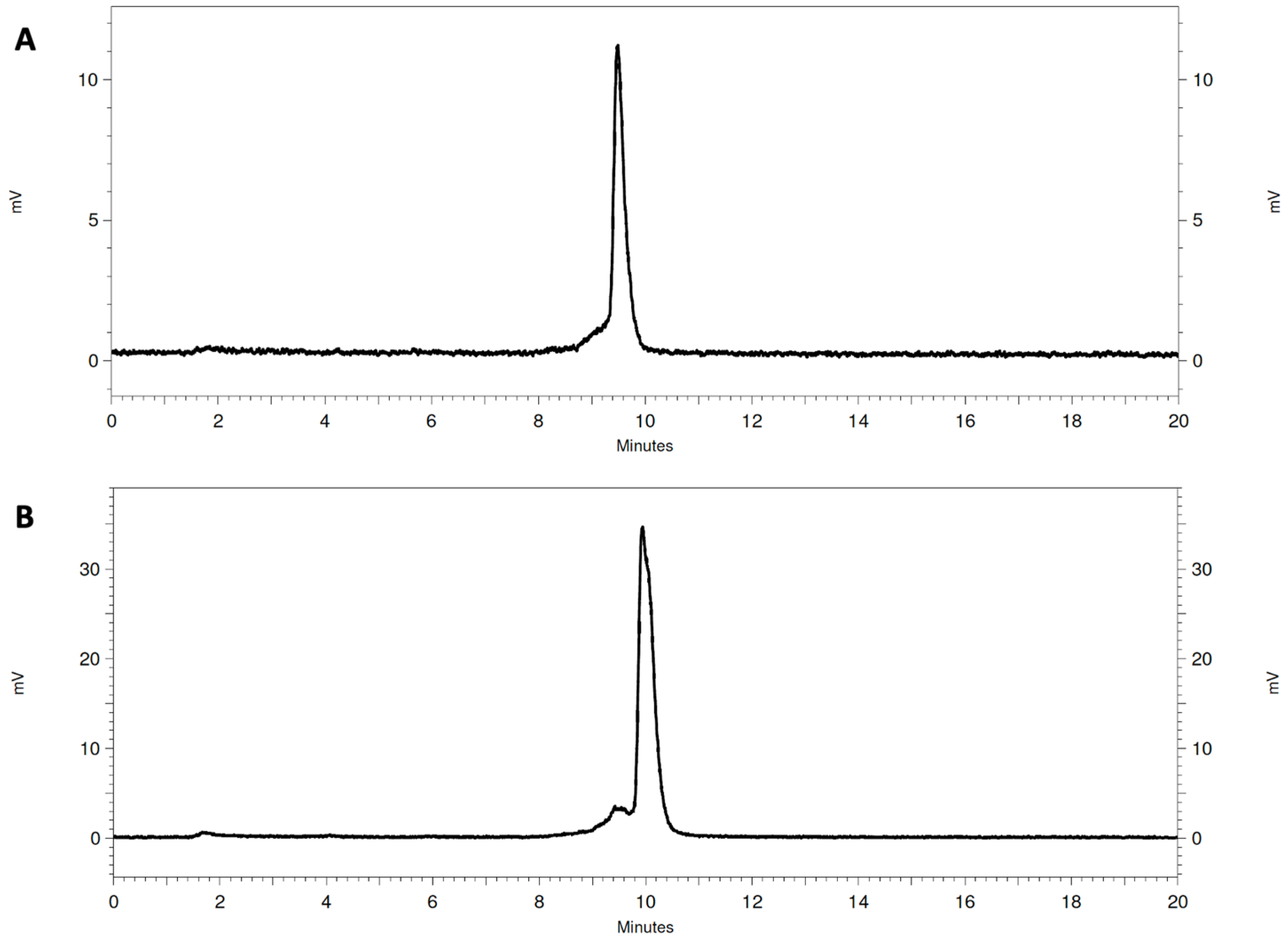
3.1. Radiolabeling and Radiochemical Stability
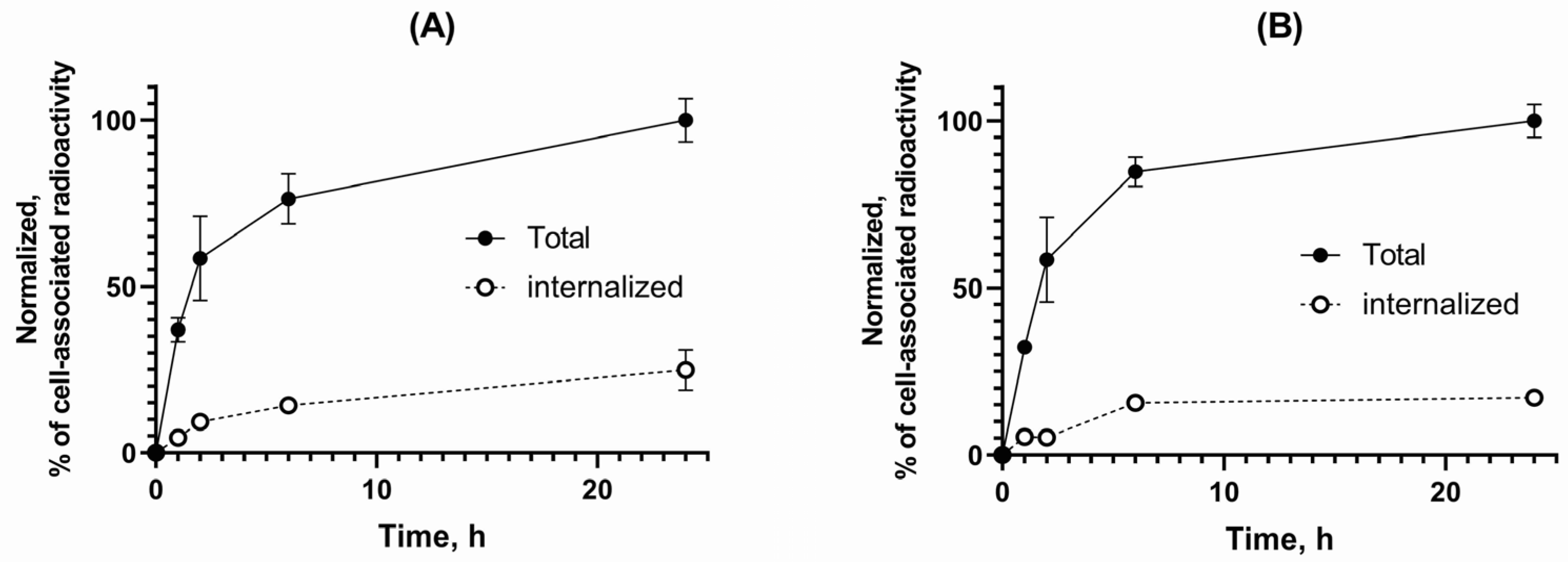
3.2. Cell Binding and Internalization
3.3. Affinity Measurements
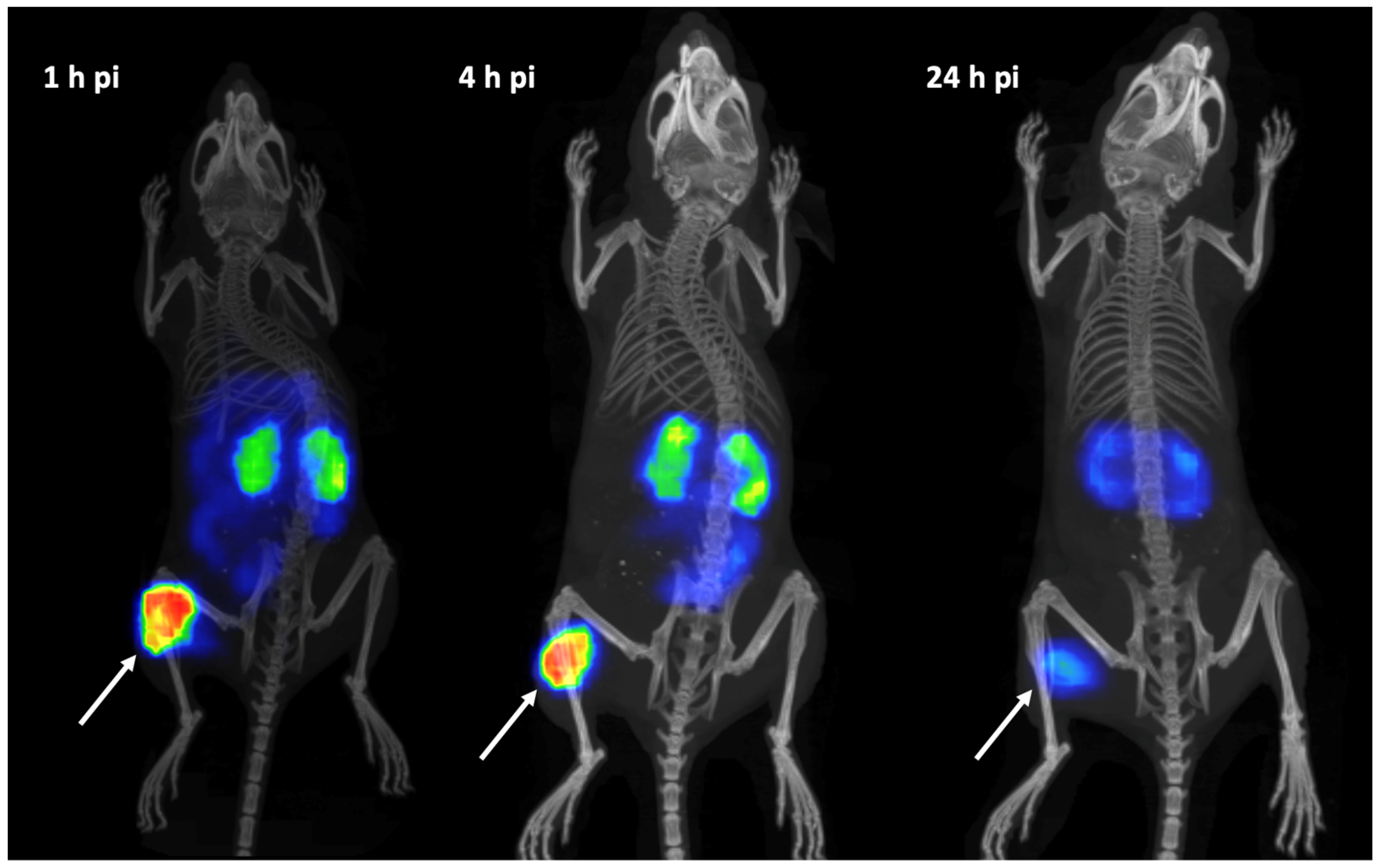
3.4. In Vivo Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| %IA/g | ||||||
|---|---|---|---|---|---|---|
| Variant Organ | [111In]In-AU-RM26-M1 | [111In]In-DOTAGA-PEG2-RM26 | ||||
| 1 h | 4 h | 24 h | 1 h | 4 h | 24 h | |
| Blood | 0.6 ± 0.1 | 0.11 ± 0.07 | 0.02 ± 0.01 | 0.5 ± 0.3 | 0.07 ± 0.01 | 0.015 ± 0.003 |
| Lungs | 0.46 ± 0.09 | 0.17 ± 0.06 | 0.03 ± 0.03 | 0.5 ± 0.2 | 0.11 ± 0.02 | 0.04 ± 0.01 |
| Liver | 0.55 ± 0.04 | 0.24 ± 0.02 | 0.10 ± 0.03 c | 0.5 ± 0.1 | 0.23 ± 0.05 | 0.16 ± 0.02 |
| Spleen | 0.3 ± 0.2 | 0.22 ± 0.07 b | 0.10 ± 0.03 | 0.21 ± 0.06 | 0.10 ± 0.02 | 0.10 ± 0.03 |
| Pancreas | 2.23 ± 0.56 | 0.29 ± 0.02 b | 0.03 ± 0.01 | 1.9 ± 1.0 | 0.18 ± 0.04 | 0.03 ± 0.01 |
| Small intestine | 1.1 ± 0.6 | 0.3 ± 0.2 | 0.03 ± 0.01 | 0.8 ± 0.2 | 0.13 ± 0.04 | 0.05 ± 0.05 |
| Kidneys | 7.2 ± 0.9 a | 6 ± 1 b | 3.0 ± 0.9 | 4.6 ± 0.9 | 3.5 ± 0.7 | 2.0 ± 0.5 |
| Tumor | 7.0 ± 0.7 | 6 ± 2 b | 1.8 ± 0.6 | 6.0 ± 0.4 | 3.0 ± 1.00 | 1.2 ± 0.4 |
| Muscle | 0.15 ± 0.08 | 0.13 ± 0.05 b | 0.01 ± 0.01 | 0.11 ± 0.08 | 0.04 ± 0.01 | 0.01 ± 0.01 |
| Bone | 0.17 ± 0.09 | 0.09 ± 0.03 | 0.06 ± 0.03 | 0.13 ± 0.08 | 0.07 ± 0.04 | 0.04 ± 0.03 |
| Tumor-to-Organ Ratios | ||||||
|---|---|---|---|---|---|---|
| Variant Organ | [111In]In-AU-RM26- M1 | [111In]In-DOTAGA-PEG2-RM26 | ||||
| 1 h | 4 h | 24 h | 1 h | 4 h | 24 h | |
| Blood | 12 ± 3 | 63 ± 38 | 119 ± 54 | 15 ± 6 | 43 ± 16 | 79 ± 34 |
| Lungs | 16 ± 31 | 38 ± 19 | 77 ± 62 | 15 ± 5 | 29 ± 11 | 30 ± 18 |
| Liver | 13 ± 2 | 24 ± 6 | 21 ± 16 | 11 ± 3 | 14 ± 6 | 7 ± 2 |
| Spleen | 31 ± 16 | 30 ± 17 | 21 ± 11 | 30 ± 8 | 30 ± 12 | 12± 3 |
| Pancreas | 3.3 ± 0.8 | 20 ± 6 | 64 ± 20 | 4 ± 2 | 17 ± 8 | 46 ± 15 |
| Small intestine | 7 ± 3 | 22 ± 11 | 76 ± 17 b | 8 ± 2 | 26 ± 14 | 28± 14 |
| Kidneys | 0.9 ± 0.1 a | 0.9 ± 0.3 | 0.6 ± 0.3 | 1.3 ± 0.3 | 0.9 ± 0.4 | 0.6 ± 0.2 |
| Muscle | 57 ± 24 | 47 ± 16 | 206 ± 97 | 78 ± 36 | 74 ± 20 | 91 ± 72 |
| Bone | 51 ± 24 | 74 ± 34 | 34 ± 12 | 61 ± 33 | 72 ± 65 | 21 ± 3 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2022, 29, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Widjaja, L.; Werner, R.A.; Bengel, F.M. 177Lu-PSMA for Extended Treatment of metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2023, 64, 54–58. [Google Scholar] [CrossRef]
- Faviana, P.; Boldrini, L.; Erba, P.A.; Di Stefano, I.; Manassero, F.; Bartoletti, R.; Galli, L.; Gentile, C.; Bardi, M. Gastrin-Releasing Peptide Receptor in Low Grade Prostate Cancer: Can It Be a Better Predictor Than Prostate-Specific Membrane Antigen? Front. Oncol. 2021, 11, 650249. [Google Scholar] [CrossRef]
- Bakht, M.L.; Derecichei, I.; Li, Y.; Ferraiuolo, R.; Dunning, M.; Oh, S.W.; Hussein, A.; Youn, H.; Stringer, K.F.; Jeong, C.W.; et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr.-Relat. Cancer 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2002, 18, 639–649. [Google Scholar] [CrossRef]
- Hildebrand, P.; Lehmann, F.S.; Ketterer, S.; Christ, A.D.; Stingelin, T.; Beltinger, J.; Gibbons, A.H.; Coy, D.H.; Calam, J.; Larsen, F.; et al. Regulation of gastric function by endogenous gastrin releasing peptide in humans: Studies with a specific gastrin releasing peptide receptor antagonist. Gut 2001, 49, 23–28. [Google Scholar] [CrossRef]
- Degen, L.P.; Peng, F.; Collet, A.; Rossi, L.; Letterer, S.; Serrano, Y.; Larsen, F.; Beglinger, C.; Hildebrand, P. Blockade of GRP Receptors Inhibits Gastric Emptying and Gallbladder Contraction but Accelerates Small Intestinal Transit. Gastroenterology 2001, 120, 361–368. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, J.; Hampton, L.L.; Weber, H.C. The human gastrin-releasing peptide receptor gene structure. Its tissue expression and promoter. Gene 2001, 264, 95–103. [Google Scholar] [CrossRef]
- Sun, B.; Halmos, G.; Schally, A.V.; Wang, X.; Martinez, M. Presence of Receptors for Bombesin/Gastrin-Releasing Peptide and mRNA for Three Receptor Subtypes in Human Prostate Cancer. Prostate 2000, 42, 295–303. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[D-Tyr6, beta-Ala11,Phe13, Nle14]bombesin(6-14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- Ananias, H.J.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the Gastrin-Releasing Peptide Receptor. the Prostate Stem Cell Antigen and the Prostate-Specific Membrane Antigen in Lymph Node and Bone Metastases of Prostate Cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing Peptide Receptors in the Human Prostate: Relation to Neoplastic Transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Müntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef]
- de Visser, M.; van Weerden, W.M.; de Ridder, C.M.; Reneman, S.; Melis, M.; Krenning, E.P.; de Jong, M. Androgen-dependent expression of the gastrin-releasing peptide receptor in human prostate tumor xenografts. J. Nucl. Med. 2007, 48, 88–93. [Google Scholar]
- Schroeder, R.P.; de Visser, M.; van Weerden, W.M.; de Ridder, C.M.; Reneman, S.; Melis, M.; Breeman, W.A.; Krenning, E.P.; de Jong, M. Androgen-regulated gastrin-releasing peptide receptor expression in androgen-dependent human prostate tumor xenografts. Int. J. Cancer 2010, 126, 2826–2834. [Google Scholar] [CrossRef]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-releasing peptide receptor (GRPr) promotes EMT. growth. and invasion in canine prostate cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef]
- Pansky, A.; De Weerth, A.; Fasler-Kan, E.; Boulay, J.L.; Schulz, M.; Ketterer, S.; Selck, C.; Beglinger, C.; VON Schrenck, T.; Hildebrand, P. Gastrin releasing peptide-preferring bombesin receptors mediate growth of human renal cell carcinoma. J. Am. Soc. Nephrol. 2000, 11, 1409–1418. [Google Scholar] [CrossRef]
- Millar, J.B.; Rozengurt, E. Chronic desensitization to bombesin by progressive down-regulation of bombesin receptors in Swiss 3T3 cells. Distinction from acute desensitization. J. Biol. Chem. 1990, 265, 12052–12058. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetraacetic Acid–Conjugated Bombesin-Based Radioantagonist for the Labeling with Single-Photon Emission Computed Tomography. Positron Emission Tomography. and Therapeutic Radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin Receptor Antagonists May Be Preferable to Agonists for Tumor Targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Sallegger, W.; Bakker, I.L.; Krenning, E.P.; de Jong, M.; Maina, T.; Nock, B.A. Radiometal-Dependent Biological Profile of the Radiolabeled Gastrin-Releasing Peptide Receptor Antagonist SB3 in Cancer Theranostics: Metabolic and Biodistribution Patterns Defined by Neprilysin. Bioconjugate Chem. 2018, 29, 1774–1784. [Google Scholar] [CrossRef]
- Linder, K.E.; Metcalfe, E.; Arunachalam, T.; Chen, J.; Eaton, S.M.; Feng, W.; Fan, H.; Raju, N.; Cagnolini, A.; Lantry, L.E.; et al. In vitro and in vivo metabolism of Lu-AMBA, a GRP-receptor binding compound, and the synthesis and characterization of its metabolites. Bioconjugate Chem. 2009, 20, 1171–1178. [Google Scholar] [CrossRef]
- Chatalic, K.L.S.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.-A.; Martinez, J.; Van Gent, D.C.; et al. In Vivo Stabilization of a Gastrin-Releasing Peptide Receptor Antagonist Enhances PET Imaging and Radionuclide Therapy of Prostate Cancer in Preclinical Studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled bombesin analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef]
- Llinares, M.; Devin, C.; Chaloin, O.; Azay, J.; Noel-Artis, A.M.; Bernad, N.; Fehrentz, J.; Martinez, J. Syntheses and biological activities of potent bombesin receptor antagonists. J. Pept. Res. 1999, 53, 275–283. [Google Scholar] [CrossRef]
- Mitran, B.; Varasteh, Z.; Selvaraju, R.K.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Selection of optimal chelator improves the contrast of GRPR imaging using bombesin analogue RM26. Int. J. Oncol. 2016, 48, 2124–2134. [Google Scholar] [CrossRef]
- Mitran, B.; Rinne, S.S.; Konijnenberg, M.W.; Maina, T.; Nock, B.A.; Altai, M.; Vorobyeva, A.; Larhed, M.; Tolmachev, V.; de Jong, M.; et al. Trastuzumab cotreatment improves survival of mice with PC-3 prostate cancer xenografts treated with the GRPR antagonist 177Lu-DOTAGA-PEG2-RM26. Int. J. Cancer 2019, 145, 3347–3358. [Google Scholar] [CrossRef]
- Nock, B.A.; Charalambidis, D.; Sallegger, W.; Waser, B.; Mansi, R.; Nicolas, G.P.; Ketani, E.; Nikolopoulou, A.; Fani, M.; Reubi, J.C.; et al. New Gastrin Releasing Peptide Receptor-Directed [99mTc]Demobesin 1 Mimics: Synthesis and Comparative Evaluation. J. Med. Chem. 2018, 61, 3138–3150. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Kanellopoulos, P.; Janota, B.; Bromińska, B.; Iżycki, D.; Mikołajczak, R.; Czepczynski, R.; Maina, T. [99mTc]Tc-DB15 in GRPR-Targeted Tumor Imaging with SPECT: From Preclinical Evaluation to the First Clinical Outcomes. Cancers 2021, 13, 5093. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.; Langenickel, T.; Pal, P.; Boggarapu, S.; Sunkara, G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): A novel angiotensin receptor-neprilysin inhibitor. Clin. Pharmacokinet. 2017, 56, 1461–1478. [Google Scholar] [CrossRef]
- Kanellopoulos, P.; Kaloudi, A.; Rouchota, M.; Loudos, G.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto. Pharmaceutics 2020, 12, 1145. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A. Slow Internalization of Anti-HER2 Synthetic Affibody Monomer 111In-DOTA-ZHER2:342-pep2: Implications for Development of Labeled Tracers. Cancer Biother Radiopharm 2008, 23, 435–442. [Google Scholar]
- Zhao, R.; Ploessl, K.; Zha, Z.; Choi, S.; Alexoff, D.; Zhu, L.; Kung, H.F. Synthesis and Evaluation of 68Ga- and 177Lu-Labeled (R)- vs (S)-DOTAGA Prostate-Specific Membrane Antigen-Targeting Derivatives. Mol. Pharm. 2020, 17, 4589–4602. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Kanellopoulos, P.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. Comparing Gly11/dAla11-Replacement vs. the in-Situ Neprilysin-Inhibition Approach on the Tumor-targeting Efficacy of the 111In-SB3/111In-SB4 Radiotracer Pair. Molecules 2019, 24, 1015. [Google Scholar] [CrossRef]
- Shipp, M.A.; Tarr, G.E.; Chen, C.Y.; Switzer, S.N.; Hersh, L.B.; Stein, H.; Sunday, M.E.; Reinherz, E.L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. PNAS 1991, 88, 10662–10666. [Google Scholar] [CrossRef]
- Xu, B.; Varasteh, Z.; Orlova, A.; Andersson, K.; Larhammar, D.; Björkelund, H. Detecting interactions with GPCR in real-time on living cells to understand receptor dynamics. Biochem. Biophys Res. Comm. 2013, 441, 820–824. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.M.; Masereeuw, R.; Melis, M.; Oyen, W.J.G.; Gotthardt, M.; Boerman, O.C. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, E.A.M.; Verhoeven, M.; Konijnenberg, M.W.; de Blois, E.; de Ridder, C.M.A.; Stuurman, D.C.; Bertarione, L.; Rolfo, K.; de Jong, M.; Dalm, S.U. Safety of [177Lu]Lu-NeoB treatment: A preclinical study characterizing absorbed dose and acute, early, and late organ toxicity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4440–4451. [Google Scholar] [CrossRef] [PubMed]





| Radiopeptide | PBS | EDTA | Serum |
|---|---|---|---|
| [111In]In-AU-RM26-M1 | 100.0 ± 0.3 | 98.7 ± 0.3 | 99.2 ± 0.3 |
| [111In]In-DOTAGA-PEG2-RM26 | 99.90 ± 0.06 | 96 ± 3 | 99.9 ± 0.2 |
| Radiopeptide | ka1 (M−1 s−1) | kd1 (s−1) | KD1 (nM) | ka2 (M−1 s−1) | kd2 (s−1) | KD2 (nM) |
|---|---|---|---|---|---|---|
| [111In]In-AU-RM26-M1 | 5.7 ± 5.6 × 104 | 1.7 ± 3.3 × 10−6 | 0.53 ± 0.46 | 3.9 ± 2.3 × 105 | 5.7 ± 2.6 × 10−4 | 1.6 ± 0.3 |
| [111In]In-DOTAGA-PEG2-RM26 | 8.54 × 104 | 9.73 × 10−6 | 0.11 | 3.03 × 105 | 5.97 × 10−4 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouzayed, A.; Kanellopoulos, P.; Gorislav, A.; Tolmachev, V.; Maina, T.; Nock, B.A.; Orlova, A. Preclinical Characterization of a Stabilized Gastrin-Releasing Peptide Receptor Antagonist for Targeted Cancer Theranostics. Biomolecules 2023, 13, 1134. https://doi.org/10.3390/biom13071134
Abouzayed A, Kanellopoulos P, Gorislav A, Tolmachev V, Maina T, Nock BA, Orlova A. Preclinical Characterization of a Stabilized Gastrin-Releasing Peptide Receptor Antagonist for Targeted Cancer Theranostics. Biomolecules. 2023; 13(7):1134. https://doi.org/10.3390/biom13071134
Chicago/Turabian StyleAbouzayed, Ayman, Panagiotis Kanellopoulos, Alisa Gorislav, Vladimir Tolmachev, Theodosia Maina, Berthold A. Nock, and Anna Orlova. 2023. "Preclinical Characterization of a Stabilized Gastrin-Releasing Peptide Receptor Antagonist for Targeted Cancer Theranostics" Biomolecules 13, no. 7: 1134. https://doi.org/10.3390/biom13071134
APA StyleAbouzayed, A., Kanellopoulos, P., Gorislav, A., Tolmachev, V., Maina, T., Nock, B. A., & Orlova, A. (2023). Preclinical Characterization of a Stabilized Gastrin-Releasing Peptide Receptor Antagonist for Targeted Cancer Theranostics. Biomolecules, 13(7), 1134. https://doi.org/10.3390/biom13071134








