Effect of Sericin Content on the Structural Characteristics and Properties of New Silk Nonwoven Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
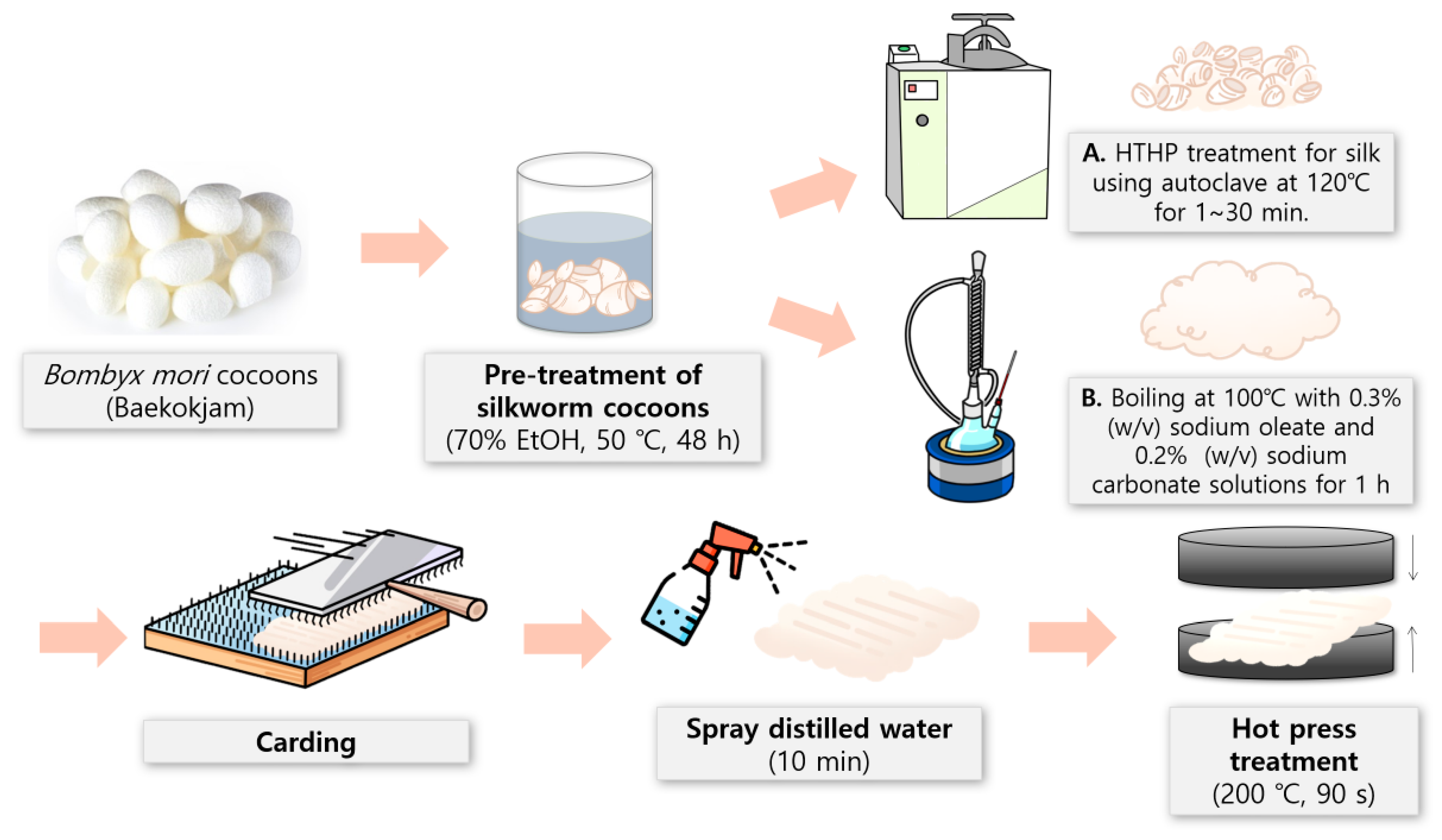
2.2. Preparation of Silk Fibers with Varying Sericin Contents
2.3. Preparation of Silk Nonwoven Fabrics
2.4. Measurement and Characterization
3. Results and Discussion
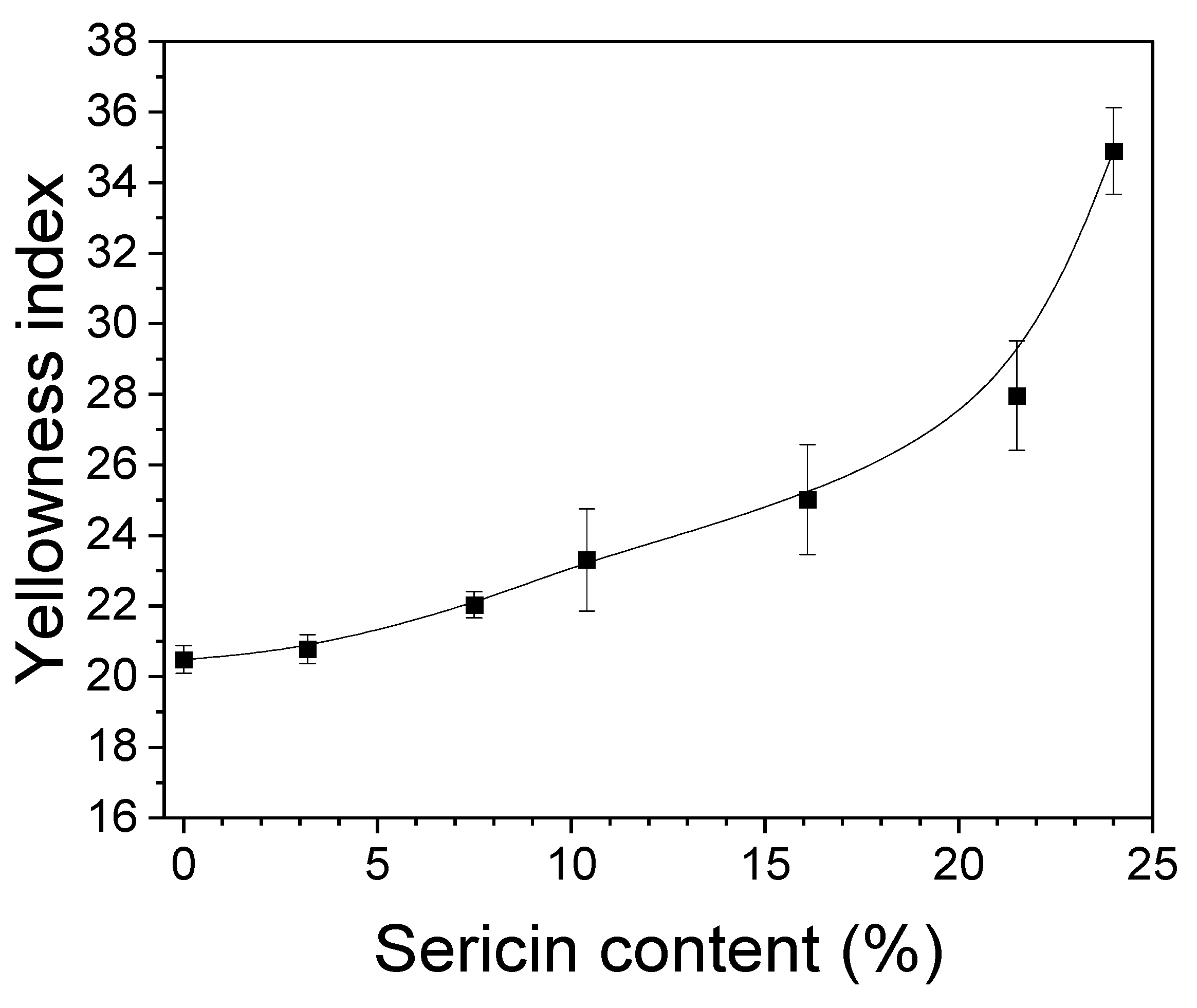
3.1. Morphology of the Silk Nonwoven Fabric
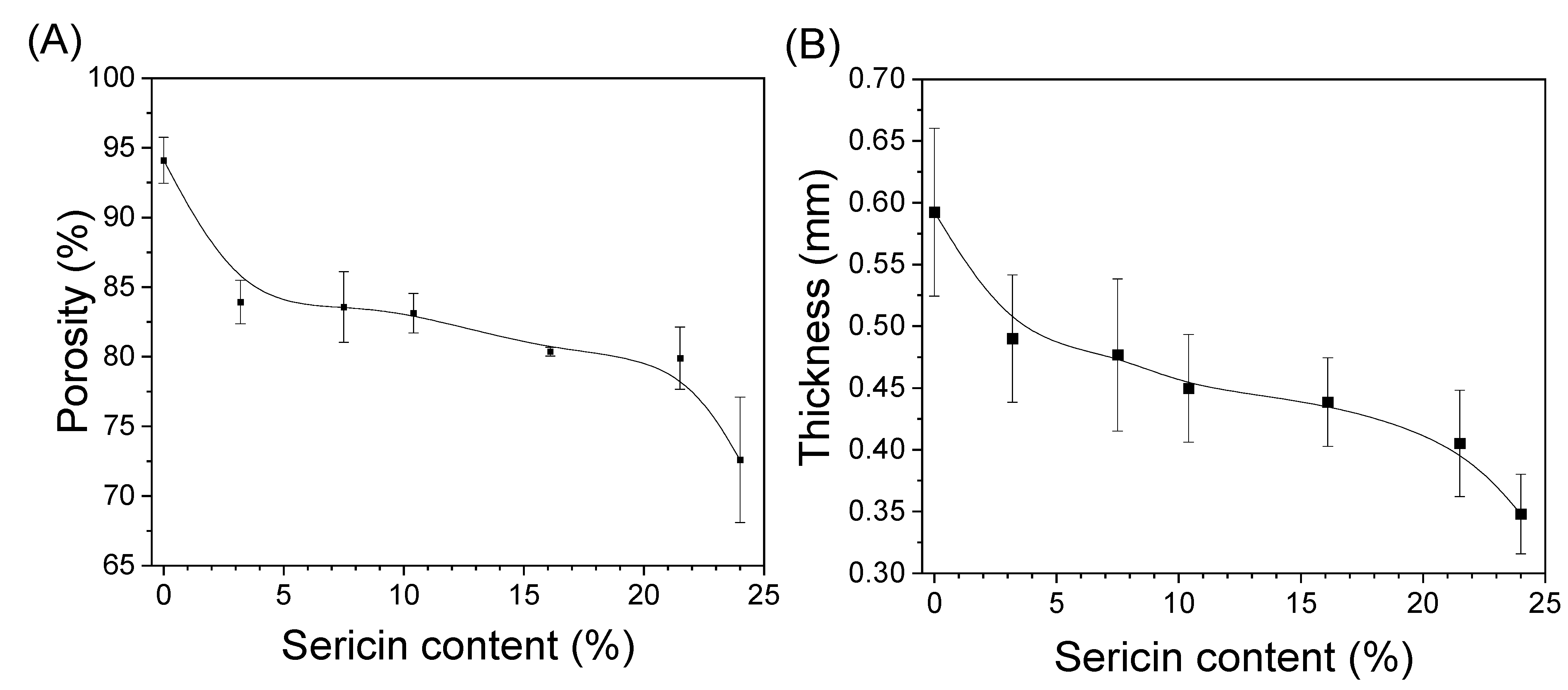
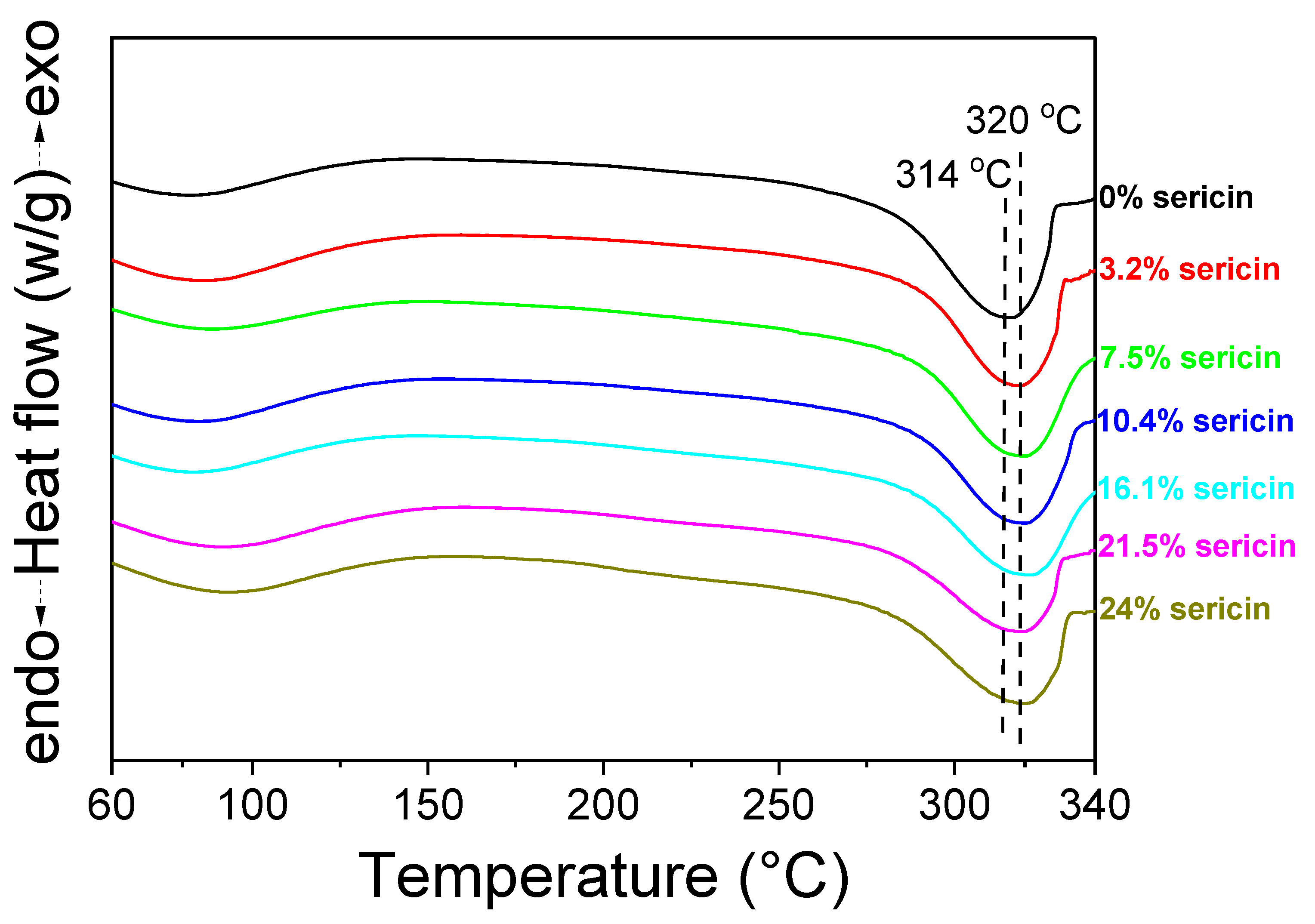
3.2. Structural Characteristics of Silk Nonwoven Fabrics
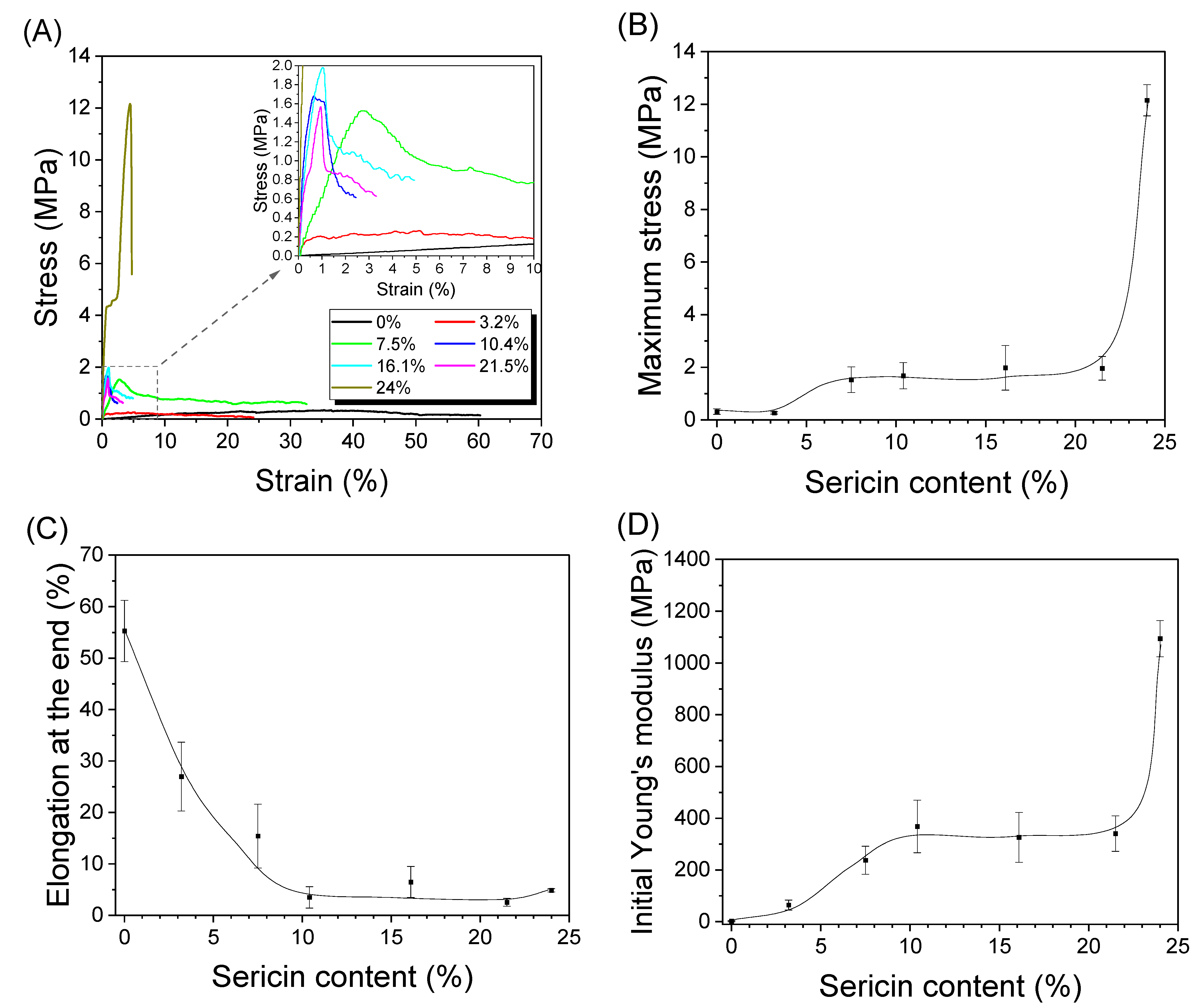
3.3. Mechanical Properties of Silk Nonwoven Fabrics
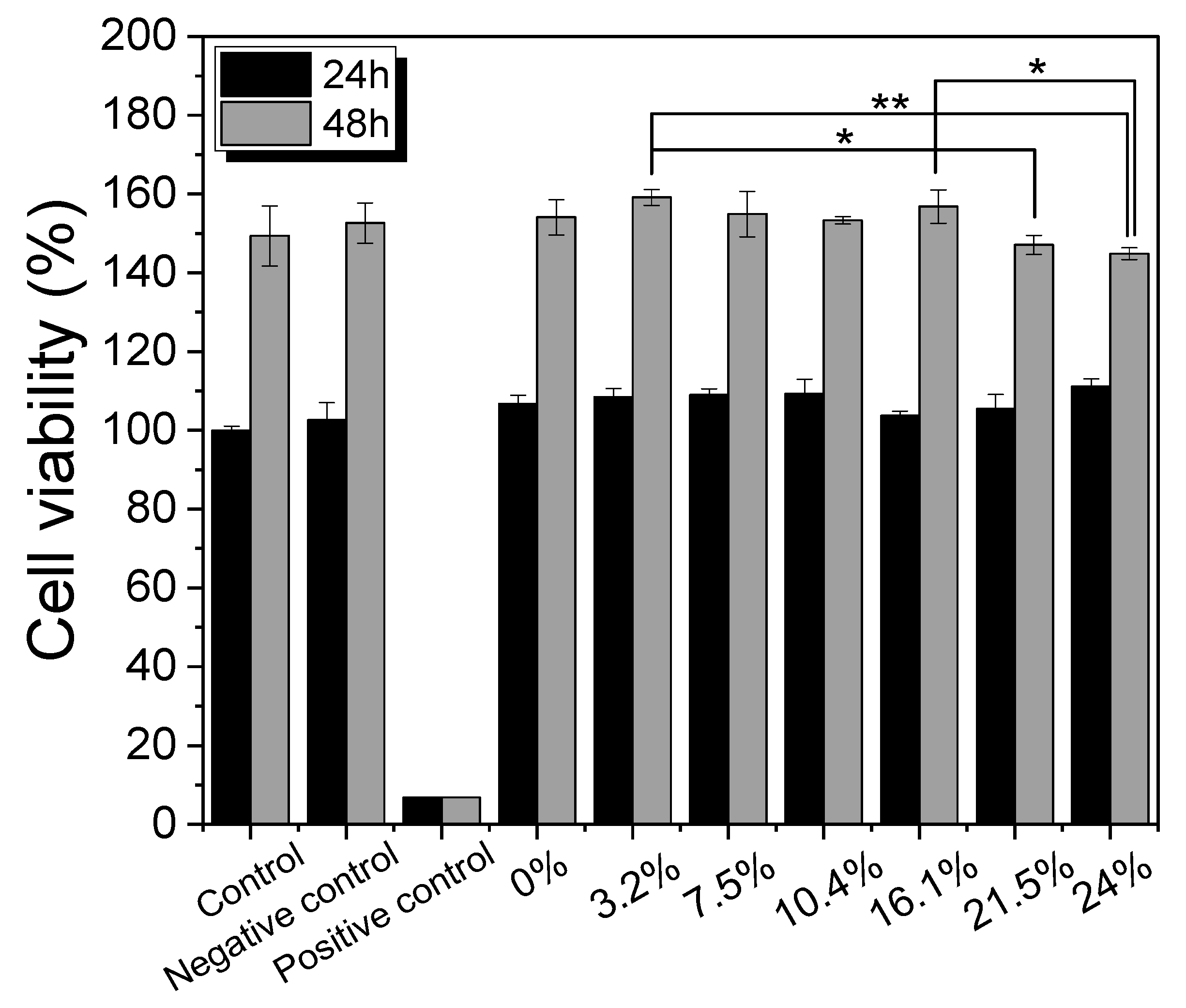
3.4. Cell Viability of Silk Nonwoven Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Zuo, B.; Dai, L.; Wu, Z. Analysis of structure and properties of biodegradable regenerated silk fibroin fibers. J. Mater. Sci. 2006, 41, 3357–3361. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Hwang, C.M.; Min, B.G.; Park, Y.H. Structural characteristics and properties of silk fibroin/polyurethane blend films. Int. J. Indust. Entomol. 2002, 5, 163–170. [Google Scholar]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef]
- Uebersax, L.; Apfel, T.; Nuss, K.M.R.; Vogt, R.; Kim, H.Y.; Meinel, L.; Kaplan, D.L.; Auer, J.A.; Merkle, H.P.; Rechenberg, B.V. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 107–118. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.I.; Gotoh, Y.; Tsukada, M.; Imai, Y.J. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef]
- Jin, H.J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Marelli, B.; Alessandrino, A.; Farè, S.; Freddi, G.; Mantovani, D.; Tanzi, M.C. Compliant electrospun silk fibroin tubes for small vessel bypass grafting. Acta Biomater. 2010, 6, 4019–4026. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Wang, Y.; Li, G.; Zheng, Z.; Kaplan, D.L.; Wang, X. Flexible Water-Absorbing Silk-Fibroin Biomaterial Sponges with Unique Pore Structure for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1641–1649. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of natural, semi-synthetic, and synthetic polymers in cosmetic formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, T.; Hashimoto, T.; Tamada, Y. Effects of supercooling and organic solvent on the formation of a silk sponge with porous 3-D structure, and its dynamical and structural characterization using solid-state NMR. J. Mater. Sci. 2011, 46, 7923–7930. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, X.; Lyu, J.; Zhao, G.; Gu, K.; Xia, J.; Chen, Z.; Shao, Z. Preparation of a novel regenerated silk fibroin-based hydrogel for extrusion bioprinting. Soft Matter 2022, 18, 7360–7368. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, A.; Ki, C.S.; Kim, J.W.; Park, Y.H.; Lee, K.H. Preparation of silk sericin beads using LiCl/DMSO solvent and their potential as a drug carrier for oral administration. Fibers Polym. 2007, 8, 470–476. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.N.; Park, B.-D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Su, D.; Jiang, L.; Chen, X.; Dong, J.; Shao, Z. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl. Mater. Interfaces 2016, 8, 9619–9628. [Google Scholar] [CrossRef]
- Um, I.C.; Ki, C.S.; Kweon, H.; Lee, K.G.; Ihm, D.W.; Park, Y.H. Wet spinning of silk polymer: II. Effect of drawing on the structural characteristics and properties of filament. Int. J. Biol. Macromol. 2004, 34, 107–119. [Google Scholar] [CrossRef]
- Ha, S.W.; Tonelli, A.E.; Hudson, S.M. Structural Studies of Bombyx mori Silk Fibroin during Regeneration from Solutions and Wet Fiber Spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef]
- Chung, D.E.; Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fibers Polym. 2014, 15, 153–160. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Silk fibroin nanofiber. Electrospinning, properties, and structure. Polym. J. 2003, 35, 185–190. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effects of electric field on the maximum electro-spinning rate of silk fibroin solutions. Int. J. Biol. Macromol. 2017, 95, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kang, K.D.; Jung, B.H.; Joo, C.H.; Nahm, J.H. Preparation of silk nonwoven fabrics by needle punching, thermal bonding and its properties. Korean J. Seric. Sci. 1999, 41, 205–210. [Google Scholar]
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk nonwoven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Preparation, Structural Characterization, and Properties of Natural Silk Nonwoven Fabrics from Different Silkworm Varieties. Fiber. Polym. 2022, 23, 1130–1141. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions. Int. J. Mol. Sci. 2016, 17, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, H.W.; Lee, K.H. Polyethylenimine-functionalized silk sericin beads for high-performance remediation of hexavalent chromium from aqueous solution. Chemosphere 2018, 207, 507–516. [Google Scholar] [CrossRef]
- Ki, C.S.; Park, S.Y.; Kim, H.J.; Jung, H.M.; Woo, K.M.; Lee, J.W.; Park, Y.H. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: Implications for bone regeneration. Biotechnol. Lett. 2008, 30, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Baughman, C.B.; Kaplan, D.L. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008, 29, 2217–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, Y.; Kojima, K.; Tamada, Y.; Tomita, N.; Kameda, T. Silk fibroin sponges with cell growth-promoting activity induced by genetically fused basic fibroblast growth factor. J. Biomed. Mater. Res. Part A 2016, 104, 82–93. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, M.-K.; Kweon, H.; Jo, Y.-Y.; Lee, K.-G.; Lee, J.K. Comparison of unprocessed silk cocoon and silk cocoon middle layer membranes for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.-M.; Yao, J.; Lee, I.-S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Wu, M.; Han, Z.; Liu, W.; Yao, J.; Zhao, B.; Shao, Z.; Chen, X. Silk-based hybrid microfibrous mats as guided bone regeneration membranes. J. Mater. Chem. B 2021, 9, 2025–2032. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Lee, K.G.; Kang, S.W.; Kweon, H.Y.; Kim, H.J. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J. Tissue Eng. Regen. Med. 2015, 9, 66–76. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Lee, K.-G.; Chae, C.-H.; Balázsi, C.; Min, S.-K.; Kim, J.-Y.; Choi, J.-Y.; Kim, S.-G. Development of Nano-Hydroxyapatite Graft With Silk Fibroin Scaffold as a New Bone Substitute. J. Oral Maxillofac. Surg. 2011, 69, 1578–1586. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Yao, J.; Shao, Z.; Chen, X. Tamoxifen-loaded silk fibroin electrospun fibers. Mater. Lett. 2016, 178, 31–34. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, B. Development and performance study of a natural silk fiber facial mask paper. J. Eng. Fibers Fabr. 2020, 15, 1558925020975756. [Google Scholar] [CrossRef]
- Choi, H.J.; Bae, Y.H.; Lee, H.H.; Yeo, S.Y. Preparation and Characterization of Alginate-gelatin/silk Wet-laid Nonwoven Fabric. Text. Color. Finish. 2020, 32, 57–64. [Google Scholar]
- Kishimoto, Y.; Morikawa, H.; Yamanaka, S.; Tamada, Y. Electrospinning of silk fibroin from all aqueous solution at low concentration. Mater. Sci. Eng. C 2017, 73, 498–506. [Google Scholar] [CrossRef]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, Y.J.; Kim, J.W.; Park, Y.H.; Bae, D.G.; Um, I.C. Effect of molecular weight and storage time on the wet-and electro-spinning of regenerated silk fibroin. Polym. Degrad. Stab. 2012, 97, 1060–1066. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Effects of Wet and Hot Press Treatments on Structure and Properties of Mechanically Fabricated Natural Silk Nonwoven Fabrics. Text. Sci. Eng. 2018, 55, 381–389. [Google Scholar]
- Bae, Y.J.; Jang, M.J.; Um, I.C. Silk/rayon webs and nonwoven fabrics: Fabrication, structural characteristics, and properties. Int. J. Mol. Sci. 2022, 23, 7511. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Bae, Y.J.; Seok, Y.S.; Um, I.C. Effect of hot press time on the structure characteristics and mechanical properties of silk nonwoven fabric. Int. J. Indust. Entomol. 2022, 44, 12–20. [Google Scholar]
- Bae, Y.-S.; Um, I.-C. Effects of Fabrication Conditions on Structure and Properties of Mechanically Prepared Natural Silk Web and Non-Woven Fabrics. Polymers 2021, 13, 1578. [Google Scholar] [CrossRef]
- Kim, H.J.; Um, I.C. Effect of degumming ratio on wet spinning and post drawing performance of regenerated silk. Int. J. Biol. Macromol. 2014, 67, 387–393. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, D.W.; Park, Y.H.; Um, I.C. Effect of residual sericin on the structural characteristics and properties of regenerated silk films. Int. J. Biol. Macromol. 2016, 89, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Um, I.C. Effect of silkworm variety on characteristics of raw sericin in silk. Fibers Polym. 2019, 20, 271–279. [Google Scholar] [CrossRef]
- Park, C.J.; Um, I.C. Effect of heat treatment on the structural characteristics and properties of silk sericin film. Int. J. Indust. Entomol. 2018, 37, 36–42. [Google Scholar]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of different sterilization methods on the properties of commercial biodegradable polyesters for single-use, disposable medical devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Um, I.C. Preparation, structural characteristics, and properties of airlaid nonwoven silk fabric. Polym. Korea 2020, 44, 809–816. [Google Scholar] [CrossRef]
- Setoyama, K. Effect of water on the heat-yellowing oh silk fabric and the changes in amino acid composition in the silk fibroin in sealed tubes by heat-treatment. J. Seric. Sci. Jpn. 1982, 51, 365–369. [Google Scholar]
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K.R. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Tao, M.-L.; Shen, W.-D.; Mao, J.-P.; Chen, Y.-H. Synthesis of silk sericin peptides-L-asparaginase bioconjugates and their characterization. J. Chem. Technol. Biotechnol. 2006, 81, 136–145. [Google Scholar] [CrossRef]
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295. [Google Scholar] [CrossRef]
- Choi, H.J.; Noh, S.K.; Um, I.C. Morphology, molecular conformation and moisture regain of cocoons of different silkworm varieties. Int. J. Indust. Entomol. 2020, 40, 6–15. [Google Scholar]
- Bae, Y.J.; Noh, S.K.; Um, I.C. Crystallinity change of silkworm variety cocoons by heat treatment. Int. J. Indust. Entomol. 2021, 42, 7–13. [Google Scholar]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Ha, S.W.; Park, Y.H.; Hudson, S.M. Dissolution of Bombyx mori silk fibroin in the calcium nitrate tetrahydrate-methanol system and aspects of wet spinning of fibroin solution. Biomacromolecules 2003, 4, 488–496. [Google Scholar] [CrossRef]
- Narita, C.; Okahisa, Y.; Wataoka, I.; Yamada, K. Characterization of ground silk fibroin through comparison of nanofibroin and higher order structures. ACS Omega 2020, 5, 22786–22792. [Google Scholar] [CrossRef]
- He, Y.; Zhou, M.; Mahmoud, M.H.H.; Lu, X.; He, G.; Zhang, L.; Huang, M.; Elnaggar, A.Y.; Lei, Q.; Liu, H.; et al. Multifunctional wearable strain/pressure sensor based on conductive carbon nanotubes/silk nonwoven fabric with high durability and low detection limit. Adv. Compos. Hybrid Mater. 2022, 5, 1939–1950. [Google Scholar] [CrossRef]
- Lee, K.G.; Lee, Y.W.; Yeo, J.H.; Nam, J.; Kweon, H.Y.; Park, Y.H. Structural charateristics of silk fibroin gel on the preparation conditions. Korean J. Seric. Sci. 1999, 41, 41–47. [Google Scholar]
- Ko, J.S.; Yoon, K.; Ki, C.S.; Kim, H.J.; Bae, D.G.; Lee, K.H.; Park, Y.H.; Um, I.C. Effect of degumming condition on the solution properties and electrospinnablity of regenerated silk solution. Int. J. Biol. Macromol. 2013, 55, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Kandasubramanian, B. Processing trends of silk fibers: Silk degumming, regeneration and physical functionalization. J. Text. Inst. 2020, 111, 1794–1810. [Google Scholar] [CrossRef]
- Iwasawa, A.; Ayaki, M.; Niwano, Y. Cell viability score (CVS) as a good indicator of critical concentration of benzalkonium chloride for toxicity in cultured ocular surface cell lines. Regul. Toxicol. Pharmacol. 2013, 66, 177–183. [Google Scholar] [CrossRef]
- López-García, L.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Func. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [Green Version]


| Sericin Content (%) | 0% | 3.2% | 7.5% | 10.4% | 16.1% | 21.5% | 24% |
|---|---|---|---|---|---|---|---|
| Sample code of silk nonwoven fabric | SNFS0 | SNFS3.2 | SNFS7.5 | SNFS10.4 | SNFS16.1 | SNFS21.5 | SNFS24 |
| Degumming ratio (%) | 24.0% | 21.5% | 17.8% | 15.2% | 9.4% | 3.2% | 0% |
| Degumming method | Soap/ soda | HTHP | HTHP | HTHP | HTHP | HTHP | — |
| Degumming time | 1 h | 30 min | 22 min | 15 min | 3 min | 1 min | — |
| SNFS0 | SNFS3.2 | SNFS7.5 | SNFS10.4 | SNFS16.1 | SNFS21.5 | SNFS24 |
|---|---|---|---|---|---|---|
 |  |  |  |  |  |  |
| SNFS0 | SNFS3.2 |
|---|---|
 |  |
| Magnification | Silk Nonwoven Fabric | ||||||
|---|---|---|---|---|---|---|---|
| SNFS0 | SNFS3.2 | SNFS7.5 | SNFS10.4 | SNFS16.1 | SNFS21.5 | SNFS24 | |
| Low magnification |  |  |  |  |  |  |  |
| High magnification |  |  |  |  |  |  |  |
| Incubation Time | Control | Negative Control | Positive Control | |
|---|---|---|---|---|
| 24 h |  |  |  | |
| 48 h |  |  |  | |
| Incubation time | 0% Sericin | 3.2% Sericin | 7.5% Sericin | |
| 24 h |  |  |  | |
| 48 h |  |  |  | |
| Incubation time | 10.4% Sericin | 16.1% Sericin | 21.5% Sericin | 24% Sericin |
| 24 h | 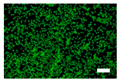 | 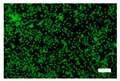 |  |  |
| 48 h |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.E.; Bae, Y.J.; Jang, M.J.; Um, I.C. Effect of Sericin Content on the Structural Characteristics and Properties of New Silk Nonwoven Fabrics. Biomolecules 2023, 13, 1186. https://doi.org/10.3390/biom13081186
Kim YE, Bae YJ, Jang MJ, Um IC. Effect of Sericin Content on the Structural Characteristics and Properties of New Silk Nonwoven Fabrics. Biomolecules. 2023; 13(8):1186. https://doi.org/10.3390/biom13081186
Chicago/Turabian StyleKim, Ye Eun, Yu Jeong Bae, Mi Jin Jang, and In Chul Um. 2023. "Effect of Sericin Content on the Structural Characteristics and Properties of New Silk Nonwoven Fabrics" Biomolecules 13, no. 8: 1186. https://doi.org/10.3390/biom13081186





