Ocular Surface Allostasis—When Homeostasis Is Lost: Challenging Coping Potential, Stress Tolerance, and Resilience
Abstract
:1. Introduction
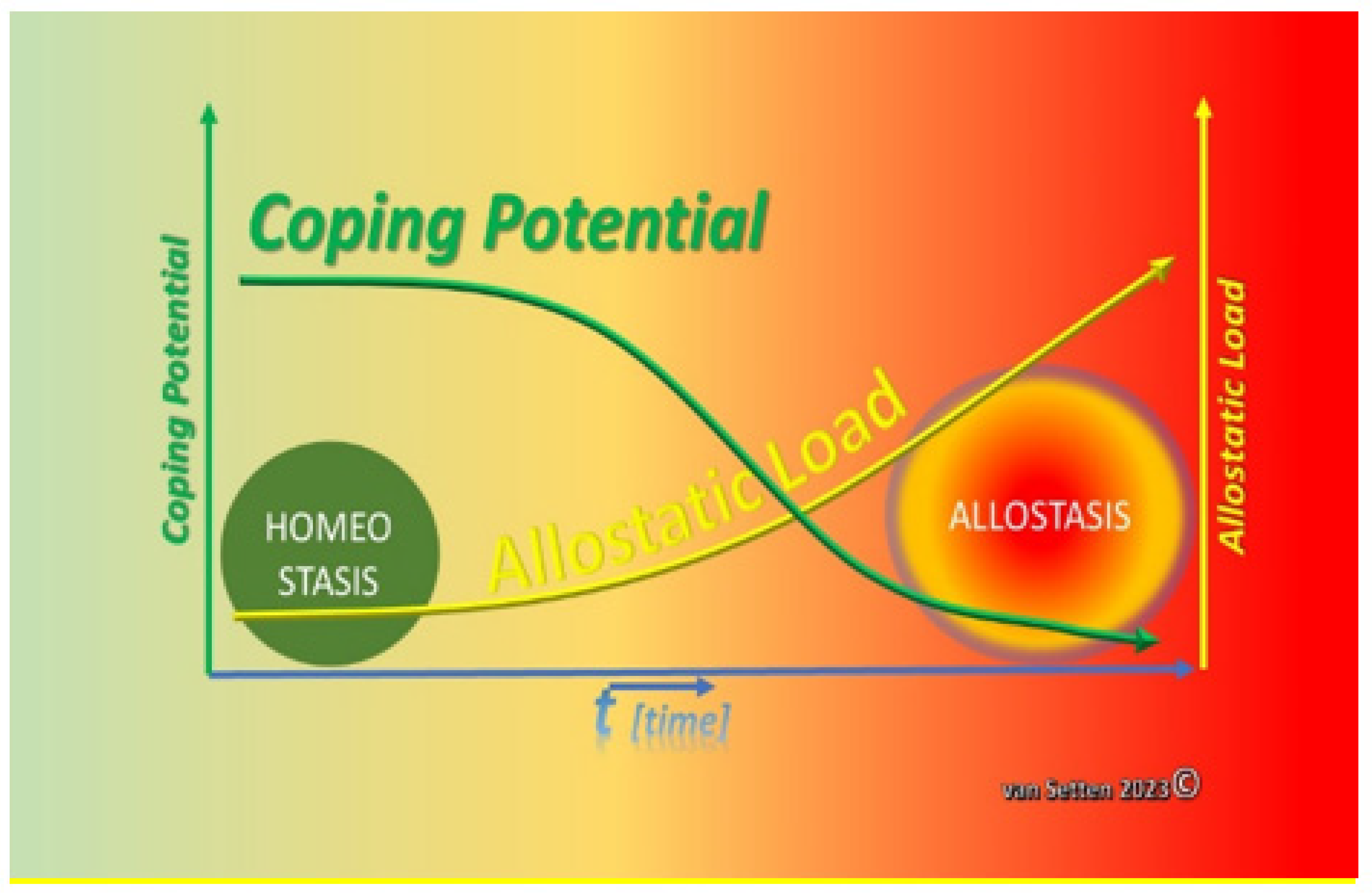
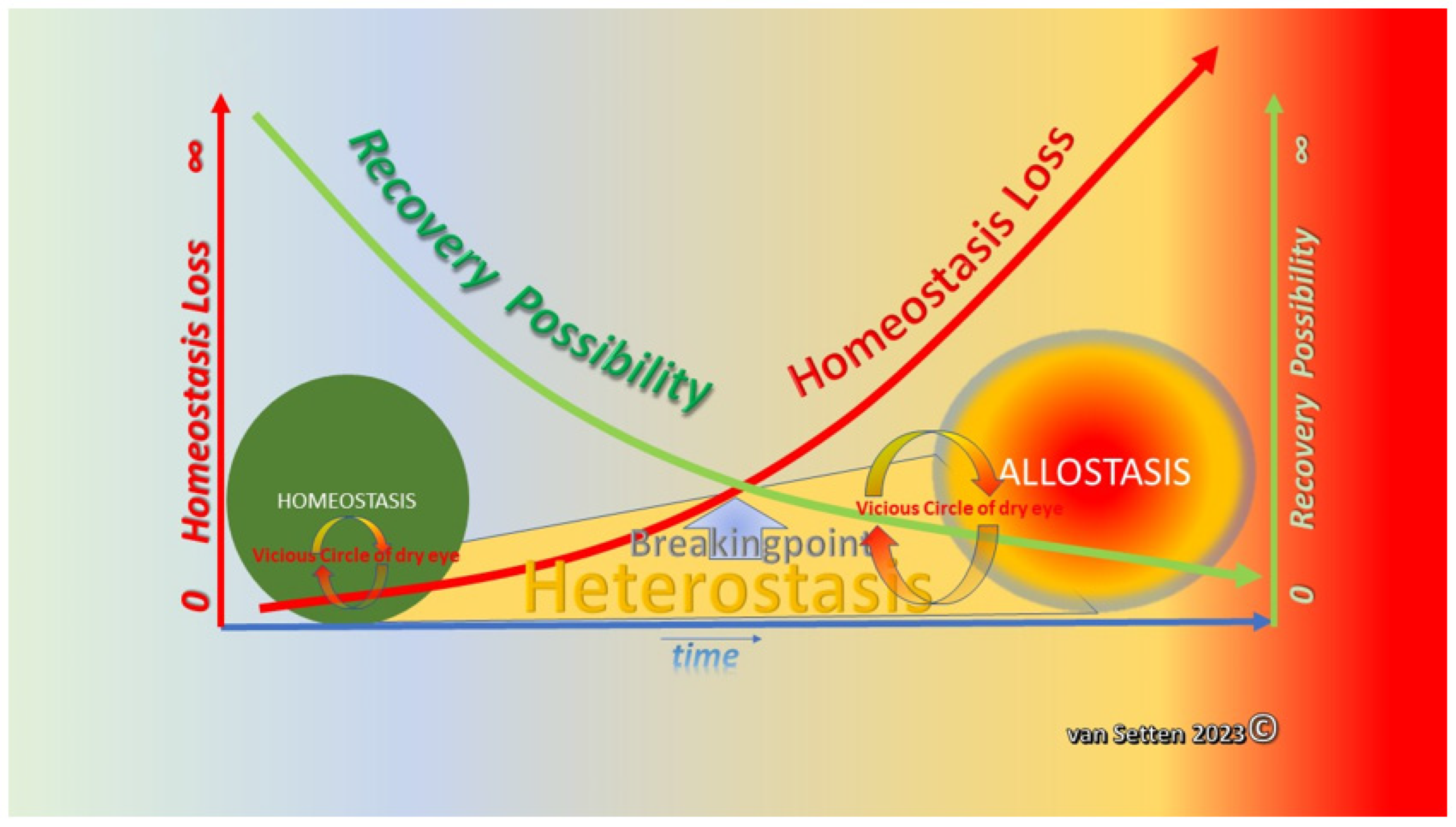
2. From Homeostasis to Heterostasis
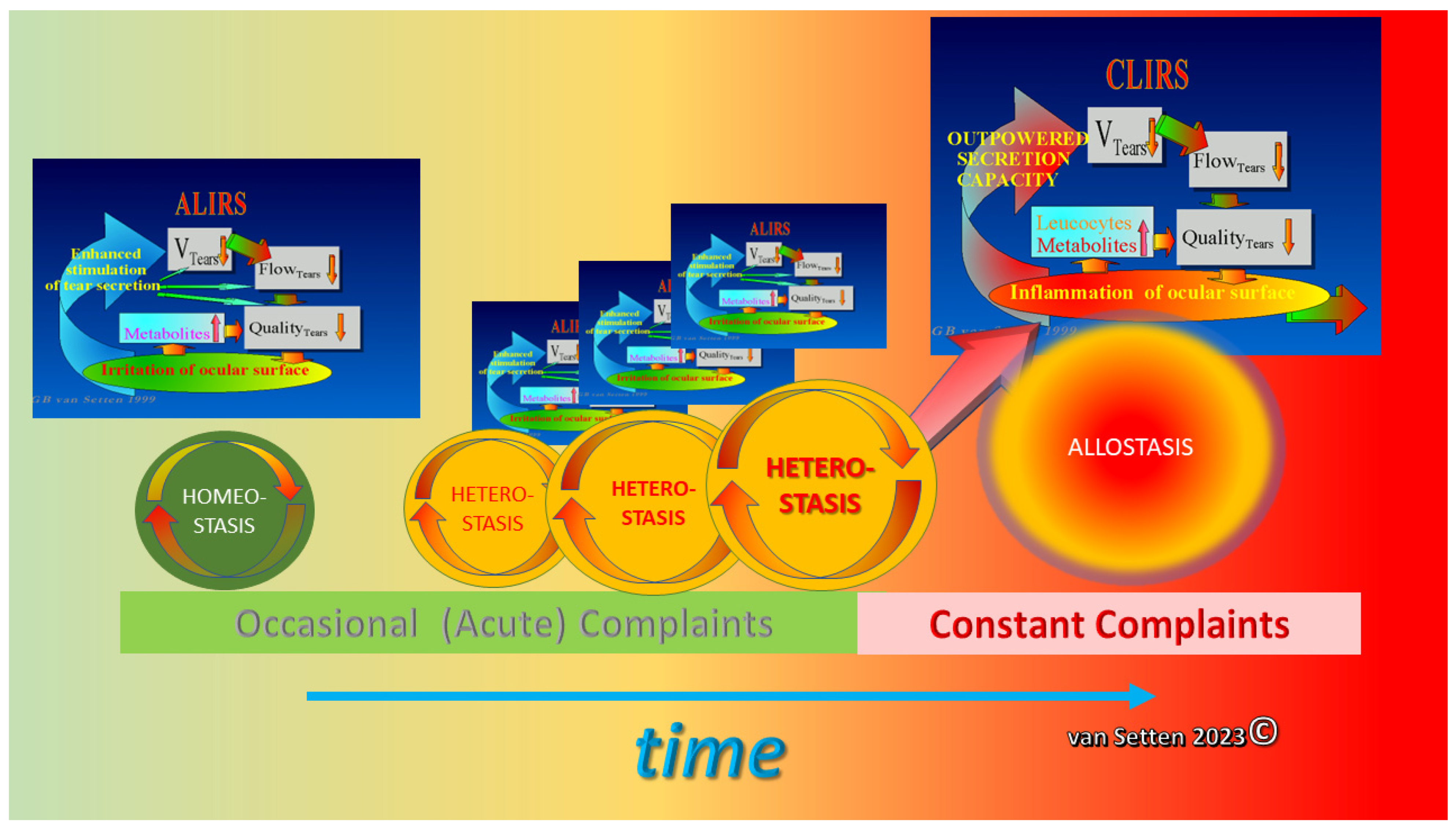
3. Heterostasis—Challenging the Coping Capacity, Increasing Cell Stress, and Touching the Limits of Resilience
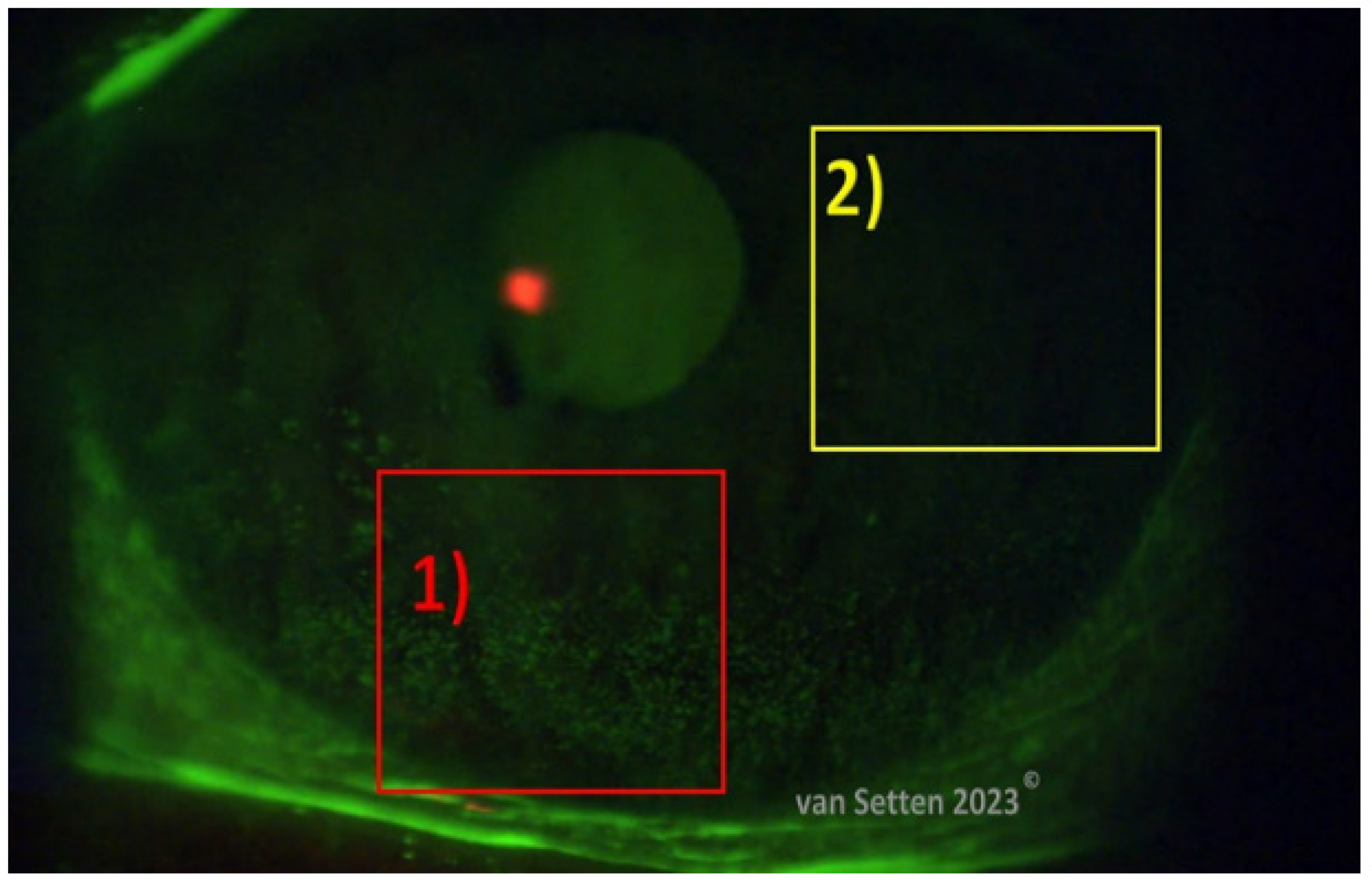
4. Ocular Surface Staining—More than Just Colour
5. Cellular Stress and Allostatic Load—Driving Forces in the Loss of Homeostasis
6. Stress, Stress Factors, and Stress Tolerance
7. Hyperosmolarity—More than a Numerical Value
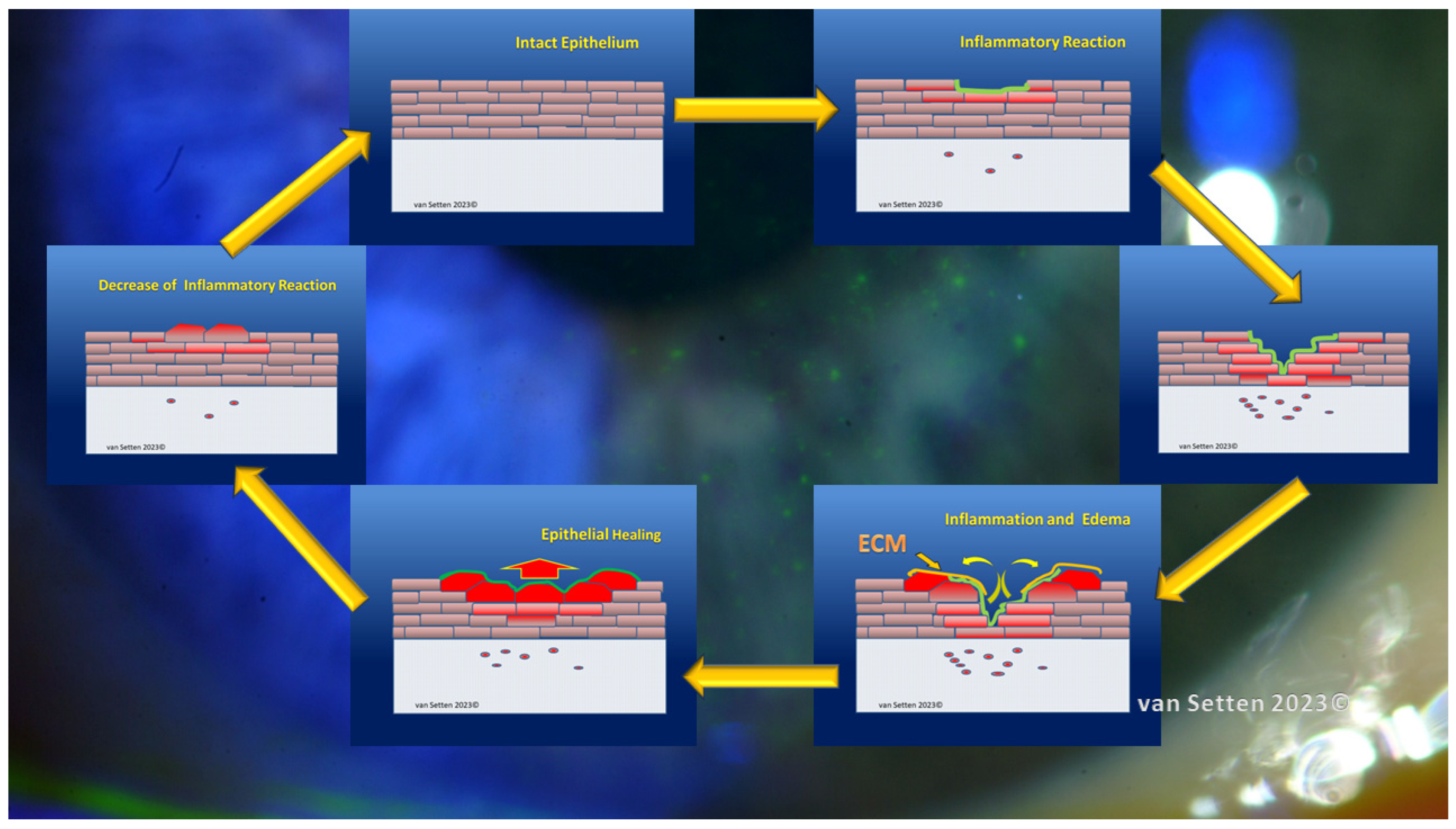
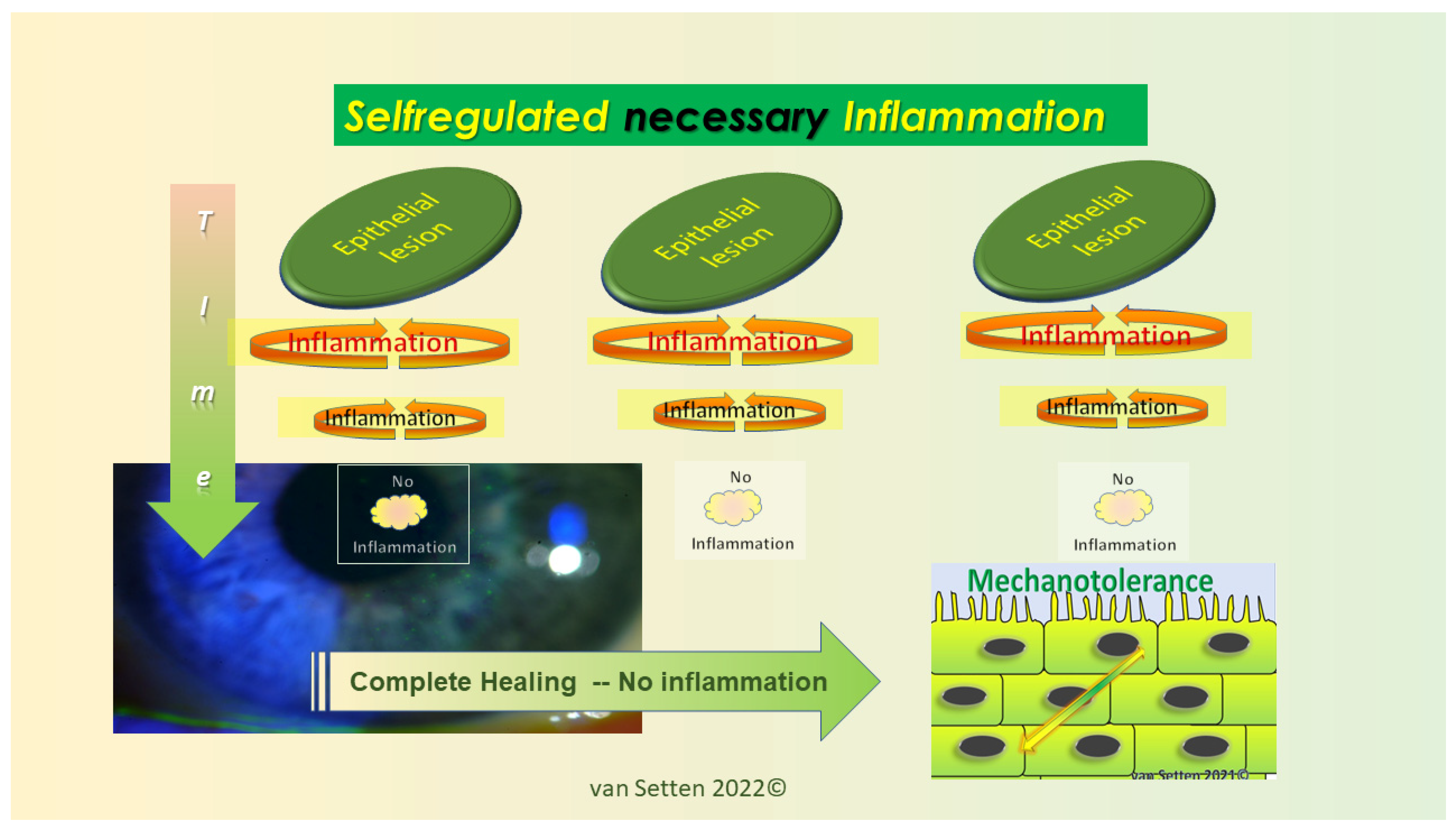
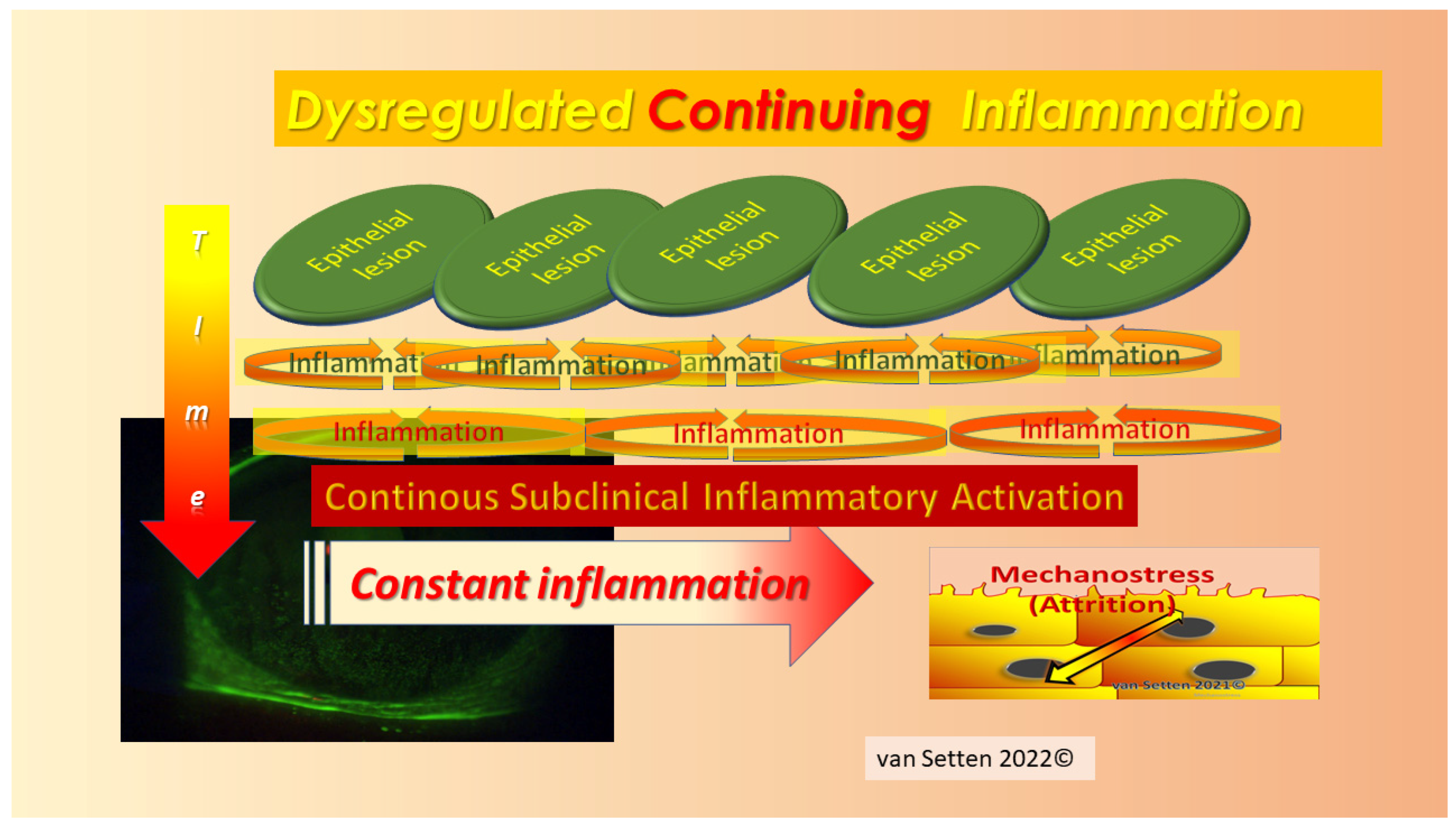
8. Inflammation—From Necessary Asset to Pathological Threat
9. Summary—Allostasis as a Situational Adaption and a Chance for Survival
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannon, W.B. The Wisdom of the Body; Kegan Paul and Co., Ltd.: London, UK, 1932; p. 12. [Google Scholar]
- Bernard, C. Introduction à L’étude de la Médecine Expérimentale; J.B.Baillière et Fils: Paris, France, 1865. [Google Scholar]
- OED. Oxford English Dictionary; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef] [Green Version]
- Krogh, E.; Epstein, R.; Langer, Á.I.; Steinebach, C. Clinical resilience: Toward a unified definition. Int. J. Qual. Health Care 2023, 35, mzad025. [Google Scholar] [CrossRef] [PubMed]
- Murube, J.; Marcos, M.G.; Javate, R. Amylase in mare lacrimale in patients with submandibular salivary gland transplantation to the lacrimal basin. Adv. Exp. Med. Biol. 1994, 350, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C. A new approach for better comprehension of diseases of the ocular surface. J. Fr. D’ophtalmol. 2007, 30, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Hawley, J.A.; Lundby, C.; Cotter, J.D.; Burke, L.M. Maximizing Cellular Adaptation to Endurance Exercise in Skeletal Muscle. Cell Metab. 2018, 27, 962–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppeler, H.; Vogt, M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001, 204, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Salmons, S.; Henriksson, J. The adaptive response of skeletal muscle to increased use. Muscle Nerve 1981, 4, 94–105. [Google Scholar] [CrossRef]
- Sorteni, C.; Clavenzani, P.; De Giorgio, R.; Portnoy, O.; Sirri, R.; Mordenti, O.; Di Biase, A.; Parmeggiani, A.; Menconi, V.; Chiocchetti, R. Enteric neuroplasticity in seawater-adapted European eel (Anguilla anguilla). J. Anat. 2014, 224, 180–191. [Google Scholar] [CrossRef]
- Barassi, G.; Bellomo, R.G.; Porreca, A.; Giannuzzo, G.; Giannandrea, N.; Pezzi, L.; Crudeli, M.; Visciano, C.; Saggini, R. The use of adaptive neuro-stimulation for rebalancing posture and muscular tone in a soccer team. J. Sports Med. Phys. Fit. 2019, 59, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Kremers, R.M.W.; Kleinegris, M.C.; Ninivaggi, M.; de Laat, B.; Ten Cate, H.; Koek, G.H.; Wagenvoord, R.J.; Hemker, H.C. Decreased prothrombin conversion and reduced thrombin inactivation explain rebalanced thrombin generation in liver cirrhosis. PLoS ONE 2017, 12, e0177020. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; An, Y.; Li, R.; Mu, C.; Wang, C. Strategy of metabolic phenotype modulation in Portunus trituberculatus exposed to low salinity. J. Agric. Food Chem. 2014, 62, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Zorbaz, T.; Madrer, N.; Soreq, H. Cholinergic blockade of neuroinflammation: From tissue to RNA regulators. Neuronal Signal. 2022, 6, Ns20210035. [Google Scholar] [CrossRef]
- Fink, G. Stress: Definition and History. Encycl. Neurosci. 2009, 549–555. [Google Scholar] [CrossRef]
- Wass, S.V. allostasis and metastasis: The yin and yang of childhood autoregulation. Dev. Psychopathol. 2023, 35, 179–190. [Google Scholar] [CrossRef]
- Sterling, P.; Eyer, J. allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition, and Health; Fisher, S., Reason, J., Eds.; John Wiley and Sons: New York, NY, USA, 1988; pp. 629–649. [Google Scholar]
- Meizlish, M.L.; Franklin, R.A.; Zhou, X.; Medzhitov, R. Tissue homeostasis and Inflammation. Annu. Rev. Immunol. 2021, 39, 557–581. [Google Scholar] [CrossRef]
- van Setten, G.-B. Coping Mechanisms of the Ocular Surface to Desiccation Challenges; 4th Ophthalmic Fiction Symposium; Coronis Foundation: Munich, Germany, 2021; Available online: https://www.coronis-foundation.org/lectures/ (accessed on 20 July 2023).
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear, and Rage, 1st ed.; Appleton-Century-Crofts: New York, NY, USA, 1915. [Google Scholar]
- McEwen, B.S. Stress, Adaptation, and Disease: allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Lacerda, R.; Menezes, J.; Candeias, M.M. Alternative Mechanisms of mRNA Translation Initiation in Cellular Stress Response and Cancer. Adv. Exp. Med. Biol. 2019, 1157, 117–132. [Google Scholar] [CrossRef]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.; Shen Lee, B.; Periman, L.M. Dry eye disease: Identification and therapeutic strategies for primary care clinicians and clinical specialists. Ann. Med. 2023, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Kanavi, M.R.; Rasaee, M.J. Inhibition of matrix metalloproteinase-9 for the treatment of dry eye syndrome; a review study. Exp. Eye Res. 2021, 205, 108523. [Google Scholar] [CrossRef]
- Gilbard, J.P.; Dartt, D.A. Changes in rabbit lacrimal gland fluid osmolarity with flow rate. Investig. Ophthalmol. Vis. Sci. 1982, 23, 804–806. [Google Scholar]
- JP, G. Tear film osmolarity and keratoconjunctivitis sicca. CLAO J. 1985, 11, 243–250. [Google Scholar]
- Li, Y.; Cui, L.; Lee, H.S.; Kang, Y.S.; Choi, W.; Yoon, K.C. Comparison of 0.3% Hypotonic and Isotonic Sodium Hyaluronate Eye Drops in the Treatment of Experimental Dry Eye. Curr. Eye Res. 2017, 42, 1108–1114. [Google Scholar] [CrossRef]
- Huh, J.; Choi, S.Y.; Eom, Y.; Kim, H.M.; Song, J.S. Changes in the Matrix Metalloproteinase 9 Point-of-Care Test Positivity According to MMP-9 Concentration and Loading Volume. Cornea 2020, 39, 234–236. [Google Scholar] [CrossRef]
- Suárez-Cortés, T.; Merino-Inda, N.; Benitez-Del-Castillo, J.M. Tear and ocular surface disease biomarkers: A diagnostic and clinical perspective for ocular allergies and dry eye disease. Exp. Eye Res. 2022, 221, 109121. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkeç, M.; Benítez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Yoon, K.C.; Kang, S.S.; Seon, S.K.; Lee, K.; Kim, B.B. Enhanced Immunomodulation, Anti-Apoptosis, and Improved Tear Dynamics of (PEG)-BHD1028, a Novel Adiponectin Receptor Agonist Peptide, for Treating Dry Eye Disease. Pharmaceutics 2022, 15, 78. [Google Scholar] [CrossRef]
- Liu, S.H.; Saldanha, I.J.; Abraham, A.G.; Rittiphairoj, T.; Hauswirth, S.; Gregory, D.; Ifantides, C.; Li, T. Topical corticosteroids for dry eye. Cochrane Database Syst. Rev. 2022, 10, Cd015070. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Jiang, X.; Zhu, L.; Li, X.; Zhou, Q.; Jie, Y.; You, Z.; Wu, M.; Jin, X.; Li, X.; et al. Cyclosporine A (0.05%) Ophthalmic Gel in the Treatment of Dry Eye Disease: A Multicenter, Randomized, Double-Masked, Phase III, COSMO Trial. Drug Des. Dev. Ther. 2022, 16, 3183–3194. [Google Scholar] [CrossRef]
- Bron, A.J.; Dogru, M.; Horwath-Wimter, J.; Kojima, T.; Kovács, I.; Müller-Lierheim, W.G.K.; van Setten, G.B.; Belmonte, C. Reflections on the Ocular Surface: Summary of the Presentations at the 4th Coronis Foundation Ophthalmic Symposium Debate: “A Multifactorial Approach to Ocular Surface Disorders” (31 August 2021). Front. Biosci. 2022, 27, 142. [Google Scholar] [CrossRef]
- Audric, J. The amazing resilience of children. Med. World 1948, 69, 329–331. [Google Scholar]
- Fossion, P.; Linkowski, P. The relevance of the concept of resiliency in the field of psychiatry. Rev. Medicale Brux. 2007, 28, 33–38. [Google Scholar]
- Snur, J. Classification of gingival resilience in partial dental prostheses. Folia Stomatol. 1950, 11, 138–150. [Google Scholar]
- Travaglini, E.A. Resilient tissue surface in complete dentures. J. Am. Dent. Assoc. 1962, 64, 512–517. [Google Scholar] [CrossRef]
- Rugh, R.; Wolff, J. Resilience of the fetal eye following radiation insult. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1955, 89, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea 2000, 19, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Foreman, D.M.; Pancholi, S.; Jarvis-Evans, J.; McLeod, D.; Boulton, M.E. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp. Eye Res. 1996, 62, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J. Diagnosis of dry eye. Surv. Ophthalmol. 2001, 45 (Suppl. S2), S221–S226. [Google Scholar] [CrossRef]
- van Setten, G.-B. The Anatomical Dry Eye—A Different Form of Ocular Surface Disease Deserves Focus. Open J. Ophthalmol. 2017, 7, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Mäkinen, P.; Beuerman, R.; Pietilä, J.; Vaajanen, A.; Uusitalo, H. Age-associated changes in human tear proteome. Clin. Proteom. 2019, 16, 11. [Google Scholar] [CrossRef]
- Segars, K.L.; Azzari, N.A.; Gomez, S.; Machen, C.; Rich, C.B.; Trinkaus-Randall, V. Age Dependent Changes in Corneal Epithelial Cell Signaling. Front. Cell Dev. Biol. 2022, 10, 886721. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. Int. J. Mol. Sci. 2019, 20, 6132. [Google Scholar] [CrossRef] [Green Version]
- Holly, F.J. Tear film physiology. Int. Ophthalmol. Clin. 1987, 27, 2–6. [Google Scholar] [CrossRef]
- Holly, F.J.; Lemp, M.A. Tear physiology and dry eyes. Surv. Ophthalmol. 1977, 22, 69–87. [Google Scholar] [CrossRef]
- Lemp, M.A. Tear film: New concepts and implications for the management of the dry eye. Trans. New Orleans Acad. Ophthalmol. 1987, 35, 53–64. [Google Scholar]
- Pflugfelder, S.C. Differential diagnosis of dry eye conditions. Adv. Dent. Res. 1996, 10, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Proust, J.E.; Arenas, E.; Petroutsos, G.; Pouliquen, Y. The lacrimal film, structure and stability. J. Fr. D’ophtalmol. 1983, 6, 963–969. [Google Scholar]
- Baudouin, C.; Rolando, M.; Benitez Del Castillo, J.M.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Hamill, J.R., Jr. Factors affecting tear film breakup in normal eyes. Arch. Ophthalmol. 1973, 89, 103–105. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.A. Tear Film-Oriented Diagnosis and Tear Film-Oriented Therapy for Dry Eye Based on Tear Film Dynamics. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des13–Des22. [Google Scholar] [CrossRef] [Green Version]
- Shimazaki, J. Definition and Diagnostic Criteria of Dry Eye Disease: Historical Overview and Future Directions. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des7–Des12. [Google Scholar] [CrossRef] [Green Version]
- Tervo, T.; van Setten, G.B.; Päällysaho, T.; Tarkkanen, A.; Tervo, K. Wound healing of the ocular surface. Ann. Med. 1992, 24, 19–27. [Google Scholar] [CrossRef]
- Tervo, T.; Salonen, E.M.; Vahen, A.; Immonen, I.; van Setten, G.B.; Himberg, J.J.; Tarkkanen, A. Elevation of tear fluid plasmin in corneal disease. Acta Ophthalmol. 1988, 66, 393–399. [Google Scholar] [CrossRef]
- Wilson, S.E. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury. Investig. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- van Setten, G.B. Expression of GPR-68 in Human Corneal and Conjunctival Epithelium. Possible indicator and mediator of attrition associated inflammation at the ocular surface. J. Fr. D’ophtalmol. 2023, 46, 19–24. [Google Scholar] [CrossRef]
- Lardner, E.; van Setten, G.B. Detection of TSG-6-like protein in human corneal epithelium. Simultaneous presence with CD44 and hyaluronic acid. J. Fr. D’ophtalmol. 2020, 43, 879–883. [Google Scholar] [CrossRef]
- van Setten, G.B. Osmokinetics: A new dynamic concept in dry eye disease. J. Fr. D’ophtalmol. 2019, 42, 221–225. [Google Scholar] [CrossRef]
- van Setten, G.-B. Attrition und Osmokinetik–Zwei Konzepte zur Pathogenese des Trockenen Auges. Spektrum Augenheilkd. 2021, 35, 150–158. [Google Scholar] [CrossRef]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- University, C. The Cambridge English Dictionary; Cambridge University Press & Assessment; University of Cambridge: Cambridge, UK, 2022. [Google Scholar]
- Ren, H.; Zhao, F.; Zhang, Q.; Huang, X.; Wang, Z. Autophagy and skin wound healing. Burn. Trauma 2022, 10, tkac003. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.S.; Ryan, K.M. New frontiers in promoting tumour cell death: Targeting apoptosis, necroptosis and autophagy. Oncogene 2012, 31, 5045–5060. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnova, L.; Harris, G.; Leist, M.; Hartung, T. Cellular resilience. Altex 2015, 32, 247–260. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Karatsoreos, I.N. Sleep Deprivation and Circadian Disruption Stress, allostasis, and Allostatic Load. Sleep Med. Clin. 2022, 17, 253–262. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Magarinos, A.M.; Reagan, L.P. Structural plasticity and tianeptine: Cellular and molecular targets. Eur. Psychiatry 2002, 17 (Suppl. S3), 318–330. [Google Scholar] [CrossRef] [PubMed]
- van Setten, G. Immunological Nudging. 2020. Available online: https://encyclopedia.pub/entry/1358 (accessed on 20 July 2023).
- Sapolsky, R.M.; Uno, H.; Rebert, C.S.; Finch, C.E. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 1990, 10, 2897–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein-Behrens, B.; Mattson, M.P.; Chang, I.; Yeh, M.; Sapolsky, R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994, 14, 5373–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapolsky, R.; Armanini, M.; Packan, D.; Tombaugh, G. Stress and glucocorticoids in aging. Endocrinol. Metab. Clin. N. Am. 1987, 16, 965–980. [Google Scholar] [CrossRef]
- Smeal, T.; Guarente, L. Mechanisms of cellular senescence. Curr. Opin. Genet. Dev. 1997, 7, 281–287. [Google Scholar] [CrossRef]
- Metcalfe, J.A.; Parkhill, J.; Campbell, L.; Stacey, M.; Biggs, P.; Byrd, P.J.; Taylor, A.M. Accelerated telomere shortening in ataxia telangiectasia. Nat. Genet. 1996, 13, 350–353. [Google Scholar] [CrossRef]
- Stefanini, M.; Orecchia, G.; Rabbiosi, G.; Nuzzo, F. Altered cellular response to UV irradiation in a patient affected by premature ageing. Hum. Genet. 1986, 73, 189–192. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- George, O.; Le Moal, M.; Koob, G.F. allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol. Behav. 2012, 106, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oster, M.; Muráni, E.; Ponsuksili, S.; D’Eath, R.B.; Turner, S.P.; Evans, G.; Thölking, L.; Kurt, E.; Klont, R.; Foury, A.; et al. Hepatic expression patterns in psychosocially high-stressed pigs suggest mechanisms following Allostatic principles. Physiol. Behav. 2014, 128, 159–165. [Google Scholar] [CrossRef]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogru, M.; Tsubota, K. New insights into the diagnosis and treatment of dry eye. Ocul. Surf. 2004, 2, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxidative Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [Green Version]
- Rhee, M.K.; Mah, F.S. Inflammation in Dry Eye Disease: How Do We Break the Cycle? Ophthalmology 2017, 124, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Sugita, S.; Kamoi, K. Immunological homeostasis of the eye. Prog. Retin. Eye Res. 2013, 33, 10–27. [Google Scholar] [CrossRef]
- Garza, A.; Diaz, G.; Hamdan, M.; Shetty, A.; Hong, B.Y.; Cervantes, J. Homeostasis and Defense at the Surface of the Eye. The Conjunctival Microbiota. Curr. Eye Res. 2021, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- van Setten, G.; Labetoulle, M.; Baudouin, C.; Rolando, M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol. 2016, 94, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [CrossRef]
- Joanito, I.; Wirapati, P.; Zhao, N.; Nawaz, Z.; Yeo, G.; Lee, F.; Eng, C.L.P.; Macalinao, D.C.; Kahraman, M.; Srinivasan, H.; et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 2022, 54, 963–975. [Google Scholar] [CrossRef]
- Kulkarni, A.; Anderson, A.G.; Merullo, D.P.; Konopka, G. Beyond bulk: A review of single cell transcriptomics methodologies and applications. Curr. Opin. Biotechnol. 2019, 58, 129–136. [Google Scholar] [CrossRef]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrvatin, S.; Hochbaum, D.R.; Nagy, M.A.; Cicconet, M.; Robertson, K.; Cheadle, L.; Zilionis, R.; Ratner, A.; Borges-Monroy, R.; Klein, A.M.; et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 2018, 21, 120–129. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Marino, S.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Vis-à-vis: A focus on genetic features of cerebral cavernous malformations and brain arteriovenous malformations pathogenesis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 243–251. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants 2022, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef]
- Carlotti, E.; Murray-Brown, W.; Blighe, K.; Caliste, M.; Astorri, E.; Sutcliffe, N.; Tappuni, A.R.; Pitzalis, C.; Corsiero, E.; Bombardieri, M. High-throughput sequencing of IgH gene in minor salivary glands from Sjögren’s syndrome patients reveals dynamic B cell recirculation between ectopic lymphoid structures. Clin. Exp. Rheumatol. 2022, 40, 2363–2372. [Google Scholar] [CrossRef]
- Chen, J.Q.; Papp, G.; Póliska, S.; Szabó, K.; Tarr, T.; Bálint, B.L.; Szodoray, P.; Zeher, M. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PLoS ONE 2017, 12, e0174585. [Google Scholar] [CrossRef]
- Debreceni, I.L.; Chimenti, M.S.; Serreze, D.V.; Geurts, A.M.; Chen, Y.G.; Lieberman, S.M. Toll-Like Receptor 7 Is Required for Lacrimal Gland Autoimmunity and Type 1 Diabetes Development in Male Nonobese Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 9478. [Google Scholar] [CrossRef]
- Kakan, S.S.; Janga, S.R.; Cooperman, B.; Craig, D.W.; Edman, M.C.; Okamoto, C.T.; Hamm-Alvarez, S.F. Small RNA Deep Sequencing Identifies a Unique miRNA Signature Released in Serum Exosomes in a Mouse Model of Sjögren’s Syndrome. Front. Immunol. 2020, 11, 1475. [Google Scholar] [CrossRef]
- Trujillo-Vargas, C.M.; Schaefer, L.; Alam, J.; Pflugfelder, S.C.; Britton, R.A.; de Paiva, C.S. The gut-eye-lacrimal gland-microbiome axis in Sjögren Syndrome. Ocul. Surf. 2020, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qu, J.; Wang, L.; Li, M.; Xu, D.; Zhao, Y.; Zhang, F.; Zeng, X. Activation of Toll-Like Receptor 7 Signaling Pathway in Primary Sjögren’s Syndrome-Associated Thrombocytopenia. Front. Immunol. 2021, 12, 637659. [Google Scholar] [CrossRef] [PubMed]
- Karolak, J.A.; Gajecka, M. Genomic strategies to understand causes of keratoconus. Mol. Genet. Genom. MGG 2017, 292, 251–269. [Google Scholar] [CrossRef] [Green Version]
- van Setten, G.B. Osmokinetics: Defining the Characteristics of Osmotic Challenge to the Ocular Surface. Klin. Monbl. Augenheilkd. 2020, 237, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.; Senchyna, M.; Jones, L. Impact of time between collection and collection method on human tear fluid osmolarity. Curr. Eye Res. 2013, 38, 428–436. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Campos, E.C. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr. Eye Res. 2010, 35, 553–564. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef] [Green Version]
- van Setten, G.B. GPR-68 in human lacrimal gland. Detection and possible role in the pathogenesis of dry eye disease. J. Fr. D’ophtalmol. 2022, 45, 921–927. [Google Scholar] [CrossRef]
- van Setten, G.B.; Tervo, T.; Viinikka, L.; Pesonen, K.; Perheentupa, J.; Tarkkanen, A. Ocular disease leads to decreased concentrations of epidermal growth factor in the tear fluid. Curr. Eye Res. 1991, 10, 523–527. [Google Scholar] [CrossRef]
- Nonami, H.; Schulze, E.D. Cell water potential, osmotic potential, and turgor in the epidermis and mesophyll of transpiring leaves: Combined measurements with the cell pressure probe and nanoliter osmometer. Planta 1989, 177, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; Rasmussen, A. Bacterial Mechanosensitive Channels. In Membrane Protein Complexes: Structure and Function; Subcellular Biochemistry; Springer: Singapore, 2018; Volume 87, pp. 83–116. [Google Scholar] [CrossRef]
- Zimmermann, U.; Rygol, J.; Balling, A.; Klöck, G.; Metzler, A.; Haase, A. Radial Turgor and Osmotic Pressure Profiles in Intact and Excised Roots of Aster tripolium: Pressure Probe Measurements and Nuclear Magnetic Resonance-Imaging Analysis. Plant Physiol. 1992, 99, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Miermont, A.; Waharte, F.; Hu, S.; McClean, M.N.; Bottani, S.; Léon, S.; Hersen, P. Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc. Natl. Acad. Sci. USA 2013, 110, 5725–5730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.H.; Trepat, X.; Park, C.Y.; Lenormand, G.; Oliver, M.N.; Mijailovich, S.M.; Hardin, C.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl. Acad. Sci. USA 2009, 106, 10632–10637. [Google Scholar] [CrossRef] [PubMed]
- van Setten, G.B. Impact of Attrition, Intercellular Shear in Dry Eye Disease: When Cells are Challenged and Neurons are Triggered. Int. J. Mol. Sci. 2020, 21, 4333. [Google Scholar] [CrossRef]
- Barlati, S.; Marchina, E.; Quaranta, C.A.; Vigasio, F.; Semeraro, F. Analysis of fibronectin, plasminogen activators and plasminogen in tear fluid as markers of corneal damage and repair. Exp. Eye Res. 1990, 51, 1–9. [Google Scholar] [CrossRef]
- Bonny, A.R.; Kochanowski, K.; Diether, M.; El-Samad, H. Stress-induced growth rate reduction restricts metabolic resource utilization to modulate osmo-adaptation time. Cell Rep. 2021, 34, 108854. [Google Scholar] [CrossRef]
- Schultz, G.; Khaw, P.T.; Oxford, K.; MaCauley, S.; Van Setten, G.; Chegini, N. Growth factors and ocular wound healing. Eye 1994, 8, 184–187. [Google Scholar] [CrossRef]
- Uchino, Y. The Ocular Surface Glycocalyx and its Alteration in Dry Eye Disease: A Review. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des157–Des162. [Google Scholar] [CrossRef] [Green Version]
- Inatomi, T.; Spurr-Michaud, S.; Tisdale, A.S.; Gipson, I.K. Human corneal and conjunctival epithelia express MUC1 mucin. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1818–1827. [Google Scholar]
- Nakamura, M.; Endo, K.; Nakata, K. Mucin-like glycoprotein secretion is mediated by cyclic-AMP and protein kinase C signal transduction pathways in rat corneal epithelium. Exp. Eye Res. 1998, 66, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H. Significance of mucin on the ocular surface. Cornea 2002, 21, S17–S22. [Google Scholar] [CrossRef]
- Watanabe, H.; Fabricant, M.; Tisdale, A.S.; Spurr-Michaud, S.J.; Lindberg, K.; Gipson, I.K. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Investig. Ophthalmol. Vis. Sci. 1995, 36, 337–344. [Google Scholar]
- Asari, A.; Miyauchi, S.; Takahashi, T.; Kohno, K.; Uchiyama, Y. Localization of hyaluronic acid, chondroitin sulfate, and CD44 in rabbit cornea. Arch. Histol. Cytol. 1992, 55, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzsimmons, T.D.; Molander, N.; Stenevi, U.; Fagerholm, P.; Schenholm, M.; von Malmborg, A. Endogenous hyaluronan in corneal disease. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2774–2782. [Google Scholar]
- Oh, J.Y.; In, Y.S.; Kim, M.K.; Ko, J.H.; Lee, H.J.; Shin, K.C.; Lee, S.M.; Wee, W.R.; Lee, J.H.; Park, M. Protective effect of uridine on cornea in a rabbit dry eye model. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Pauloin, T.; Dutot, M.; Liang, H.; Chavinier, E.; Warnet, J.M.; Rat, P. Corneal protection with high-molecular-weight hyaluronan against in vitro and in vivo sodium lauryl sulfate-induced toxic effects. Cornea 2009, 28, 1032–1041. [Google Scholar] [CrossRef]
- Elsheikh, A.; Alhasso, D.; Rama, P. Assessment of the epithelium’s contribution to corneal biomechanics. Exp. Eye Res. 2008, 86, 445–451. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef] [Green Version]
- Okada, Y.; Zhang, Y.; Zhang, L.; Yeh, L.K.; Wang, Y.C.; Saika, S.; Liu, C.Y. Shp2-mediated MAPK pathway regulates ΔNp63 in epithelium to promote corneal innervation and homeostasis. Lab. Investig. A J. Technol. Methods Pathol. 2020, 100, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Ritchey, E.R.; Code, K.; Zelinka, C.P.; Scott, M.A.; Fischer, A.J. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol. Vis. 2011, 17, 2440–2454. [Google Scholar] [PubMed]
- Yam, G.H.; Williams, G.P.; Setiawan, M.; Yusoff, N.Z.; Lee, X.W.; Htoon, H.M.; Zhou, L.; Fuest, M.; Mehta, J.S. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci. Rep. 2017, 7, 45396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratay, M.L.; Balmert, S.C.; Acharya, A.P.; Greene, A.C.; Meyyappan, T.; Little, S.R. TRI Microspheres prevent key signs of dry eye disease in a murine, inflammatory model. Sci. Rep. 2017, 7, 17527. [Google Scholar] [CrossRef] [Green Version]
- Ratay, M.L.; Glowacki, A.J.; Balmert, S.C.; Acharya, A.P.; Polat, J.; Andrews, L.P.; Fedorchak, M.V.; Schuman, J.S.; Vignali, D.A.A.; Little, S.R. Treg-recruiting microspheres prevent inflammation in a murine model of dry eye disease. J. Control. Release Off. J. Control. Release Soc. 2017, 258, 208–217. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capó-Aponte, J.E.; Pleyer, U.; et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell. Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef]
- Guindolet, D.; Woodward, A.M.; Gabison, E.E.; Argüeso, P. Alleviation of Endoplasmic Reticulum Stress Enhances Human Corneal Epithelial Cell Viability under Hyperosmotic Conditions. Int. J. Mol. Sci. 2022, 23, 4528. [Google Scholar] [CrossRef]
- Dolinay, T.; Aonbangkhen, C.; Zacharias, W.; Cantu, E.; Pogoriler, J.; Stablow, A.; Lawrence, G.G.; Suzuki, Y.; Chenoweth, D.M.; Morrisey, E.; et al. Protein kinase R-like endoplasmatic reticulum kinase is a mediator of stretch in ventilator-induced lung injury. Respir. Res. 2018, 19, 157. [Google Scholar] [CrossRef]
- van Setten, G.B.; Mueller-Lierheim, W.; Baudouin, C. Dry Eye Etiology: Focus on Friction. Klin. Monatsblatter Augenheilkd. 2020, 237, 1235–1236. [Google Scholar] [CrossRef]
- Levy, A.; Georgeon, C.; Knoeri, J.; Tourabaly, M.; Leveziel, L.; Bouheraoua, N.; Borderie, V.M. Corneal Epithelial Thickness Mapping in the Diagnosis of Ocular Surface Disorders Involving the Corneal Epithelium: A Comparative Study. Cornea 2022, 41, 1353–1361. [Google Scholar] [CrossRef]
- Abou Shousha, M.; Wang, J.; Kontadakis, G.; Feuer, W.; Canto, A.P.; Hoffmann, R.; Perez, V.L. Corneal epithelial thickness profile in dry-eye disease. Eye 2020, 34, 915–922. [Google Scholar] [CrossRef]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z. Resident Innate Immune Cells in the Cornea. Front. Immunol. 2021, 12, 620284. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.L.; Stern, M.E.; Pflugfelder, S.C. Inflammatory basis for dry eye disease flares. Exp. Eye Res. 2020, 201, 108294. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C. The pathology of dry eye. Surv. Ophthalmol. 2001, 45 (Suppl. S2), S211–S220. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef] [Green Version]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef]
- Krysko, D.V.; D’Herde, K.; Vandenabeele, P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis Int. J. Program. Cell Death 2006, 11, 1709–1726. [Google Scholar] [CrossRef]
- Birge, R.B.; Ucker, D.S. Innate apoptotic immunity: The calming touch of death. Cell Death Differ. 2008, 15, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Májai, G.; Petrovski, G.; Fésüs, L. Inflammation and the apopto-phagocytic system. Immunol. Lett. 2006, 104, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Toda, S.; Nishi, C.; Yanagihashi, Y.; Segawa, K.; Nagata, S. Clearance of Apoptotic Cells and Pyrenocytes. Curr. Top. Dev. Biol. 2015, 114, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Amin, D.; Wu, R.; Jacob, A.; Zhou, M.; Dong, W.; Yang, W.L.; Ravikumar, T.S.; Wang, P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int. J. Mol. Med. 2008, 22, 743–748. [Google Scholar] [PubMed]
- Kim, Y.H.; Oh, T.W.; Park, E.; Yim, N.H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Anti-Inflammatory and Anti-Apoptotic Effects of Acer Palmatum Thumb. Extract, KIOM-2015EW, in a Hyperosmolar-Stress-Induced in vitro Dry Eye Model. Nutrients 2018, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Albert, B.J.A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P.; Wilson, J.; Hunt, T. Molecular Biology of the Cell, 6th ed.; Garland Science, Taylor & Francis Group (Publ) (Informa Business): New York, NY, USA, 2017; p. 857. ISBN 978-0-8153-4464-3. [Google Scholar]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Ma, X.; Zou, J.; He, L.; Zhang, Y. Dry eye management in a Sjögren’s syndrome mouse model by inhibition of p38-MAPK pathway. Diagn. Pathol. 2014, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.J.; Kim, J.M.; Lee, M.J.; Sohn, Y.S.; Kang, K.K.; Yoo, M. The therapeutic effect of DA-6034 on ocular inflammation via suppression of MMP-9 and inflammatory cytokines and activation of the MAPK signaling pathway in an experimental dry eye model. Curr. Eye Res. 2010, 35, 165–175. [Google Scholar] [CrossRef]
- Liu, H.; Nan, B.; Yang, C.; Li, X.; Yan, H.; Yuan, Y. Elaidic acid induced NLRP3 inflammasome activation via ERS-MAPK signaling pathways in Kupffer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159061. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Chen, S.J.; Zhou, S.C.; Wu, S.Z.; Wang, H. Inflammasomes and Fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bi, Y.; Wang, R.; Wang, X. Self-eating and self-defense: Autophagy controls innate immunity and adaptive immunity. J. Leukoc. Biol. 2013, 93, 511–519. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Eissa, N.T. Autophagy as a Stress Response Pathway in the Immune System. Int. Rev. Immunol. 2015, 34, 382–402. [Google Scholar] [CrossRef]
- Panigrahi, T.; Shivakumar, S.; Shetty, R.; D’Souza, S.; Nelson, E.J.R.; Sethu, S.; Jeyabalan, N.; Ghosh, A. Trehalose augments autophagy to mitigate stress induced inflammation in human corneal cells. Ocul. Surf. 2019, 17, 699–713. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Matsuzawa-Ishimoto, Y.; Hwang, S.; Cadwell, K. Autophagy and Inflammation. Annu. Rev. Immunol. 2018, 36, 73–101. [Google Scholar] [CrossRef]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Akira, S. Regulation of inflammasomes by autophagy. J. Allergy Clin. Immunol. 2016, 138, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018, 281, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. Homeostasis vs allostasis: Implications for brain function and mental disorders. JAMA Psychiatry 2014, 71, 1192–1193. [Google Scholar] [CrossRef]
- Peter, S. Principles of allostasis: Optimal Design, Predictive Regulation, Pathophysiology, and Rational Therapeutics. In Allostasis; Schulkin, J., Ed.; Cambridge University Press: Cambridge, UK, 2004; pp. 17–64. [Google Scholar]
- Hille, K.; Hille, A.; Ruprecht, K.W. Medium term results in keratoprostheses with biocompatible and biological haptic. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2006, 244, 696–704. [Google Scholar] [CrossRef]
- Su, J.Z.; Zheng, B.; Wang, Z.; Liu, X.J.; Cai, Z.G.; Zhang, L.; Peng, X.; Wu, J.; Liu, X.H.; Lv, L.; et al. Submandibular Gland Transplantation vs Minor Salivary Glands Transplantation for Treatment of Dry Eye: A Retrospective Cohort Study. Am. J. Ophthalmol. 2022, 241, 238–247. [Google Scholar] [CrossRef]
- Sendama, W. The effect of ageing on the resolution of inflammation. Ageing Res. Rev. 2020, 57, 101000. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Setten, G.-B. Ocular Surface Allostasis—When Homeostasis Is Lost: Challenging Coping Potential, Stress Tolerance, and Resilience. Biomolecules 2023, 13, 1246. https://doi.org/10.3390/biom13081246
van Setten G-B. Ocular Surface Allostasis—When Homeostasis Is Lost: Challenging Coping Potential, Stress Tolerance, and Resilience. Biomolecules. 2023; 13(8):1246. https://doi.org/10.3390/biom13081246
Chicago/Turabian Stylevan Setten, Gysbert-Botho. 2023. "Ocular Surface Allostasis—When Homeostasis Is Lost: Challenging Coping Potential, Stress Tolerance, and Resilience" Biomolecules 13, no. 8: 1246. https://doi.org/10.3390/biom13081246
APA Stylevan Setten, G.-B. (2023). Ocular Surface Allostasis—When Homeostasis Is Lost: Challenging Coping Potential, Stress Tolerance, and Resilience. Biomolecules, 13(8), 1246. https://doi.org/10.3390/biom13081246






