Associations of High-Density Lipoprotein Functionality with Coronary Plaque Characteristics in Diabetic Patients with Coronary Artery Disease: Integrated Backscatter Intravascular Ultrasound Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Laboratory Data Analyses
2.3. Measurement of the HDL-Mediated Cholesterol Efflux Capacity
2.4. Measurement of HDL Inflammatory Index (HII)
2.5. IVUS Procedures
2.6. Analysis of Acquired IVUS Images
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Gray-Scale and IB-IVUS Parameters at the Culprit Lesion
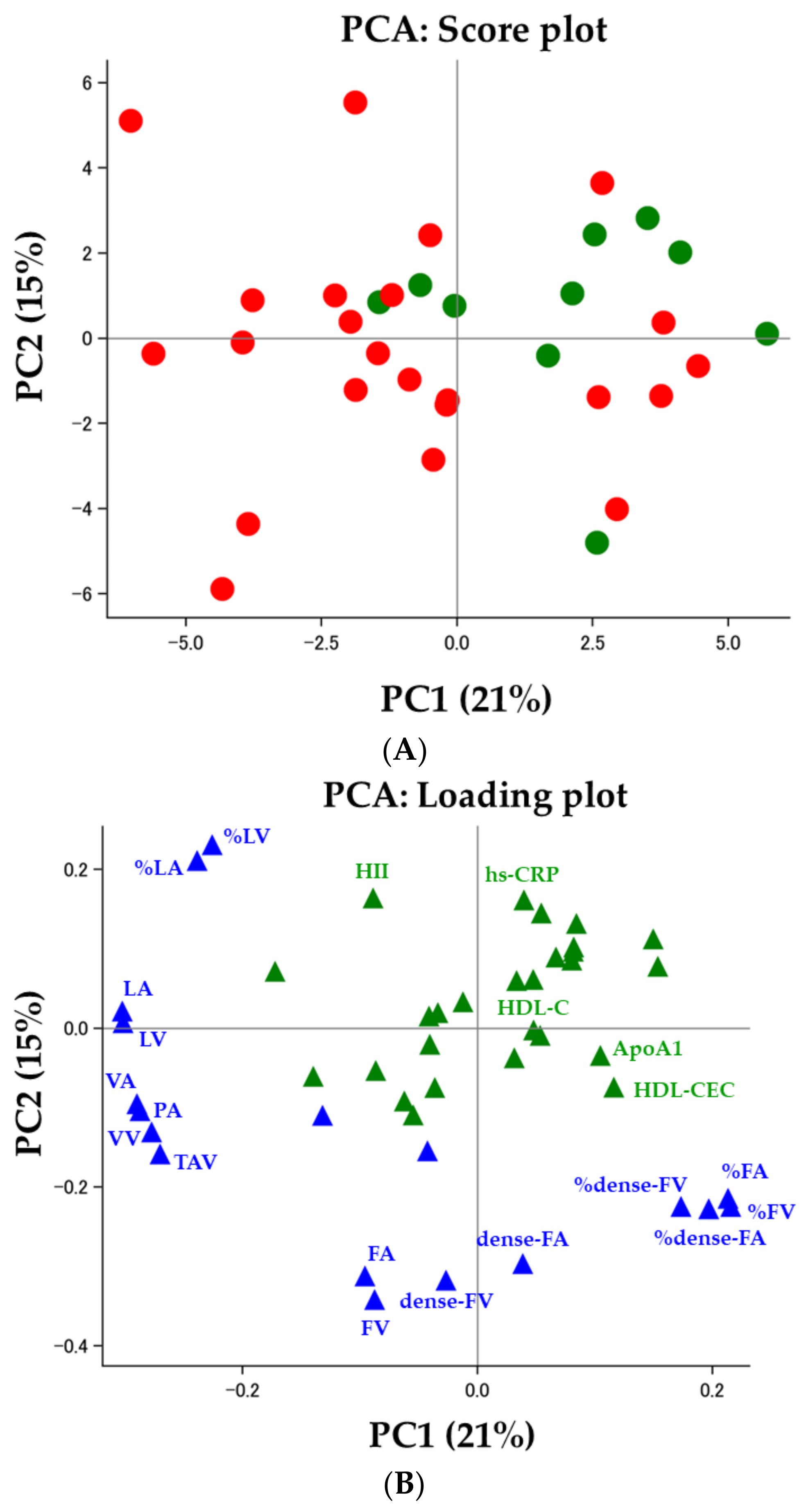
3.3. Correlations between IVUS-Derived Measures and Clinical Demographics, including HDL Functionality
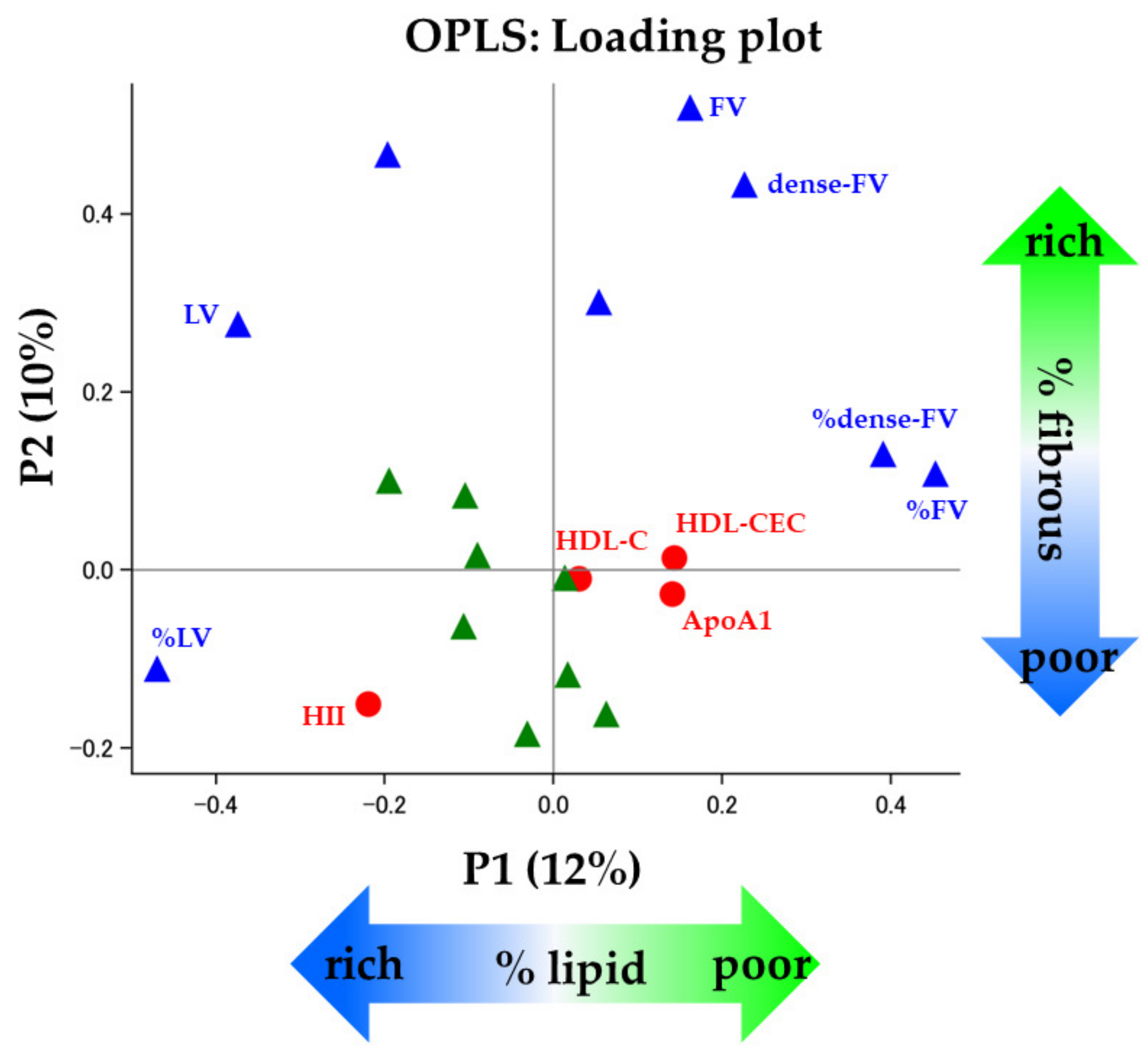
3.4. HDL Functionality and Coronary Plaques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Lopez, A.D. Regional patterns of disability-free life expectancy and disability-adjusted life expectancy: Global Burden of Disease Study. Lancet 1997, 349, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Nicholls, S.J. Tackling Residual Atherosclerotic Risk in Statin-Treated Adults: Focus on Emerging Drugs. Am. J. Cardiovasc. Drugs 2019, 19, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Rifkind, B.M. High-density lipoprotein--the clinical implications of recent studies. N. Engl. J. Med. 1989, 321, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.J.; Bittner, V.; Fruchart, J.C. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Holm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Ansell, B.J.; Navab, M.; Hama, S.; Kamranpour, N.; Fonarow, G.; Hough, G.; Rahmani, S.; Mottahedeh, R.; Dave, R.; Reddy, S.T.; et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003, 108, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D.; Kamranpour, N.; Fogelman, A.M.; Navab, M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007, 72, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Morgantini, C.; Natali, A.; Boldrini, B.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Ferrannini, E.; Reddy, S.T. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011, 60, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Khera, A.V.; Jafri, K.; Wilensky, R.L.; Rader, D.J. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J. Am. Coll. Cardiol. 2011, 58, 2068–2075. [Google Scholar] [CrossRef]
- Kawasaki, M.; Takatsu, H.; Noda, T.; Sano, K.; Ito, Y.; Hayakawa, K.; Tsuchiya, K.; Arai, M.; Nishigaki, K.; Takemura, G.; et al. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation 2002, 105, 2487–2492. [Google Scholar] [CrossRef]
- Okubo, M.; Kawasaki, M.; Ishihara, Y.; Takeyama, U.; Kubota, T.; Yamaki, T.; Ojio, S.; Nishigaki, K.; Takemura, G.; Saio, M.; et al. Development of integrated backscatter intravascular ultrasound for tissue characterization of coronary plaques. Ultrasound Med. Biol. 2008, 34, 655–663. [Google Scholar] [CrossRef]
- Sano, K.; Kawasaki, M.; Ishihara, Y.; Okubo, M.; Tsuchiya, K.; Nishigaki, K.; Zhou, X.; Minatoguchi, S.; Fujita, H.; Fujiwara, H. Assessment of vulnerable plaques causing acute coronary syndrome using integrated backscatter intravascular ultrasound. J. Am. Coll. Cardiol. 2006, 47, 734–741. [Google Scholar] [CrossRef]
- Amano, T.; Matsubara, T.; Uetani, T.; Kato, M.; Kato, B.; Yoshida, T.; Harada, K.; Kumagai, S.; Kunimura, A.; Shinbo, Y.; et al. Lipid-rich plaques predict non-target-lesion ischemic events in patients undergoing percutaneous coronary intervention. Circ. J. 2011, 75, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Kolodgie, F.D.; Zieske, A.; Fowler, D.R.; Weber, D.K.; Varghese, P.J.; Farb, A.; Virmani, R. Morphologic findings of coronary atherosclerotic plaques in diabetics: A postmortem study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). Circ. J. 2013, 77, 231–248. [CrossRef] [PubMed]
- Teramoto, T.; Sasaki, J.; Ueshima, H.; Egusa, G.; Kinoshita, M.; Shimamoto, K.; Daida, H.; Biro, S.; Hirobe, K.; Funahashi, T.; et al. Risk factors of atherosclerotic diseases. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerosis cardiovascular diseases for Japanese. J. Atheroscler. Thromb. 2007, 14, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society of Nephrology. Evidence-based practice guideline for the treatment of CKD. Clin. Exp. Nephrol. 2009, 13, 537–566. [Google Scholar] [CrossRef]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M.; et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [PubMed]
- Eriksson, L.B.T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis Basic Principles and Applications, 3rd ed.; Umetrics Academy: Umeå, Sweden, 2013. [Google Scholar]
- Vajargah, K.F.; Sadeghi-Bazargani, H.; Mehdizadeh-Esfanjani, R.; Savadi-Oskouei, D.; Farhoudi, M. OPLS statistical model versus linear regression to assess sonographic predictors of stroke prognosis. Neuropsychiatr. Dis. Treat. 2012, 8, 387–392. [Google Scholar] [CrossRef]
- Agarwala, A.P.; Rodrigues, A.; Risman, M.; McCoy, M.; Trindade, K.; Qu, L.; Cuchel, M.; Billheimer, J.; Rader, D.J. High-Density Lipoprotein (HDL) Phospholipid Content and Cholesterol Efflux Capacity Are Reduced in Patients With Very High HDL Cholesterol and Coronary Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1515–1519. [Google Scholar] [CrossRef]
- Kataoka, Y.; Puri, R.; Hammadah, M.; Duggal, B.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; King, P.; Nicholls, S.J. Sex Differences in Nonculprit Coronary Plaque Microstructures on Frequency-Domain Optical Coherence Tomography in Acute Coronary Syndromes and Stable Coronary Artery Disease. Circ. Cardiovasc. Imaging 2016, 9, 004506. [Google Scholar] [CrossRef]
- Moreno, P.R.; Murcia, A.M.; Palacios, I.F.; Leon, M.N.; Bernardi, V.H.; Fuster, V.; Fallon, J.T. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000, 102, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Inaba, S.; Okayama, H.; Funada, J.; Higashi, H.; Saito, M.; Yoshii, T.; Hiasa, G.; Sumimoto, T.; Takata, Y.; Nishimura, K.; et al. Impact of type 2 diabetes on serial changes in tissue characteristics of coronary plaques: An integrated backscatter intravascular ultrasound analysis. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hatley, M.E.; Bolick, D.T.; Palmer, L.A.; Edelstein, D.; Brownlee, M.; Hedrick, C.C. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 2004, 47, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Barnett, J.; Fong, L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta 1990, 1044, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, S.T.; Jarvis, M.R.; Hamilton, R.L.; Kane, J.P. Binding of transition metals by apolipoprotein A-I-containing plasma lipoproteins: Inhibition of oxidation of low density lipoproteins. Proc. Natl. Acad. Sci. USA 1992, 89, 6993–6997. [Google Scholar] [CrossRef] [PubMed]
- Distelmaier, K.; Schrutka, L.; Seidl, V.; Winter, M.; Wurm, R.; Mangold, A.; Perkmann, T.; Maurer, G.; Adlbrecht, C.; Lang, I.M. Pro-oxidant HDL predicts poor outcome in patients with ST-elevation acute coronary syndrome. Thromb. Haemost. 2015, 114, 133–138. [Google Scholar] [CrossRef]
- Kaartinen, M.; Penttila, A.; Kovanen, P.T. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-alpha. Circulation 1996, 94, 2787–2792. [Google Scholar] [CrossRef]
- Robbesyn, F.; Garcia, V.; Auge, N.; Vieira, O.; Frisach, M.F.; Salvayre, R.; Negre-Salvayre, A. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J. 2003, 17, 743–745. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.S.; Vengrenyuk, Y.; Shameer, K.; Maehara, A.; Purushothaman, M.; Yoshimura, T.; Matsumura, M.; Aquino, M.; Haider, N.; Johnson, K.W.; et al. Intracoronary Imaging, Cholesterol Efflux, and Transcriptomes After Intensive Statin Treatment: The YELLOW II Study. J. Am. Coll. Cardiol. 2017, 69, 628–640. [Google Scholar] [CrossRef]
- Takata, K.; Imaizumi, S.; Kawachi, E.; Suematsu, Y.; Shimizu, T.; Abe, S.; Matsuo, Y.; Tsukahara, H.; Noda, K.; Yahiro, E.; et al. Impact of cigarette smoking cessation on high-density lipoprotein functionality. Circ. J. 2014, 78, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Ayaori, M.; Uto-Kondo, H.; Furuyama, F.; Yokota, Y.; Tsunoda, F.; Shoji, M.; Ikewaki, K.; Kobayashi, Y. Beneficial Effects of Exercise-Based Cardiac Rehabilitation on High-Density Lipoprotein-Mediated Cholesterol Efflux Capacity in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2016, 23, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Berliner, J.A.; Subbanagounder, G.; Hama, S.; Lusis, A.J.; Castellani, L.W.; Reddy, S.; Shih, D.; Shi, W.; Watson, A.D.; et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Andrews, J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.K.; Schwartz, G.G.; Worthley, S.G.; Keyserling, C.; Dasseux, J.L.; et al. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 815–822. [Google Scholar] [CrossRef]
- Gibson, C.M.; Kastelein, J.J.P.; Phillips, A.T.; Aylward, P.E.; Yee, M.K.; Tendera, M.; Nicholls, S.J.; Pocock, S.; Goodman, S.G.; Alexander, J.H.; et al. Rationale and design of ApoA-I Event Reducing in Ischemic Syndromes II (AEGIS-II): A phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am. Heart J. 2021, 231, 121–127. [Google Scholar] [CrossRef]
- Michael Gibson, C.; Korjian, S.; Tricoci, P.; Daaboul, Y.; Yee, M.; Jain, P.; Alexander, J.H.; Steg, P.G.; Lincoff, A.M.; Kastelein, J.J.; et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016, 134, 1918–1930. [Google Scholar] [CrossRef]
- Solly, E.L.; Dimasi, C.G.; Bursill, C.A.; Psaltis, P.J.; Tan, J.T.M. MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis. J. Clin. Med. 2019, 8, 2199. [Google Scholar] [CrossRef]
- Rayner, K.J.; Moore, K.J. MicroRNA control of high-density lipoprotein metabolism and function. Circ. Res. 2014, 114, 183–192. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, M.; Song, Q.; Cheng, L.; Zhang, X.; Song, G.; Sun, X.; Gu, M.; Zhou, C.; Zhang, Y.; et al. Development of the novel ACLY inhibitor 326E as a promising treatment for hypercholesterolemia. Acta Pharm. Sin. B 2023, 13, 739–753. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef] [PubMed]



| Variables | Overall (n = 38) | Male (n = 26) | Female (n = 12) | p Value |
|---|---|---|---|---|
| Age, years | 70 ± 9 | 70 ± 8 | 70 ± 11 | 0.90 |
| BMI, kg/m2 | 25 ± 4 | 25 ± 4 | 24 ± 5 | 0.50 |
| Smoking, n (%) | 5 (13) | 4 (15) | 1 (8) | 0.55 |
| Hypertension, n (%) | 30 (79) | 21 (81) | 9 (75) | 0.69 |
| Dyslipidemia, n (%) | 32 (84) | 22 (85) | 10 (83) | 0.92 |
| Hyperuricemia, n (%) | 6 (16) | 6 (23) | 0 (0) | 0.07 |
| CKD, n (%) | 20 (53) | 15 (58) | 5 (42) | 0.36 |
| Prior MI, n (%) | 8 (21) | 5 (19) | 3 (25) | 0.69 |
| Prior PCI, n (%) | 15 (39) | 9 (35) | 6 (50) | 0.37 |
| Prior CABG, n (%) | 1 (3) | 1 (4) | 0 (0) | 0.49 |
| Number of diseased vessel, n (%) 1/2/3 | 18/11/9 (47/29/24) | 14/8/4 (54/31/15) | 4/3/5 (33/25/42) | 0.20 |
| Target vessel, n (%) LAD/LCx/RCA/LMT | 14/5/18/1 (37/13/47/3) | 8/3/14/1 (31/12/54/4) | 6/2/4/0 (50/17/33) | 0.54 |
| Stent, n (%) DES/BMS | 37/1 (97/3) | 25/1 (96/4) | 12/0 (100/0) | 0.49 |
| Medications, n (%) | ||||
| Statin | 37 (97) | 25 (96) | 12 (100) | 0.49 |
| Ezetimibe | 3 (8) | 3 (12) | 0 (0) | 0.22 |
| EPA | 7 (18) | 5 (19) | 2 (17) | 0.85 |
| ARB | 25 (66) | 18 (69) | 7 (58) | 0.51 |
| ACE-I | 2 (5) | 1 (4) | 1 (8) | 0.56 |
| CCB | 26 (68) | 16 (62) | 10 (83) | 0.18 |
| β-blocker | 3 (8) | 3 (12) | 0 (0) | 0.22 |
| Aspirin | 38 (100) | 26 (100) | 12 (100) | 1.00 |
| Thienopyridine | 38 (100) | 26 (100) | 12 (100) | 1.00 |
| Variables | Overall (n = 38) | Male (n = 26) | Female (n = 12) | p Value |
|---|---|---|---|---|
| Lipids | ||||
| TG, mg/dL | 149 ± 76 | 152 ± 75 | 144 ± 82 | 0.69 |
| LDL-C, mg/dL | 88 ± 24 | 84 ± 26 | 96 ± 20 | 0.12 |
| sd-LDL-C, mg/dL | 32 ± 15 | 32 ± 15 | 34 ± 16 | 0.64 |
| HDL-C, mg/dL | 50 ± 14 | 47 ± 11 | 55 ± 18 | 0.18 |
| HDL functionality | ||||
| HDL-CEC, % | 14.2 ± 1.4 | 13.9 ± 1.3 | 14.7 ± 1.5 | 0.12 |
| HII, AU | 1.10 ± 0.20 | 1.12 ± 0.19 | 1.05 ± 0.24 | 0.18 |
| Apolipoproteins | ||||
| ApoA1, mg/dL | 94 ± 22 | 89 ± 21 | 103 ± 22 | 0.10 |
| ApoB, mg/dL | 22 ± 8 | 20 ± 6 | 26 ± 10 | 0.10 |
| ApoC2, mg/dL | 4.8 ± 1.8 | 4.7 ± 1.7 | 4.9 ± 2.0 | 0.63 |
| ApoC3, mg/dL | 12.9 ± 3.3 | 12.8 ± 3.1 | 13.2 ± 3.7 | 0.55 |
| Free fatty acids | ||||
| LnA, µg/mL | 34 ± 11 | 33 ± 12 | 37 ± 9 | 0.18 |
| AA, µg/mL | 173 ± 31 | 165 ± 28 | 190 ± 33 | 0.025 |
| EPA, µg/mL | 80 ± 57 | 82 ± 53 | 77 ± 68 | 0.15 |
| EPA/AA | 0.50 ± 0.42 | 0.53 ± 0.42 | 0.43 ± 0.43 | 0.06 |
| DHA, µg/mL | 125 ± 35 | 125 ± 37 | 127 ± 34 | 0.76 |
| Others | ||||
| hs-CRP, mg/dL | 0.13 ± 0.20 | 0.13 ± 0.23 | 0.11 ± 0.10 | 0.75 |
| eGFR, mL/min/1.73 m2 | 61 ± 15 | 59 ± 14 | 64 ± 17 | 0.43 |
| UA, mg/dL | 5.2 ± 1.4 | 5.6 ± 1.4 | 4.4 ± 1.1 | 0.015 |
| HbA1c, % | 7.0 ± 1.0 | 7.0 ± 1.1 | 7.1 ± 0.8 | 0.38 |
| FBS, mg/dL | 103 ± 23 | 106 ± 21 | 98 ± 26 | 0.20 |
| SBP, mmHg | 129 ± 17 | 131 ± 19 | 124 ± 11 | 0.43 |
| DBP, mmHg | 69 ± 12 | 72 ± 12 | 64 ± 8 | 0.038 |
| LVEF, % | 66 ± 9 | 64 ± 10 | 69 ± 8 | 0.18 |
| Variables | Overall (n = 38) | Male (n = 26) | Female (n = 12) | p Value |
|---|---|---|---|---|
| Gray-scale IVUS parameters | ||||
| 3D parameters | ||||
| PAV, % | 73.3 ± 5.5 | 73.4 ± 5.5 | 72.9 ± 5.8 | 1.00 |
| TAV, m3 | 87.4 (67.9 to 102.6) | 91.6 (82.3 to 113.0) | 72.8 (54.2 to 84.9) | 0.005 |
| Vessel volume, m3 | 117.8 (87.7 to 137.9) | 125.3 (108.8 to 152.5) | 94.0 (77.9 to 110.8) | 0.005 |
| Lumen volume, m3 | 27.4 (23.8 to 39.3) | 32.3 (24.8 to 41.3) | 24.5 (20.9 to 27.2) | 0.01 |
| 2D parameters | ||||
| PAA, % | 82.8 ± 5.5 | 83.4 ± 5.9 | 81.6 ± 4.4 | 0.23 |
| TAA, m2 | 10.4 (7.5 to 13.3) | 11.5 (9.0 to 15.3) | 7.9 (6.8 to 9.4) | 0.01 |
| Vessel area, m2 | 12.1 (9.3 to 15.5) | 14.0 (10.8 to 16.9) | 9.5 (8.8 to 11.6) | 0.01 |
| Lumen area, m2 | 1.8 (1.5 to 2.5) | 1.9 (1.6 to 2.5) | 1.6 (1.3 to 2.3) | 0.16 |
| IB-IVUS parameters | ||||
| 3D parameters | ||||
| Plaque components | ||||
| LV, m3 | 48.8 (31.8 to 62.6) | 58.9 (43.2 to 77.5) | 37.7 (29.4 to 46.9) | 0.01 |
| FV, m3 | 28.8 (23.6 to 35.3) | 30.2 (26.4 to 37.0) | 25.3 (19.9 to 33.0) | 0.15 |
| dense-FV, m3 | 3.1 (2.2 to 5.5) | 3.6 (2.3 to 6.1) | 2.6 (1.8 to 4.2) | 0.26 |
| CV, m3 | 0.9 (0.4 to 1.8) | 0.9 (0.4 to 1.8) | 0.7 (0.3 to 1.9) | 0.68 |
| Plaque composition | ||||
| %LV | 57.1 (45.0 to 66.4) | 62.0 (45.0 to 68.2) | 56.2 (47.0 to 58.3) | 0.13 |
| %FV | 36.5 (28.3 to 44.0) | 33.8 (27.1 to 42.5) | 37.6 (31.6 to 51.0) | 0.24 |
| %dense-FV | 4.4 (2.5 to 5.8) | 4.2 (2.4 to 5.8) | 4.7 (2.6 to 5.8) | 0.63 |
| %CV | 1.2 (0.4 to 1.9) | 1.2 (0.4 to 1.4) | 1.3 (0.5 to 2.9) | 0.58 |
| 2D parameters | ||||
| Plaque components | ||||
| LA, m2 | 6.7 (4.2 to 8.8) | 7.9 (6.4 to 10.4) | 4.3 (4.0 to 5.4) | 0.01 |
| FA, m2 | 2.7 (2.3 to 4.0) | 3.1 (2.3 to 4.0) | 2.4 (2.3 to 2.6) | 0.16 |
| dense-FA, m2 | 0.4 (0.2 to 0.5) | 0.4 (0.3 to 0.6) | 0.2 (0.2 to 0.4) | 0.17 |
| CA, m2 | 0.1 (0.0 to 0.2) | 0.1 (0.0 to 0.2) | 0.1 (0.0 to 0.2) | 0.63 |
| Plaque composition | ||||
| %LA | 62.2 (55.3 to 72.0) | 66.9 (55.3 to 75.7) | 59.4 (55.3 to 62.8) | 0.12 |
| %FA | 31.1 (24.7 to 36.4) | 30.2 (22.1 to 35.7) | 32.7 (29.7 to 40.3) | 0.14 |
| %dense-FA | 3.5 (2.1 to 6.0) | 3.1 (2.1 to 6.0) | 3.9 (2.1 to 5.4) | 0.94 |
| %CA | 0.8 (0.2 to 2.1) | 0.7 (0.2 to 2.1) | 0.9 (0.3 to 2.0) | 0.79 |
| Dependent Variable | Variations by P1 | Variations by P2 |
|---|---|---|
| PAV | <0.001 | 0.03 |
| TAV | 0.15 | 0.24 |
| LV | 0.46 | 0.08 |
| FV | 0.03 | 0.35 |
| dense-FV | 0.04 | 0.10 |
| %LV | 0.49 | 0.004 |
| %FV | 0.47 | 0.03 |
| %dense-FV | 0.18 | 0.001 |
| Loading Value of P1 | Loading Value of P2 | |
|---|---|---|
| X variables | ||
| BMI | −0.09 | 0.02 |
| TG | 0.02 | −0.12 |
| LDL-C | 0.06 | −0.16 |
| HDL-C | 0.03 | −0.01 |
| HDL-CEC | 0.14 | 0.01 |
| HII | −0.22 | −0.15 |
| ApoA1 | 0.14 | −0.03 |
| hs-CRP | −0.03 | −0.18 |
| eGFR | 0.01 | −0.01 |
| HbA1c | −0.11 | −0.06 |
| SBP | −0.10 | 0.08 |
| DBP | −0.19 | 0.10 |
| Y variables | ||
| PAV | 0.05 | 0.30 |
| TAV | −0.20 | 0.47 |
| LV | −0.37 | 0.28 |
| FV | 0.16 | 0.52 |
| dense-FV | 0.23 | 0.43 |
| %LV | −0.47 | −0.11 |
| %FV | 0.45 | 0.11 |
| %dense-FV | 0.39 | 0.13 |
| Variables | %LV | FV | ||
|---|---|---|---|---|
| Coefficient | 95% CI | Coefficient | 95% CI | |
| HII | 0.34 | (0.08, 0.60) | −0.25 | (−0.68, 0.17) |
| DBP | 0.28 | (0.01, 0.54) | 0.20 | (−0.37, 0.77) |
| hs-CRP | 0.06 | (−0.13, 0.25) | −0.25 | (−0.55, 0.04) |
| LDL-C | −0.17 | (−0.47, 0.14) | −0.11 | (−0.66, 0.43) |
| HDL-CEC | −0.26 | (−0.49, −0.04) | 0.15 | (−0.31, 0.60) |
| ApoA1 | −0.20 | (−0.44, 0.05) | 0.06 | (−0.25, 0.37) |
| SBP | 0.04 | (−0.43, 0.52) | 0.09 | (−0.25, 0.43) |
| HbA1c | 0.24 | (−0.20, 0.68) | −0.20 | (−0.57, 0.17) |
| TG | −0.03 | (−0.25, 0.19) | −0.19 | (−0.62, 0.25) |
| BMI | −0.01 | (−0.26, 0.23) | −0.01 | (−0.42, 0.40) |
| HDL-C | 0.09 | (−0.16, 0.33) | −0.12 | (−0.48, 0.24) |
| eGFR | −0.07 | (−0.38, 0.24) | 0.10 | (−0.46, 0.66) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, K.; Imaizumi, S.; Iwata, A.; Zhang, B.; Kawachi, E.; Miura, S.-i.; Ogawa, M. Associations of High-Density Lipoprotein Functionality with Coronary Plaque Characteristics in Diabetic Patients with Coronary Artery Disease: Integrated Backscatter Intravascular Ultrasound Analysis. Biomolecules 2023, 13, 1278. https://doi.org/10.3390/biom13091278
Takata K, Imaizumi S, Iwata A, Zhang B, Kawachi E, Miura S-i, Ogawa M. Associations of High-Density Lipoprotein Functionality with Coronary Plaque Characteristics in Diabetic Patients with Coronary Artery Disease: Integrated Backscatter Intravascular Ultrasound Analysis. Biomolecules. 2023; 13(9):1278. https://doi.org/10.3390/biom13091278
Chicago/Turabian StyleTakata, Kohei, Satoshi Imaizumi, Atsushi Iwata, Bo Zhang, Emi Kawachi, Shin-ichiro Miura, and Masahiro Ogawa. 2023. "Associations of High-Density Lipoprotein Functionality with Coronary Plaque Characteristics in Diabetic Patients with Coronary Artery Disease: Integrated Backscatter Intravascular Ultrasound Analysis" Biomolecules 13, no. 9: 1278. https://doi.org/10.3390/biom13091278
APA StyleTakata, K., Imaizumi, S., Iwata, A., Zhang, B., Kawachi, E., Miura, S.-i., & Ogawa, M. (2023). Associations of High-Density Lipoprotein Functionality with Coronary Plaque Characteristics in Diabetic Patients with Coronary Artery Disease: Integrated Backscatter Intravascular Ultrasound Analysis. Biomolecules, 13(9), 1278. https://doi.org/10.3390/biom13091278






