Beneficial Effects of Lactobacilli Species on Intestinal Homeostasis in Low-Grade Inflammation and Stress Rodent Models and Their Implication in the Modulation of the Adhesive Junctional Complex
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Culture Preparation
2.2. In Vivo Models of Induced-Intestinal Hyperpermeability
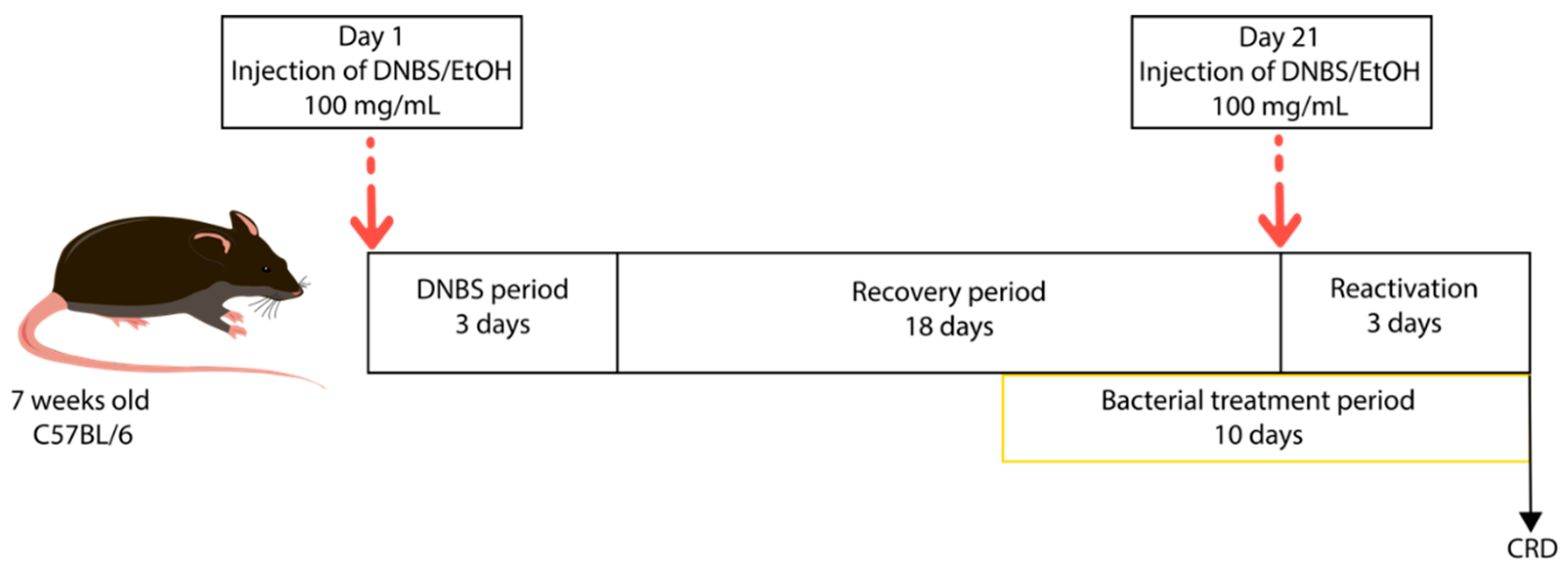
2.2.1. Low-Grade Inflammation
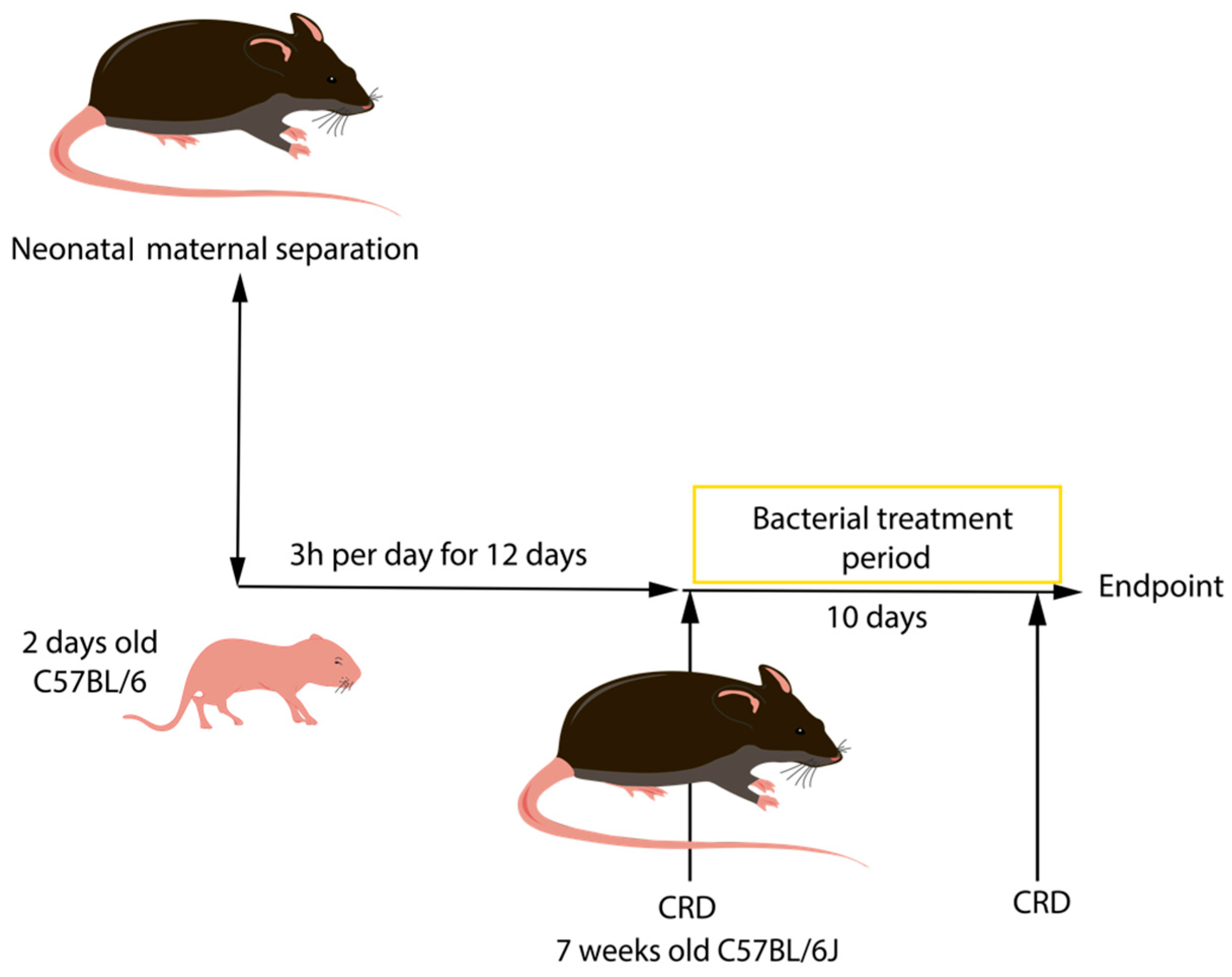
2.2.2. Neonatal Maternal Separation (NMS)
2.3. Assessment of Tissue Damage
2.4. Quantification of Early Inflammation Biomarker: Lipocalin-2/NGAL (LCN2)
2.5. Study of Mucus Production
2.6. In Vivo Permeability Assay
2.7. Evaluation of the CHS
2.8. Study of the Expression of Tight Junction Complex by Quantitative Real-Time PCR (qPCR)
2.9. Statistics
3. Results
3.1. Assessment of Tissue Damage
3.1.1. Low-Grade Inflammation
3.1.2. Neonatal Maternal Separation (NMS)
3.2. Evaluation of Early Inflammation (Lipocalin-2)
3.3. Lactobacillus Strains Enhance the Epithelial Barrier Functions
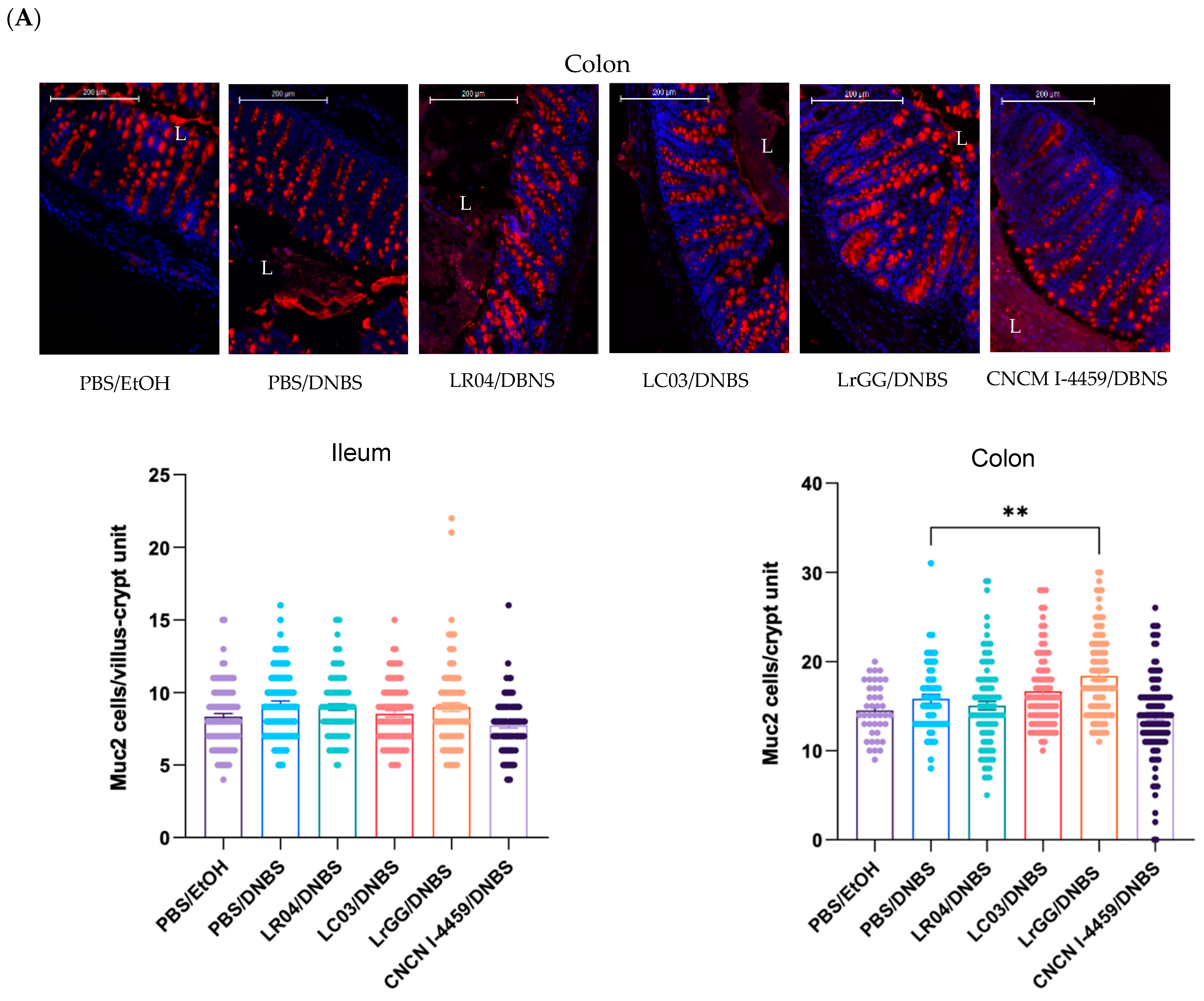
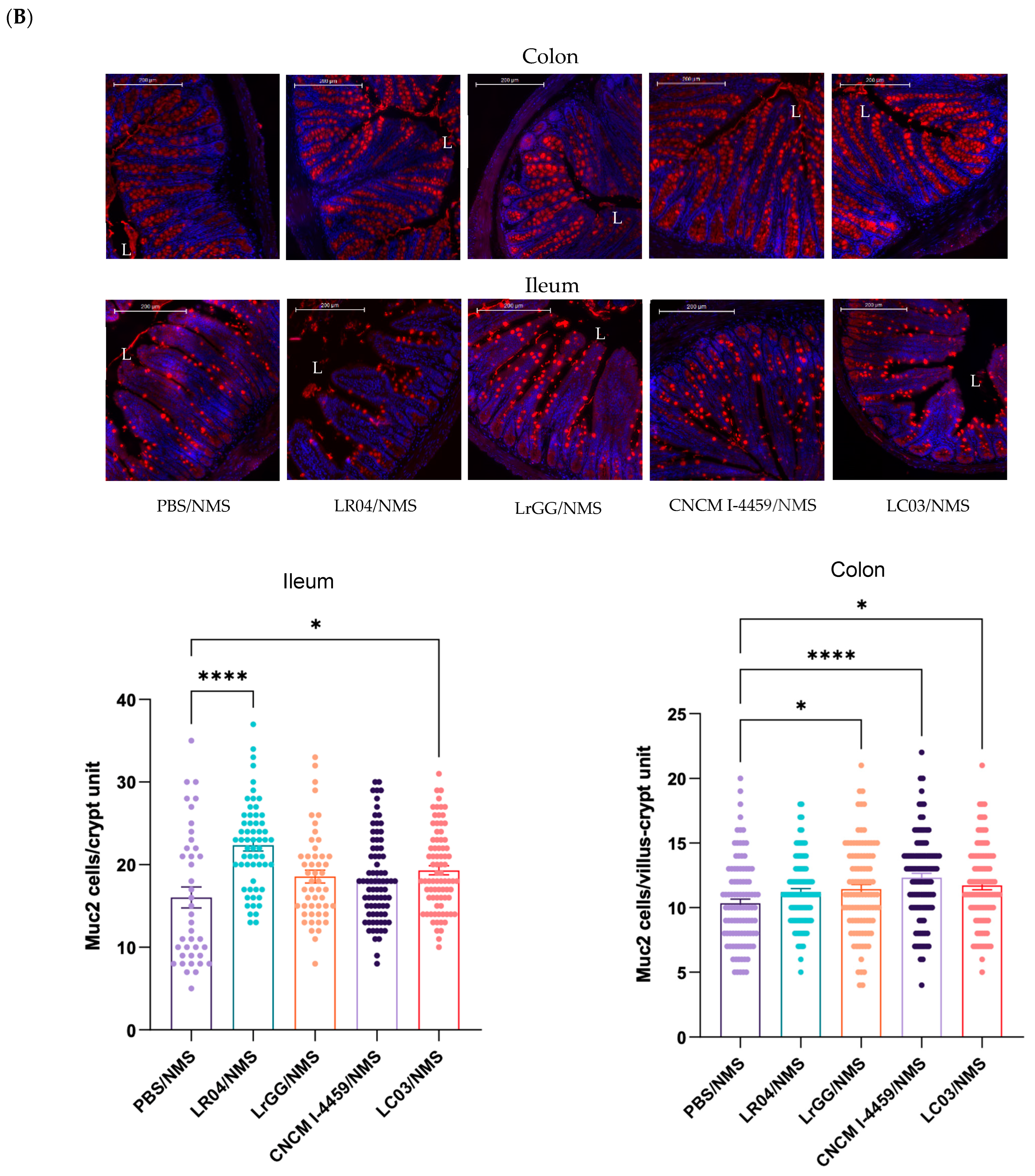
3.3.1. Effects on Mucus Production
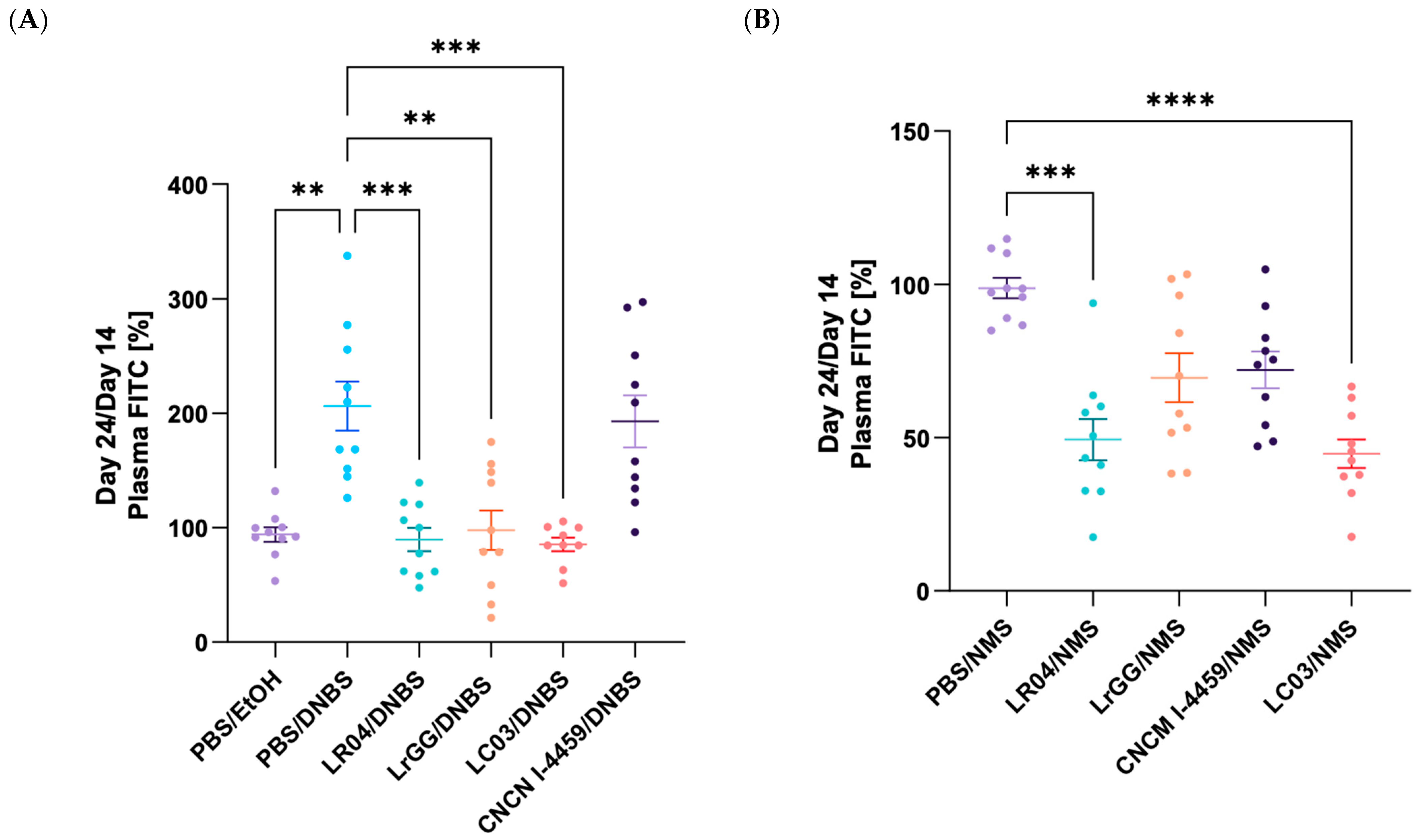
3.3.2. Modulation of the Gut Permeability
3.3.3. Modulation of the CHS
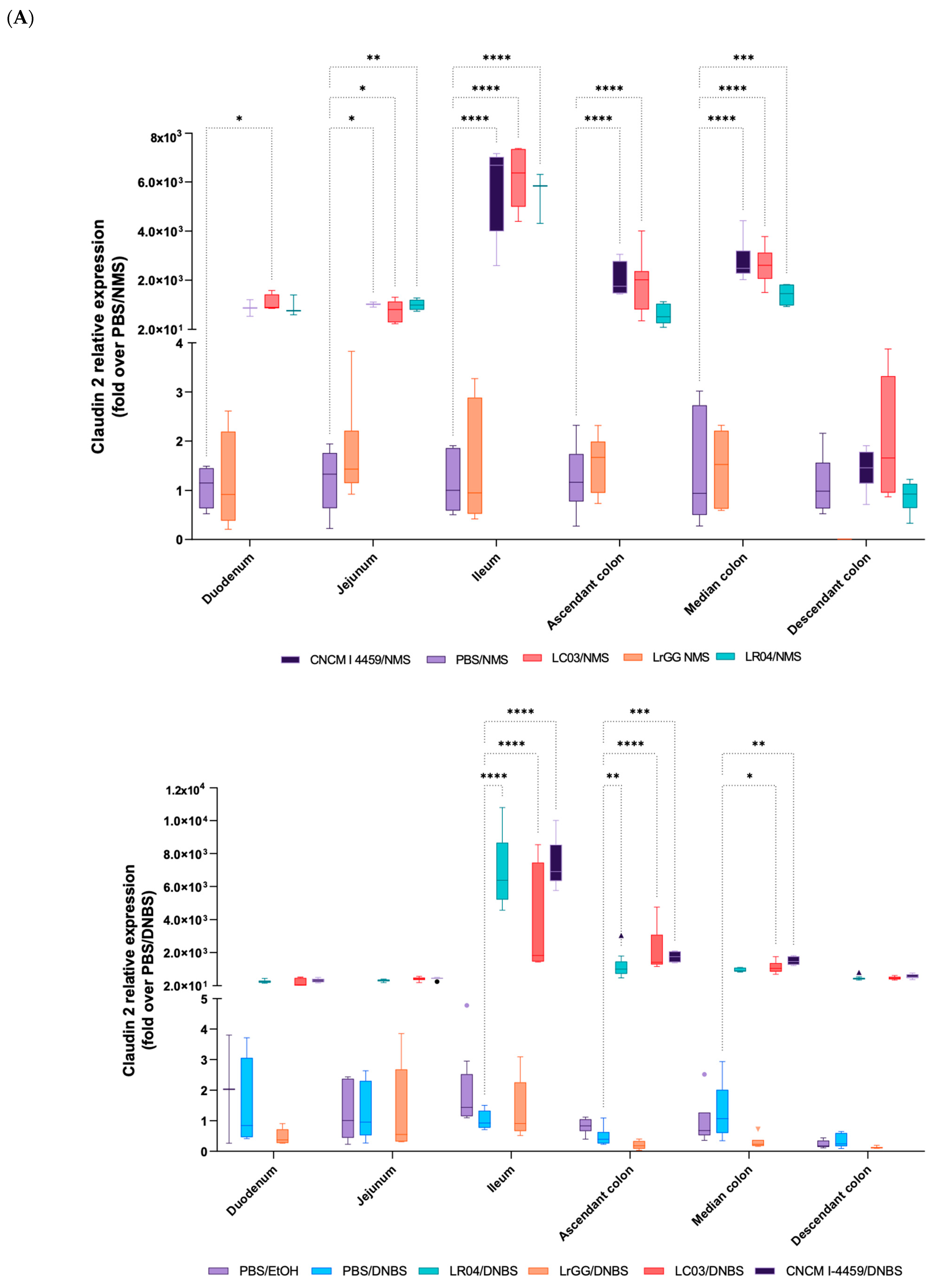
3.4. Expression of Gene Involved in the Complex of Epithelial Cell Junctions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaladanki, R.N.; Wang, J.-Y. Regulation of Gastrointestinal Mucosal Growth; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef]
- Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis inf High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG—Host interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef]
- Sreeja, V.; Prajapati, J.B. Probiotic Formulations: Application and Status as Pharmaceuticals—A Review. Probiotics Antimicrob. Proteins 2013, 5, 81–91. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, D.-M.; Sharkey, K.A.; Wallace, J.L. Beneficial effects of local or systemic lidocaine in experimental colitis. Am. J. Physiol. 1994, 266, G560–G567. [Google Scholar] [CrossRef]
- Ameho, C.K.; Adjei, A.A.; Harrison, E.K.; Takeshita, K.; Morioka, T.; Arakaki, Y.; Ito, E.; Suzuki, I.; Kulkarni, A.D.; Kawajiri, A.; et al. Prophylactic eVect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor a production in trinitrobenzene sulphonic acid induced colitis. Gut 1997, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Mizutani, T.; Mochizuki, W.; Matsumoto, T.; Nozaki, K.; Sakamaki, Y.; Ichinose, S.; Okada, Y.; Tanaka, T.; Watanabe, M.; et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes. Dev. 2014, 28, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Taché, Y. Stress and visceral pain: From animal models to clinical therapies. Exp. Neurol. 2012, 233, 49–67. [Google Scholar] [CrossRef]
- Sharma, S.; Awasthi, A.; Singh, S. Altered gut microbiota and intestinal permeability in Parkinson’s disease: Pathological highlight to management. Patient Prefer. Adherence 2020, 14, 927–937. [Google Scholar] [CrossRef]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef]
- Singh, P.; Silvester, J.; Chen, X.; Xu, H.; Sawhney, V.; Rangan, V.; Iturrino, J.; Nee, J.; Duerksen, D.R.; Lembo, A. Serum zonulin is elevated in IBS and correlates with stool frequency in IBS-D. United Eur. Gastroenterol. J. 2019, 7, 709–715. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Ran, Y.; Fukui, H.; Xu, X.; Wang, X.; Ebisutani, N.; Tanaka, Y.; Maeda, A.; Makizaki, Y.; Ohno, H.; Kondo, T.; et al. Alteration of Colonic Mucosal Permeability during Antibiotic-Induced Dysbiosis. Int. J. Mol. Sci. 2020, 21, 6108. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Yahya, G.; Gobba, N.A.; Sharaf, H.; Alshaman, R.; Alattar, A.; Amin, N.A.; El-Shedody, R.; Aboutouk, F.H.; El-Galeel, Y.A.; et al. The Supportive Role of NSC328332, a P2X7R Antagonist, in Enhancing the Inhibitory Effect of CRID3 on NLRP3 Inflammasome Activation in Rats with Dextran Sodium Sulfate-Induced Colitis. J. Inflamm. Res. 2021, 14, 3443–3463. [Google Scholar] [CrossRef]
- Liu, H.; Hong, X.L.; Sun, T.T.; Huang, X.W.; Wang, J.L.; Xiong, H. Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J. Dig. Dis. 2020, 21, 385–398. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kurihara, C.; Furuhashi, H.; Takajo, T.; Maruta, K.; Yasutake, Y.; Sato, H.; Narimatsu, K.; Okada, Y.; Higashiyama, M.; et al. Psychological stress exacerbates NSAID-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J. Gastroenterol. 2017, 52, 61–71. [Google Scholar] [CrossRef]
- Martín, R.; Chain, F.; Miquel, S.; Motta, J.P.; Vergnolle, N.; Sokol, H.; Langella, P. Using murine colitis models to analyze probiotics–host interactions. FEMS Microbiol. Rev. 2017, 41, S49–S70. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef]
- Hsieh, H.; Morin, J.; Filliettaz, C.; Varada, R.; LaBarre, S.; Radi, Z. Fecal Lipocalin-2 as a Sensitive and Noninvasive Biomarker in the TNBS Crohn’s Inflammatory Bowel Disease Model. Toxicol. Pathol. 2016, 44, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Lee, S.; Park, D.H.; Kook, H.; Park, K.G.; Lee, I.K.; Suk, K. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci. Biobehav. Rev. 2015, 49, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Yadav, H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab. 2017, 71, 11–16. [Google Scholar] [CrossRef]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–120. [Google Scholar] [CrossRef] [PubMed]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin Function in Inflammatory Bowel Disease An Update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.; Robbe-Masselot, C.; Ait-Belgnaoui, A.; Mancuso, A.; Mercade-Loubière, M.; Salvador-Cartier, C.; Gillet, M.; Ferrier, L.; Loubière, P.; Dague, E.; et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: Prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G420–G429. [Google Scholar] [CrossRef] [PubMed]
- Arike, L.; Hansson, G.C. The densely O-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J. Mol. Biol. 2016, 428, 3221–3229. [Google Scholar] [CrossRef]
- Martín, R.; Laval, L.; Chain, F.; Miquel, S.; Natividad, J.; Cherbuy, C.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.; Bermudez-Humaran, L.G.; et al. Bifidobacterium animalis ssp. lactis CNCM-I2494 Restores Gut Barrier Permeability in Chronically Low-Grade Inflamed Mice. Front. Microbiol. 2016, 7, 608. [Google Scholar] [CrossRef]
- Barone, M.; Chain, F.; Sokol, H.; Brigidi, P.; Bermúdez-Humarán, L.G.; Langella, P.; Martín, R. A Versatile New Model of Chemically Induced Chronic Colitis Using an Outbred Murine Strain. Front. Microbiol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef]
- Li, B.; Yu, F.Z.; Minich, A.; Hock, A.; Lee, C.; Pierro, A. Neonatal Intestinal Injury Induced by Maternal Separation: Pathogenesis and Pharmacological Targets. Can. J. Physiol. Pharmacol. 2019, 97, 193–196. [Google Scholar] [CrossRef]
- Rincel, M.; Olier, M.; Minni, A.; Monchaux de Oliveira, C.; Matime, Y.; Gaultier, E.; Grit, I.; Helbling, J.C.; Costa, A.M.; Lépinay, A.; et al. Pharmacological restoration of gut barrier function in stressed neonates partially reverses long-term alterations associated with maternal separation. Psychopharmacology 2019, 236, 1583–1596. [Google Scholar] [CrossRef] [PubMed]
- Deiteren, A.; Vermeulen, W.; Moreels, T.G.; Pelckmans, P.A.; De Man, J.G.; De Winter, B.Y. The effect of chemically induced colitis, psychological stress and their combination on visceral pain in female Wistar rats. Stress 2014, 17, 431–444. [Google Scholar] [CrossRef]
- Yi, L.; Zhang, H.; Sun, H.; Zhou, L.; Chen, Y.; Xuan, L.; Jiang, Y.; Xu, S. Maternal Separation Induced Visceral Hypersensitivity from Childhood to Adulthood. J. Neurogastroenterol. Motil. 2017, 23, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Ait Belgnaoui, A.; Agostini, S.; Eutamene, H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes 2014, 5, 430–436. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Tramullas, M.; Fitzgerald, P.; Cryan, J.F. Rodent Models of Colorectal Distension. Curr. Protoc. Neurosci 2012, 61, 9.40.1–9.40.13. [Google Scholar] [CrossRef]
- Chamignon, C.; Guéneau, V.; Medina, S.; Deschamps, J.; Gil-Izquierdo, A.; Briandet, R.; Mousset, P.Y.; Langella, P.; Lafay, S.; Bermúdez-Humarán, L.G. Evaluation of the Probiotic Properties and the Capacity to Form Biofilms of Various Lactobacillus Strains. Microorganisms 2020, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Bradesi, S.; Fioramonti, J.; Theodorou, V.; Bueno, L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: Role of myosin light chain kinase. Pain 2005, 113, 141–147. [Google Scholar] [CrossRef]
- Martínez, C.; Vicario, M.; Ramos, L.; Lobo, B.; Mosquera, J.L.; Alonso, C.; Sánchez, A.; Guilarte, M.; Antolín, M.; de Torres, I.; et al. The Jejunum of Diarrhea-Predominant Irritable Bowel Syndrome Shows Molecular Alterations in the Tight Junction Signaling Pathway That Are Associated With Mucosal Pathobiology and Clinical Manifestations. Am. J. Gastroenterol. 2012, 107, 736–746. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Weber, C.R.; Raleigh, D.R.; Su, L.; Shen, L.; Sullivan, E.A.; Wang, Y.; Turner, J.R. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010, 285, 12037–12046. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Frey, M.R.; Washington, M.K.; Chaturvedi, R.; Kuhnhein, L.A.; Matta, P.; Revetta, F.L.; Wilson, K.T.; Polk, D.B. STAT6 Activation in Ulcerative Colitis: A New Target for Prevention of IL-13-Induced Colon Epithelial Cell Dysfunction. Inflamm. Bowel Dis. 2011, 17, 2224–2234. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Günzel, D.; Conrad, M.P.; Lee, I.F.; Amasheh, S.; Fromm, M.; Yu, A.S. Charge-selective claudin channels. Ann. N. Y Acad. Sci. 2012, 1257, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Chaturvedi, R.; Olivares-Villagómez, D.; Habib, T.; Asim, M.; Shivesh, P.; Polk, D.B.; Wilson, K.T.; Washington, M.K.; Van Kaer, L.; et al. Targeted Colonic Claudin-2 Expression Renders Resistance to Epithelial Injury, Induces Immune Suppression and Protects from Colitis. Mucosal Immunol. 2014, 7, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Yoshida, M.; Nishiumi, S.; Furuse, M.; Azuma, T. Claudin-2 Regulates Colorectal Inflammation via Myosin Light Chain Kinase-Dependent Signaling. Dig. Dis. Sci. 2013, 58, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Anwer, S.; Szaszi, K. Claudin-2: Roles beyond Permeability Functions. Int. J. Mol. Sci. 2019, 20, 5655. [Google Scholar] [CrossRef]
- Guillemot, L.; Schneider, Y.; Brun, P.; Castagliuolo, I.; Pizzuti, D.; Martines, D.; Jond, L.; Bongiovanni, M.; Citi, S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J. Cell Sci. 2012, 125, 5005–5014. [Google Scholar] [CrossRef]
- Schossleitner, K.; Rauscher, S.; Gröger, M.; Friedl, H.P.; Finsterwalder, R.; Habertheuer, A.; Sibilia, M.; Brostjan, C.; Födinger, D.; Citi, S.; et al. Evidence That Cingulin Regulates Endothelial Barrier Function In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, L.; Citi, S. Cingulin Regulates Claudin-2 Expression and Cell Proliferation through the Small GTPase RhoA. Mol. Biol. Cell 2006, 17, 3569–3577. [Google Scholar] [CrossRef]
- Dan, Q.; Shi, Y.; Rabani, R.; Venugopal, S.; Xiao, J.; Anwer, S.; Ding, M.; Speight, P.; Pan, W.; Alexander, R.T.; et al. Claudin-2 suppresses GEF-H1, RHOA, and MRTF thereby impacting proliferation and profibrotic phenotype of tubular cells. J. Biol. Chem. 2019, 294, 15446–15465. [Google Scholar] [CrossRef]
- Terry, S.; Nie, M.; Matter, K.; Balda, M.S. Rho Signaling and Tight Junction Functions. Physiology 2010, 25, 16–26. [Google Scholar] [CrossRef]
- Tian, Y.; Gawlak, G.; Tian, X.; Shah, A.S.; Sarich, N.; Citi, S.; Birukova, A.A. Role of Cingulin in Agonist-induced Vascular Endothelial Permeability. J. Biol. Chem. 2016, 291, 23681–23692. [Google Scholar] [CrossRef]
- Guillemot, L.; Spadaro, D.; Citi, S. The Junctional Proteins Cingulin and Paracingulin Modulate the Expression of Tight Junction Protein Genes through GATA-4. PLoS ONE 2013, 8, e55873. [Google Scholar] [CrossRef] [PubMed]





| Lactobacillus Species | Strains | Origin | Growth Conditions |
|---|---|---|---|
| L. rhamnosus | LrGG ATCC 53103 | ATCC | Aerobic MRS 37 °C |
| L. casei | LC03 DSM 27537 | Probiotical | |
| L. rhamnosus | LR04 DSM 16605 | Probiotical | |
| L. plantarum | CNCM I-4459 | CNCM |
| Genes | Name | Assay ID |
|---|---|---|
| Claudin 1 | CLDN1 | Mm00516701_m1 |
| Claudin 2 | CLDN2 | Mm00516703_s1 |
| Claudin 3 | CLDN3 | Mm00515499_s1 |
| Claudin 5 | CLDN5 | Mm00727012_s1 |
| Occludin | OCLN | Mm00500912_m1 |
| Junctional Adhesive Molecule (JAM) | F11-R | Mm00554113_m1 |
| Cingulin | CGN | Mm01263534_m1 |
| Zonula Occludens 1 | TJP1 | Mm00493699_m1 |
| Zonula Occludens 2 | TJP2 | Mm00495620_m1 |
| E-cadherin | CDH1 | Mm01247357_m1 |
| Vinculin | VCL | Mm00447745_m1 |
| Desmoglein 2 | DSG2 | Mm00514608_m1 |
| Myosin Chain Kinase | MYLK | Mm00653039_m1 |
| Tata Binding Protein | TBP | Mm01277042_m1 |
| Ribosomal Protein L19 | RPL19 | Mm02601633_g1 |
| Actin Beta | ACTB | Mm02619580_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamignon, C.; Mallaret, G.; Rivière, J.; Vilotte, M.; Chadi, S.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Carvalho, F.A.; Pane, M.; Mousset, P.-Y.; et al. Beneficial Effects of Lactobacilli Species on Intestinal Homeostasis in Low-Grade Inflammation and Stress Rodent Models and Their Implication in the Modulation of the Adhesive Junctional Complex. Biomolecules 2023, 13, 1295. https://doi.org/10.3390/biom13091295
Chamignon C, Mallaret G, Rivière J, Vilotte M, Chadi S, de Moreno de LeBlanc A, LeBlanc JG, Carvalho FA, Pane M, Mousset P-Y, et al. Beneficial Effects of Lactobacilli Species on Intestinal Homeostasis in Low-Grade Inflammation and Stress Rodent Models and Their Implication in the Modulation of the Adhesive Junctional Complex. Biomolecules. 2023; 13(9):1295. https://doi.org/10.3390/biom13091295
Chicago/Turabian StyleChamignon, Célia, Geoffroy Mallaret, Julie Rivière, Marthe Vilotte, Sead Chadi, Alejandra de Moreno de LeBlanc, Jean Guy LeBlanc, Frédéric Antonio Carvalho, Marco Pane, Pierre-Yves Mousset, and et al. 2023. "Beneficial Effects of Lactobacilli Species on Intestinal Homeostasis in Low-Grade Inflammation and Stress Rodent Models and Their Implication in the Modulation of the Adhesive Junctional Complex" Biomolecules 13, no. 9: 1295. https://doi.org/10.3390/biom13091295
APA StyleChamignon, C., Mallaret, G., Rivière, J., Vilotte, M., Chadi, S., de Moreno de LeBlanc, A., LeBlanc, J. G., Carvalho, F. A., Pane, M., Mousset, P.-Y., Langella, P., Lafay, S., & Bermúdez-Humarán, L. G. (2023). Beneficial Effects of Lactobacilli Species on Intestinal Homeostasis in Low-Grade Inflammation and Stress Rodent Models and Their Implication in the Modulation of the Adhesive Junctional Complex. Biomolecules, 13(9), 1295. https://doi.org/10.3390/biom13091295







