Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells
Abstract
:1. Introduction
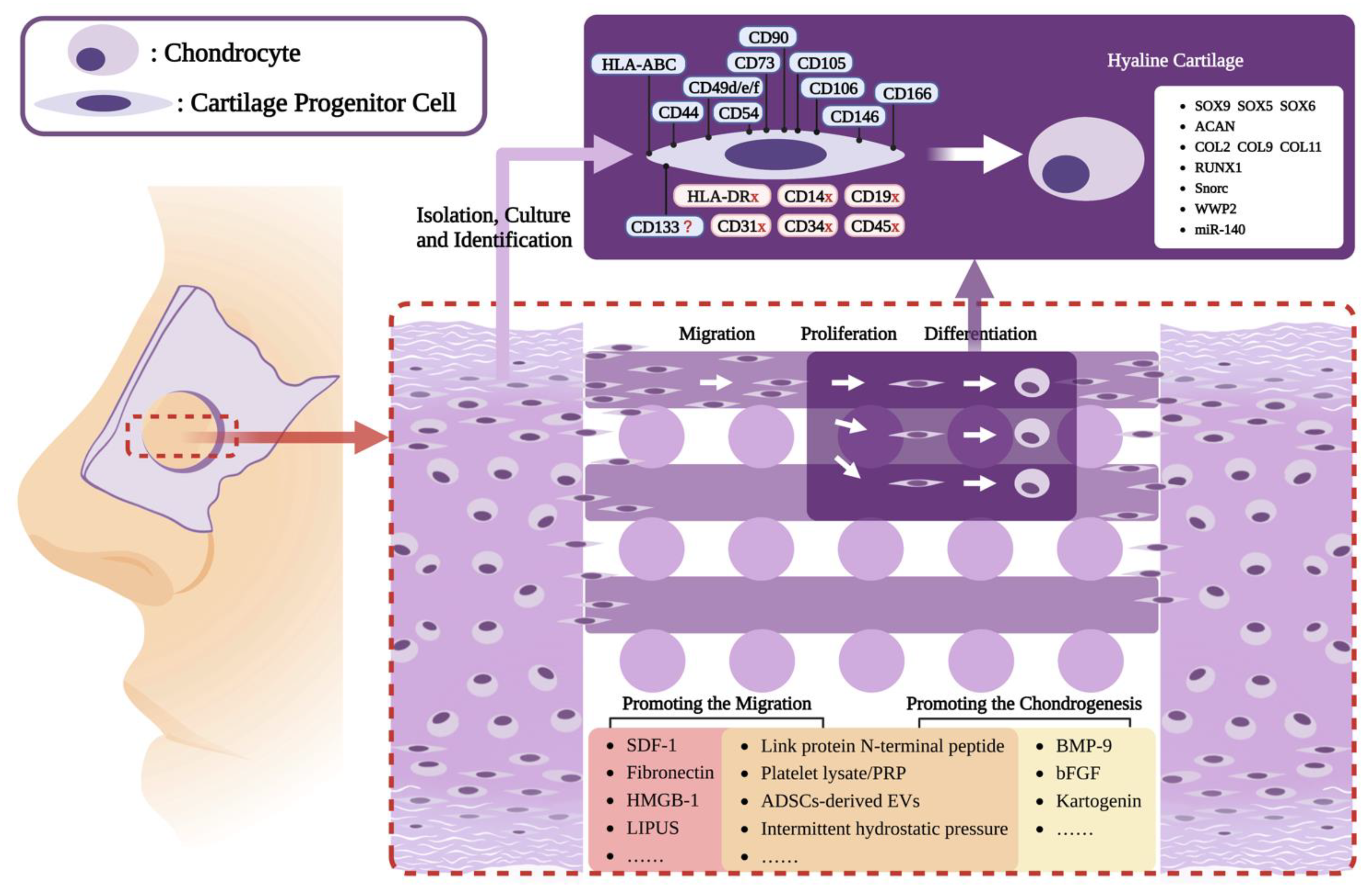
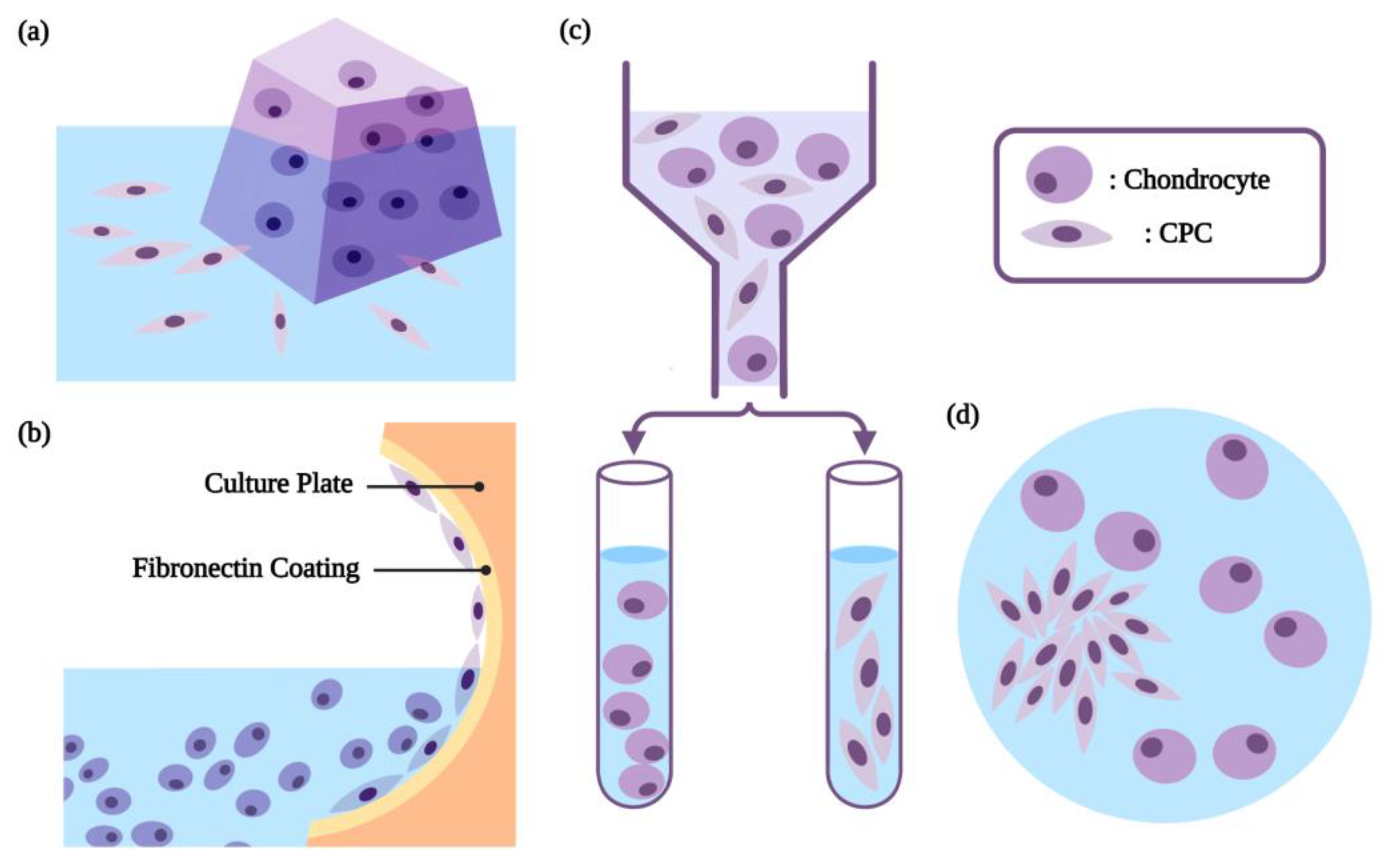
2. Isolation and Characterization of CPCs
2.1. Histological Distribution and Characteristics of NCPCs
2.2. The Characteristic-Based Isolation of CPCs
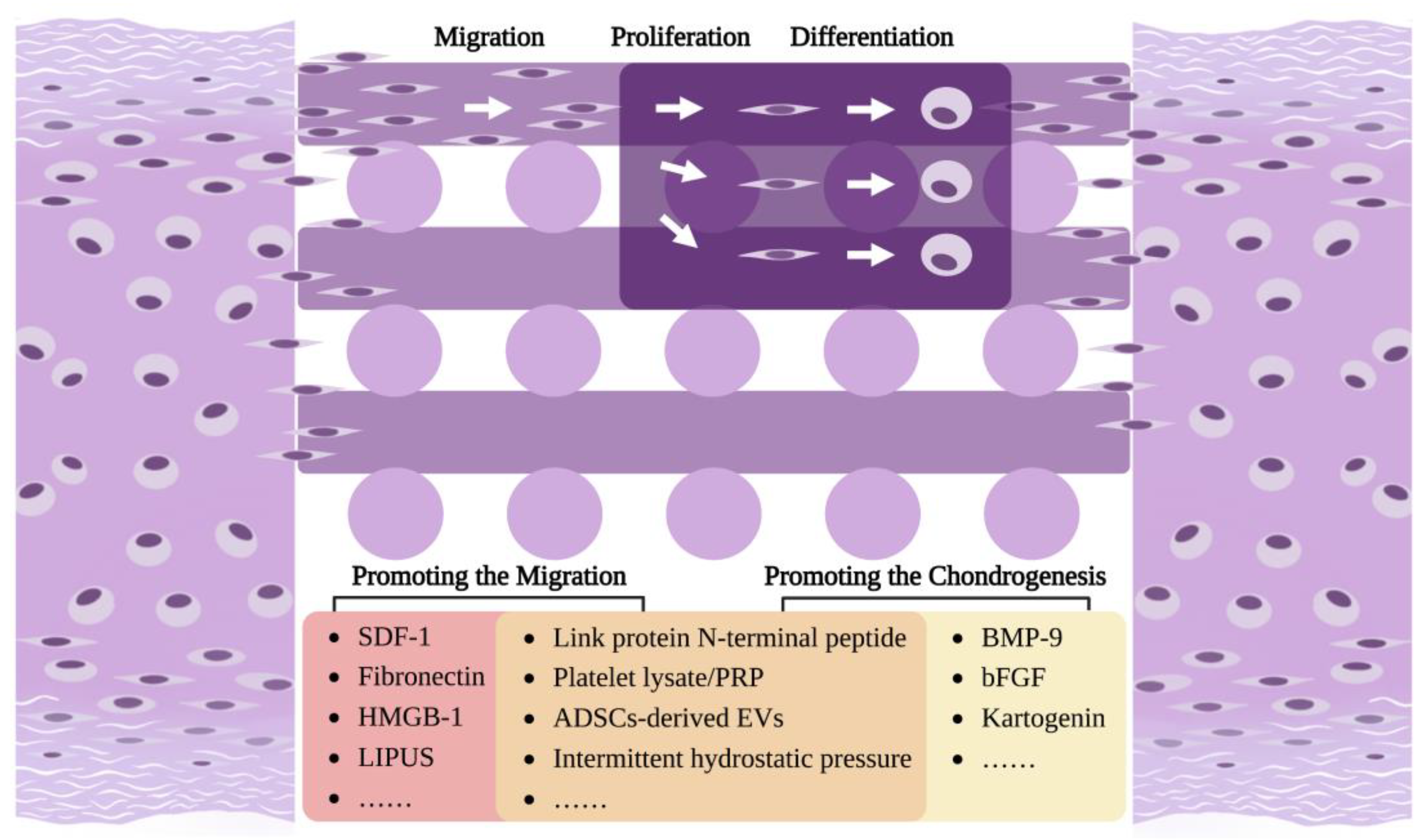
3. The Role of Migratory CPCs in Cartilage Regeneration
3.1. The Migrating CPCs in Cartilage
3.1.1. Migrating CPCs in the Injury Cartilage and Inflammatory Environment
3.1.2. Migrating CPCs in Osteoarthritis
3.2. The Migratory CPCs and Cartilage Regeneration
4. CPCs-Based Cartilage Tissue Engineering Strategies
5. Strategies to Promote CPCs Migration and Chondrogenesis
5.1. Promoting the Migration of CPCs
5.2. Promoting the Chondrogenesis of CPCs
| No. | Reference | Seed Cell | Scaffold | Implanted Site | Result |
|---|---|---|---|---|---|
| 1 | Williams et al., 2010 [65] | Human/Goat ACPC | Type I/III collagen membrane | Goat lateral femoral condyle | The ACPCs group and the chondrocytes group showed comparable histological repair scores. |
| 2 | Shafiee et al., 2014 [20] | Human NCPC | Nanofibrous PLLA-PCL | — | The chondrogenic differentiation of NCPCs was enhanced when cultured on aligned scaffolds compared with randomly oriented scaffolds. |
| 3 | Marcus et al., 2014 [107] | Bovine ACPC | — | SCID mouse thigh muscle | The ACPCs were able to survive in vivo, but failed to create a robust cartilage pellet, suggesting the requirement of further signals for chondrogenic differentiation. |
| 4 | Frisbie et al., 2015 [71] | Equine ACPC | Fibrin | Equine femur medial trochlear ridge | Autologous cells significantly reduced central osteophyte formation compared with allogenic cells and fibrin alone. |
| 5 | Yu et al., 2015 [68] | — | HA-fibrin | Bovine femoral condyle osteochondral explants | The use of rhSDF-1α improved ACPC recruitment and achieved functional repair of full-thickness bovine articular cartilage. |
| 6 | Neumann et al., 2015 [72] | Human ACPC | Fibrin + PU | — | Mechanical stimulation induced chondrogenesis; over-expression of BMP-2 increased hypertrophy markers. |
| 7 | Shafiee et al., 2015 [21] | Human NCPC/ACPC | Nanofibrous PLLA-PCL | — | NCPCs showed a higher proliferation potential and chondrogenic capacity than did BMSCs, ADSCs, and ACPCs. |
| 8 | Li et al., 2016 [74] | Rabbit ACPC | Alginate | — | Intermittent hydrostatic pressure enhanced the migration and chondrogenic differentiation of ACPCs, which were more prominent than in FPSCs and ACs. |
| 9 | Jiang et al., 2016 [73] | Human ACPC | Fibrin | Immunodeficient mouse back; human femoral condyle | 2DLL-cultured ACPCs proved efficient in cartilage formation, both in vitro and in vivo, and in repairing large knee cartilage defects (6–13 cm2) in patients. |
| 10 | Studer et al., 2016 [66] | Human ACPC | Alginate + porous porcine collagen I/III sponge | Nude mouse subcutaneous pocket | Human ACPCs in alginate in collagen hybrid scaffolds produced stable cartilage in vivo. |
| 11 | Levato et al., 2017 [76] | Equine ACPC Equine BMSC | GelMA | — | Combining ACPC-laden and BMSC-laden bioink, articular cartilage consisting of defined superficial and deep regions was generated. |
| 12 | Tao et al., 2018 [75] | Mouse ACPC | Fibronectin/Pluronic F-127 | Mouse knee (OA) | Fibronectin enhanced ACPCs proliferation, migration, and chondrogenic differentiation through the integrin α5β1-dependent signaling pathway. |
| 13 | Lim et al., 2018 [77] | Equine ACPC | Bio-resin (PVA-MA/GelMA) | — | DLP-printed bio-resin supported the chondrogenic differentiation of ACPCs. |
| 14 | Mouser et al., 2018 [78] | Equine ACPC Equine BMSC | GelMA/gellan/HAMA | — | The incorporation of HAMA improved shape-fidelity; chondrogenic differentiation was confirmed, while printing influenced this response. |
| 15 | Xue et al., 2019 [85] | Swine ACPC | PHBV | Nude mouse subcutaneous pocket | CPCs underwent chondrogenesis without chondrogenic induction and were better at performing chondrogenesis than were BMSCs, but worse than ACs. |
| 16 | Wang et al., 2019 [60] | Human ACPC | PRP | Rabbit knee | ACPCs exhibited superiority over BMSCs and ACs in PRP-based scaffold for cartilage regeneration. |
| 17 | Bernal et al., 2019 [79] | Equine ACPC | GelMA-based resin | — | ACPCs synthesized teh neo-fibrocartilage matrix in volumetric rapidly bioprinted meniscus-shaped constructs. |
| 18 | Newberry et al., 2019 [87] | Human CPC cell line | HPC | Human explant meniscus tissue | CPCs migrated in response to SDF-1 and successfully dispersed into injured tissues to help facilitate tissue reintegration. |
| 19 | Mancini et al., 2020 [70] | Equine ACPC Equine BMSC | HA/poly(glycidol)-based hydrogel + PCL | Equine knee | The repair tissue was significantly stiffer in defects repaired with ACPC/BMSC zonal constructs. |
| 20 | Peiffer et al., 2020 [80] | Equine ACPC | GelMA + MEW-PCL | — | The implant composed of ACPCs-laden hydrogel reinforced with an MEW scaffold retained its personalized shape, improved its compressive properties, and supported neocartilage formation. |
| 21 | Schmidt et al., 2020 [82] | Equine ACPC | Agarose | — | Higher production of glycosaminoglycans, weaker type I collagen staining, and lower alkaline phosphatase activity were observed in the ACPC constructs compared to BMSC constructs. |
| 22 | Bauza et al., 2020 [67] | Human ACPC | Collagen-chondroitin sulfate | — | OA-ACPCs with immunosuppressive potential had a higher proliferation rate and a higher propensity toward chondrogenesis compared to BMSCs. |
| 23 | Piluso et al., 2020 [83] | Equine ACPC | Silk fibroin | — | Rapid gelation of silk fibroin could be achieved by combining it with riboflavin and electron acceptors, while the contained ACPCs maintained their viability. |
| 24 | Wang et al., 2021 [88] | Human ACPC | PLGA | Rabbit knee | ACPC-loaded PLGA scaffolds produced a hyaline-like cartilaginous tissue, which showed good integration with the host tissue and subchondral bone. |
| 25 | Xue et al., 2022 [86] | Swine ACPC | PHBV-Bioglass | Nude mouse back subcutaneous tissue | The addition of bioglass improved the cell adherence, cartilage matrix formation, and biomechanical performance. |
6. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavernia, L.; Brown, W.E.; Wong, B.J.; Hu, J.C.; Athanasiou, K.A. Toward tissue-engineering of nasal cartilages. Acta Biomater. 2019, 88, 42–56. [Google Scholar] [CrossRef]
- Bagher, Z.; Asgari, N.; Bozorgmehr, P.; Kamrava, S.K.; Alizadeh, R.; Seifalian, A. Will Tissue-Engineering Strategies Bring New Hope for the Reconstruction of Nasal Septal Cartilage? Curr. Stem Cell Res. Ther. 2020, 15, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kafienah, W.; Jakob, M.; Démarteau, O.; Frazer, A.; Barker, M.D.; Martin, I.; Hollander, A.P. Three-dimensional tissue engineering of hyaline cartilage: Comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002, 8, 817–826. [Google Scholar] [CrossRef]
- Acevedo Rua, L.; Mumme, M.; Manferdini, C.; Darwiche, S.; Khalil, A.; Hilpert, M.; Buchner, D.A.; Lisignoli, G.; Occhetta, P.; von Rechenberg, B.; et al. Engineered nasal cartilage for the repair of osteoarthritic knee cartilage defects. Sci. Transl. Med. 2021, 13, eaaz4499. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; von Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine collagen scaffolds for nasal cartilage repair: Prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Ramezani, K.; Jamalpour, M.R.; Mohammadi, C.; Vahdatinia, F.; Irani, A.D.; Sharifi, E.; Haddadi, R.; Jamshidi, S.; Amirabad, L.M.; et al. In vivo efficacy of 3D-printed elastin–gelatin–hyaluronic acid scaffolds for regeneration of nasal septal cartilage defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 614–624. [Google Scholar] [CrossRef]
- Von Bomhard, A.; Elsaesser, A.; Riepl, R.; Pippich, K.; Faust, J.; Schwarz, S.; Koerber, L.; Breiter, R.; Rotter, N. Cartilage regeneration using decellularized cartilage matrix: Long-term comparison of subcutaneous and intranasal placement in a rabbit model. J. Cranio-Maxillofac. Surg. 2019, 47, 682–694. [Google Scholar] [CrossRef]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef]
- Shafiee, A.; Kabiri, M.; Ahmadbeigi, N.; Yazdani, S.O.; Mojtahed, M.; Amanpour, S.; Soleimani, M. Nasal septum-derived multipotent progenitors: A potent source for stem cell-based regenerative medicine. Stem Cells Dev. 2011, 20, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, A.F.; Schwarz, S.; Joos, H.; Koerber, L.; Brenner, R.E.; Rotter, N. Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies. Cell Biosci. 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.L.; Karam, A.M.; Sepehr, A.; Wong, H.; Liaw, L.-H.L.; Vokes, D.E.; Wong, B.J. Cartilage regeneration in the rabbit nasal septum. Laryngoscope 2006, 116, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Popko, M.; Bleys, R.L.; De Groot, J.W.; Huizing, E.H. Histological structure of the nasal cartilages and their perichondrial envelope. I. The septal and lobular cartilage. Rhinology 2007, 45, 148–152. [Google Scholar] [PubMed]
- Bleys, R.L.; Popko, M.; De Groot, J.W.; Huizing, E.H. Histological structure of the nasal cartilages and their perichondrial envelope. II. The perichondrial envelope of the septal and lobular cartilage. Rhinology 2007, 45, 153–157. [Google Scholar]
- Do Amaral, R.J.; Pedrosa, C.d.S.; Kochem, M.C.; da Silva, K.R.; Aniceto, M.; Claudio-Da-Silva, C.; Borojevic, R.; Baptista, L.S. Isolation of human nasoseptal chondrogenic cells: A promise for cartilage engineering. Stem Cell Res. 2012, 8, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, F.; Yildirim, Y.S.; Demirhan, H.; Özturan, O.; Solakoglu, S. Structural characteristics of septal cartilage and mucoperichondrium. J. Laryngol. Otol. 2012, 126, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Pirsig, W.; Bean, J.; Lenders, H.; Verwoerd, C.; Verwoerd-Verhoef, H. Cartilage transformation in a composite graft of demineralized bovine bone matrix and ear perichondrium used in a child for the reconstruction of the nasal septum. Int. J. Pediatr. Otorhinolaryngol. 1995, 32, 171–181. [Google Scholar] [CrossRef]
- Tcacencu, I.; Carlsoo, B.; Stierna, P. Cell origin in experimental repair of cricoid cartilage defects treated with recombinant human bone morphogenetic protein-2. Wound Repair Regen. 2005, 13, 341–349. [Google Scholar] [CrossRef]
- Hayes, A.J.; MacPherson, S.; Morrison, H.; Dowthwaite, G.; Archer, C.W. The development of articular cartilage: Evidence for an appositional growth mechanism. Anat. Embryol. 2001, 203, 469–479. [Google Scholar] [CrossRef]
- Shafiee, A.; Seyedjafari, E.; Taherzadeh, E.S.; Dinarvand, P.; Soleimani, M.; Ai, J. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater. Sci. Eng. C 2014, 40, 445–454. [Google Scholar] [CrossRef]
- Shafiee, A.; Kabiri, M.; Langroudi, L.; Soleimani, M.; Ai, J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J. Biomed. Mater. Res. A 2016, 104, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.P.; Matsui, R.A.M.; Santos, M.F.S.; Côrtes, I.; Azevedo, M.S.; Silva, K.R.; Beatrici, A.; Leite, P.E.C.; Falagan-Lotsch, P.; Granjeiro, J.M.; et al. Successful Low-Cost Scaffold-Free Cartilage Tissue Engineering Using Human Cartilage Progenitor Cell Spheroids Formed by Micromolded Nonadhesive Hydrogel. Stem Cells Int. 2017, 2017, 7053465. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lim, J.Y.; Kim, S.W.; Lee, W.; Park, S.H.; Kwon, M.Y.; Park, S.H.; Lim, M.H.; Back, S.A.; Yun, B.G.; et al. Characteristics of Nasal Septal Cartilage–Derived Progenitor Cells during Prolonged Cultivation. Otolaryngol. Neck Surg. 2018, 159, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Simoes, I.N.; Burnell, S.E.A.; Pieper, I.L.; Thornton, C.A.; Whitaker, I.S. Isolation and characterisation of nasoseptal cartilage stem/progenitor cells and their role in the chondrogenic niche. Stem Cell Res. Ther. 2020, 11, 177. [Google Scholar] [CrossRef]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef]
- Seol, D.; McCabe, D.J.; Choe, H.; Zheng, H.J.; Yu, Y.; Jang, K.; Walter, M.W.; Lehman, A.D.; Ding, L.; Buckwalter, J.A.; et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012, 64, 3626–3637. [Google Scholar] [CrossRef]
- Alsalameh, S.; Amin, R.; Gemba, T.; Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004, 50, 1522–1532. [Google Scholar] [CrossRef]
- Xia, Z.; Ma, P.; Wu, N.; Su, X.; Chen, J.; Jiang, C.; Liu, S.; Chen, W.; Ma, B.; Yang, X.; et al. Altered function in cartilage derived mesenchymal stem cell leads to OA-related cartilage erosion. Am. J. Transl. Res. 2016, 8, 433–446. [Google Scholar]
- Peng, X.; Yang, L.; Chang, H.; Dai, G.; Wang, F.; Duan, X.; Guo, L.; Zhang, Y.; Chen, G. Wnt/beta-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE 2014, 9, e97283. [Google Scholar] [CrossRef]
- Su, X.; Zuo, W.; Wu, Z.; Chen, J.; Wu, N.; Ma, P.; Xia, Z.; Jiang, C.; Ye, Z.; Liu, S.; et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015, 33, 84–91. [Google Scholar] [CrossRef]
- Pretzel, D.; Linss, S.; Rochler, S.; Endres, M.; Kaps, C.; Alsalameh, S.; Kinne, R.W. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 2011, 13, R64. [Google Scholar] [CrossRef]
- Barbero, A.; Ploegert, S.; Heberer, M.; Martin, I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003, 48, 1315–1325. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, H.; Buckwalter, J.; Martin, J. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthr. Cartil. 2014, 22, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Vinod, E.; Johnson, N.N.; Kumar, S.; Amirtham, S.M.; James, J.V.; Livingston, A.; Rebekah, G.; Daniel, A.J.; Ramasamy, B.; Sathishkumar, S. Migratory chondroprogenitors retain superior intrinsic chondrogenic potential for regenerative cartilage repair as compared to human fibronectin derived chondroprogenitors. Sci. Rep. 2021, 11, 23685. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Lehmann, C.; Bode, C.; Miosge, N.; Schubert, A. High Mobility Group Box 1 Protein in Osteoarthritic Knee Tissue and Chondrogenic Progenitor Cells: An Ex Vivo and In Vitro Study. Cartilage 2021, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Seol, D.; Yu, Y.; Choe, H.; Jang, K.; Brouillette, M.J.; Zheng, H.; Lim, T.-H.; Buckwalter, J.A.; Martin, J.A. Effect of short-term enzymatic treatment on cell migration and cartilage regeneration: In vitro organ culture of bovine articular cartilage. Tissue Eng. Part A 2014, 20, 1807–1814. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, C.; Yu, S.; Yan, J.; Peng, H.; Ouyang, H.W.; Tuan, R.S. Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1beta/nerve growth factor signaling. Arthritis Res. Ther. 2015, 17, 327. [Google Scholar] [CrossRef]
- Joos, H.; Wildner, A.; Hogrefe, C.; Reichel, H.; Brenner, R.E. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res. Ther. 2013, 15, R119. [Google Scholar] [CrossRef]
- Riegger, J.; Palm, H.G.; Brenner, R.E. The functional role of chondrogenic stem/progenitor cells: Novel evidence for immunomodulatory properties and regenerative potential after cartilage injury. Eur. Cells Mater. 2018, 36, 110–127. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, C.; Zheng, H.; Wang, Q.; Song, H.; Buckwalter, J.A.; Martin, J.A. Migrating Progenitor Cells Derived from Injured Cartilage Surface Respond to Damage-Associated Molecular Patterns. Cartilage 2021, 13 (Suppl. S2), 755S–765S. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, H.; Buckwalter, J.; Martin, J. Enhanced phagocytic capacity endows chondrogenic progenitor cells with a novel scavenger function within injured cartilage. Osteoarthr. Cartil. 2016, 24, 1648–1655. [Google Scholar] [CrossRef]
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef]
- Tong, W.; Geng, Y.; Huang, Y.; Shi, Y.; Xiang, S.; Zhang, N.; Qin, L.; Shi, Q.; Chen, Q.; Dai, K.; et al. In Vivo Identification and Induction of Articular Cartilage Stem Cells by Inhibiting NF-kappaB Signaling in Osteoarthritis. Stem Cells 2015, 33, 3125–3137. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.; Bova, W.; Boehm, C.; Piuzzi, N.; Obuchowski, N.; Midura, R.; Muschler, G. Primary Cells Isolated from Human Knee Cartilage Reveal Decreased Prevalence of Progenitor Cells but Comparable Biological Potential During Osteoarthritic Disease Progression. J. Bone Jt. Surg. Am. 2018, 100, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhao, Z.D.; Wang, Q.; Li, Z.L.; Huang, Y.; Zhao, S.; Hu, W.; Liang, J.W.; Li, P.L.; Wang, H.; et al. Biological potential alterations of migratory chondrogenic progenitor cells during knee osteoarthritic progression. Arthritis Res. Ther. 2020, 22, 62. [Google Scholar] [CrossRef]
- Jacob, J.; Aggarwal, A.; Aggarwal, A.; Bhattacharyya, S.; Kumar, V.; Sharma, V.; Sahni, D. Senescent chondrogenic progenitor cells derived from articular cartilage of knee osteoarthritis patients contributes to senescence-associated secretory phenotype via release of IL-6 and IL-8. Acta Histochem. 2022, 124, 151867. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, Y.; Zhang, J.; Li, D.; Hu, G.; Yao, J.; Li, Y.; Huang, P.; Zhang, M.; Huang, Z.; et al. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 2016, 7, e2188. [Google Scholar] [CrossRef]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef]
- Koelling, S.; Miosge, N. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum. 2010, 62, 1077–1087. [Google Scholar] [CrossRef]
- Jang, K.W.; Ding, L.; Seol, D.; Lim, T.-H.; Buckwalter, J.A.; Martin, J.A. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med. Biol. 2014, 40, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zheng, H.; Seol, D.; Yu, Y.; Martin, J.A. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J. Orthop. Res. 2014, 32, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Fodor, J.; Miosge, N.; Takács, R.; Juhász, T.; Rybaltovszki, H.; Tóth, A.; Csernoch, L.; Zákány, R. Purinergic signalling is required for calcium oscillations in migratory chondrogenic progenitor cells. Pflug. Arch. 2015, 467, 429–442. [Google Scholar] [CrossRef]
- Schminke, B.; Trautmann, S.; Mai, B.; Miosge, N.; Blaschke, S. Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage. Eur. J. Immunol. 2016, 46, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Seol, D.; Zhou, C.; Brouillette, M.J.; Song, I.; Yu, Y.; Choe, H.H.; Lehman, A.D.; Jang, K.W.; Fredericks, D.C.; Laughlin, B.J.; et al. Characteristics of meniscus progenitor cells migrated from injured meniscus. J. Orthop. Res. 2017, 35, 1966–1972. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, C.; Zheng, H.; Zhang, Z.; Mei, Y.; Martin, J.A. Chondrogenic progenitor cells promote vascular endothelial growth factor expression through stromal-derived factor-1. Osteoarthr. Cartil. 2017, 25, 742–749. [Google Scholar] [CrossRef]
- Batschkus, S.; Atanassov, I.; Lenz, C.; Meyer-Marcotty, P.; Cingoz, G.; Kirschneck, C.; Urlaub, H.; Miosge, N. Mapping the secretome of human chondrogenic progenitor cells with mass spectrometry. Ann. Anat. 2017, 212, 4–10. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Cancedda, R.; Descalzi, F. Platelet lysate activates quiescent cell proliferation and reprogramming in human articular cartilage: Involvement of hypoxia inducible factor 1. J. Tissue Eng. Regen. Med. 2018, 12, e1691–e1703. [Google Scholar] [CrossRef]
- Janssen, J.N.; Batschkus, S.; Schimmel, S.; Bode, C.; Schminke, B.; Miosge, N. The Influence of TGF-beta3, EGF, and BGN on SOX9 and RUNX2 Expression in Human Chondrogenic Progenitor Cells. J. Histochem. Cytochem. 2019, 67, 117–127. [Google Scholar] [CrossRef]
- Wang, K.; Li, J.; Li, Z.; Wang, B.; Qin, Y.; Zhang, N.; Zhang, H.; Su, X.; Wang, Y.; Zhu, H. Chondrogenic Progenitor Cells Exhibit Superiority Over Mesenchymal Stem Cells and Chondrocytes in Platelet-Rich Plasma Scaffold-Based Cartilage Regeneration. Am. J. Sports Med. 2019, 47, 2200–2215. [Google Scholar] [CrossRef]
- Matta, C.; Boocock, D.J.; Fellows, C.R.; Miosge, N.; Dixon, J.E.; Liddell, S.; Smith, J.; Mobasheri, A. Molecular phenotyping of the surfaceome of migratory chondroprogenitors and mesenchymal stem cells using biotinylation, glycocapture and quantitative LC-MS/MS proteomic analysis. Sci. Rep. 2019, 9, 9018. [Google Scholar] [CrossRef]
- Carluccio, S.; Martinelli, D.; Palamà, M.E.F.; Pereira, R.C.; Benelli, R.; Guijarro, A.; Cancedda, R.; Gentili, C. Progenitor Cells Activated by Platelet Lysate in Human Articular Cartilage as a Tool for Future Cartilage Engineering and Reparative Strategies. Cells 2020, 9, 1052. [Google Scholar] [CrossRef]
- Morgan, B.J.; Bauza-Mayol, G.; Gardner, O.F.W.; Zhang, Y.; Levato, R.; Archer, C.W.; van Weeren, R.; Malda, J.; Conlan, R.S.; Francis, L.W.; et al. Bone Morphogenetic Protein-9 Is a Potent Chondrogenic and Morphogenic Factor for Articular Cartilage Chondroprogenitors. Stem Cells Dev. 2020, 29, 882–894. [Google Scholar] [CrossRef]
- Schminke, B.; Kauffmann, P.; Schubert, A.; Altherr, M.; Gelis, T.; Miosge, N. SMURF1 and SMURF2 in Progenitor Cells from Articular Cartilage and Meniscus during Late-Stage Osteoarthritis. Cartilage 2021, 13 (Suppl. S2), 117S–128S. [Google Scholar] [CrossRef]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and Clonal Characterisation of a Progenitor Cell Sub-Population in Normal Human Articular Cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef]
- Studer, D.; Cavalli, E.; Formica, F.A.; Kuhn, G.A.; Salzmann, G.; Mumme, M.; Steinwachs, M.R.; Laurent-Applegate, L.A.; Maniura-Weber, K.; Zenobi-Wong, M. Human chondroprogenitors in alginate-collagen hybrid scaffolds produce stable cartilage in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 3014–3026. [Google Scholar] [CrossRef] [PubMed]
- Bauza, G.; Pasto, A.; Mcculloch, P.; Lintner, D.; Brozovich, A.; Niclot, F.B.; Khan, I.; Francis, L.W.; Tasciotti, E.; Taraballi, F. Improving the immunosuppressive potential of articular chondroprogenitors in a three-dimensional culture setting. Sci. Rep. 2020, 10, 16610. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Brouillette, M.J.; Seol, D.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015, 67, 1274–1285. [Google Scholar] [CrossRef]
- Cai, Z.; Hong, M.; Xu, L.; Yang, K.; Li, C.; Sun, T.; Feng, Y.; Zeng, H.; Lu, W.W.; Chiu, K.-Y. Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 2020, 126, 109733. [Google Scholar] [CrossRef]
- Mancini, I.A.D.; Schmidt, S.; Brommer, H.; Pouran, B.; Schäfer, S.; Tessmar, J.; Mensinga, A.; van Rijen, M.H.P.; Groll, J.; Blunk, T.; et al. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: In vivo performance in a long-term equine model. Biofabrication 2020, 12, 035028. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; McCarthy, H.E.; Archer, C.W.; Barrett, M.F.; McIlwraith, C.W. Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model. J. Bone Jt. Surg. 2015, 97, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.J.; Gardner, O.F.W.; Williams, R.; Alini, M.; Archer, C.W.; Stoddart, M.J. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS ONE 2015, 10, e0136229. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells from Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Yang, X.; Jiang, Y.; Gui, J. Intermittent hydrostatic pressure maintains and enhances the chondrogenic differentiation of cartilage progenitor cells cultivated in alginate beads. Dev. Growth Differ. 2016, 58, 180–193. [Google Scholar] [CrossRef]
- Tao, T.; Li, Y.; Gui, C.; Ma, Y.; Ge, Y.; Dai, H.; Zhang, K.; Du, J.; Guo, Y.; Jiang, Y.; et al. Fibronectin Enhances Cartilage Repair by Activating Progenitor Cells Through Integrin alpha5beta1 Receptor. Tissue Eng. Part A 2018, 24, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Levato, R.; Costa, P.F.; Castilho, M.D.; Alcala-Orozco, C.R.; van Dorenmalen, K.M.A.; Melchels, F.P.W.; Gawlitta, D.; Hooper, G.J.; Malda, J.; et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 2018, 10, 034101. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Levato, R.; Mensinga, A.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 2020, 61, 137–151. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef]
- Peiffer, Q.C.; de Ruijter, M.; van Duijn, J.; Crottet, D.; Dominic, E.; Malda, J.; Castilho, M. Melt electrowriting onto anatomically relevant biodegradable substrates: Resurfacing a diarthrodial joint. Mater. Des. 2020, 195, 109025. [Google Scholar] [CrossRef]
- Diloksumpan, P.; de Ruijter, M.; Castilho, M.; Gbureck, U.; Vermonden, T.; van Weeren, P.R.; Malda, J.; Levato, R. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication 2020, 12, 025014. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Abinzano, F.; Mensinga, A.; Teßmar, J.; Groll, J.; Malda, J.; Levato, R.; Blunk, T. Differential Production of Cartilage ECM in 3D Agarose Constructs by Equine Articular Cartilage Progenitor Cells and Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2020, 21, 7071. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Gomez, D.F.; Dokter, I.; Teixeira, L.M.; Li, Y.; Leijten, J.; van Weeren, R.; Vermonden, T.; Karperien, M.; Malda, J. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B 2020, 8, 9566–9575. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.P.; Ketzmar, A.-K.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma induces chondrogenic differentiation of subchondral progenitor cells in polyglycolic acid-hyaluronan scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, X.D.; Gao, Z.X.; Xia, W.Y.; Qi, L.; Liu, K. Cartilage progenitor cells combined with PHBV in cartilage tissue engineering. J. Transl. Med. 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, S.; Ge, J.; Wang, Q.; Qi, L.; Liu, K. Integration of Bioglass Into PHBV-Constructed Tissue-Engineered Cartilages to Improve Chondrogenic Properties of Cartilage Progenitor Cells. Front. Bioeng. Biotechnol. 2022, 10, 868719. [Google Scholar] [CrossRef] [PubMed]
- Newberry, J.; Desai, S.; Adler, C.; Li, N.; Karamchedu, N.P.; Fleming, B.C.; Jayasuriya, C.T. SDF-1 preconditioned HPC scaffolds mobilize cartilage-derived progenitors and stimulate meniscal fibrocartilage repair in human explant tissue culture. Connect. Tissue Res. 2020, 61, 338–348. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, T.H.; Hsu, C.C.; Yeh, M.L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Twomey-Kozak, J.; Newberry, J.; Desai, S.; Feltman, P.; Franco, J.R.; Li, N.; Terek, R.; Ehrlich, M.G.; Owens, B.D. Human Cartilage-Derived Progenitors Resist Terminal Differentiation and Require CXCR4 Activation to Successfully Bridge Meniscus Tissue Tears. Stem Cells 2019, 37, 102–114. [Google Scholar] [CrossRef]
- Kalkreuth, R.H.; Krüger, J.P.; Lau, S.; Niemeyer, P.; Endres, M.; Kreuz, P.C.; Kaps, C. Fibronectin stimulates migration and proliferation, but not chondrogenic differentiation of human subchondral progenitor cells. Regen. Med. 2014, 9, 759–773. [Google Scholar] [CrossRef]
- He, R.; Wang, B.; Cui, M.; Xiong, Z.; Lin, H.; Zhao, L.; Li, Z.; Wang, Z.; Peggrem, S.; Xia, Z.; et al. Link Protein N-Terminal Peptide as a Potential Stimulating Factor for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 3217895. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Scaranari, M.; Benelli, R.; Strada, P.; Reis, R.L.; Cancedda, R.; Gentili, C.; Sakata, R.; Reddi, A.H.; Mishra, A.; et al. Dual effect of platelet lysate on human articular cartilage: A maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng. Part A 2013, 19, 1476–1488. [Google Scholar] [CrossRef]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, W.; Zhang, L.; Xie, S.; Zhang, S.; Yuan, S.; Jin, Y.; Zhou, G. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Jiang, Y.; Zhang, X.; Wu, J.; Qi, L.; Liu, K. Hypoxic ADSCs-derived EVs promote the proliferation and chondrogenic differentiation of cartilage stem/progenitor cells. Adipocyte 2021, 10, 322–337. [Google Scholar] [CrossRef]
- Phelps, J.; Leonard, C.; Shah, S.; Krawetz, R.; Hart, D.A.; A Duncan, N.; Sen, A. Production of Mesenchymal Progenitor Cell-Derived Extracellular Vesicles in Suspension Bioreactors for Use in Articular Cartilage Repair. Stem Cells Transl. Med. 2022, 11, 73–87. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Muschler, G.F. Improved biological performance of human cartilage-derived progenitors in platelet lysate xenofree media in comparison to fetal bovine serum media. Curr. Res. Transl. Med. 2022, 70, 103353. [Google Scholar] [CrossRef]
- Takebayashi, T.; Iwamoto, M.; Jikko, A.; Matsumura, T.; Enomoto-Iwamoto, M.; Myoukai, F.; Koyama, E.; Yamaai, T.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor/scatter factor modulates cell motility, proliferation, and proteoglycan synthesis of chondrocytes. J. Cell Biol. 1995, 129, 1411–1419. [Google Scholar] [CrossRef]
- Mishima, Y.; Lotz, M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. 2008, 26, 1407–1412. [Google Scholar] [CrossRef]
- Padmaja, K.; Amirtham, S.M.; Rebekah, G.; Sathishkumar, S.; Vinod, E. Supplementation of articular cartilage-derived chondroprogenitors with bone morphogenic protein-9 enhances chondrogenesis without affecting hypertrophy. Biotechnol. Lett. 2022, 44, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhu, T.; Xue, J.; Zhang, Y.; Lu, Y.; Yang, H.; Yu, Z.; Zhu, Y.; Zhu, X. Influence of bFGF on in vitro expansion and chondrogenic construction of articular cartilage-derived progenitor cells. Ann. Transl. Med. 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, D.; Shen, Y.; Xu, Z.; Dai, J.; Chen, D.; Teng, H.; Jiang, Q. Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res. Ther. 2015, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Wang, T.; Chen, X.; Zhang, S.; Liao, J.; Wang, W.; Zou, X.; Zhou, G. Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem. Biophys. Res. Commun. 2020, 532, 385–392. [Google Scholar] [CrossRef]
- Boushell, M.K.; Mosher, C.Z.; Suri, G.K.; Doty, S.B.; Strauss, E.J.; Hunziker, E.B.; Lu, H.H. Polymeric mesh and insulin-like growth factor 1 delivery enhance cell homing and graft−cartilage integration. Ann. N. Y. Acad. Sci. 2019, 1442, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P.; De Bari, C.; Dell’accio, F.; Archer, C.W. Articular Chondroprogenitor Cells Maintain Chondrogenic Potential but Fail to Form a Functional Matrix When Implanted into Muscles of SCID Mice. Cartilage 2014, 5, 231–240. [Google Scholar] [CrossRef]


| No. | Reference | Isolation | Culture | Characteristics and Results |
|---|---|---|---|---|
| 1 | Shafiee et al., 2011 [10] | Collagenase type I and II (0.2%, 16 h); Colony culture | DMEM-low glucose, 15% FBS, 10 μg/mL ascorbic acid, 1% penicillin-streptomycin solution, 1.25 μg/mL amphotericin-B | (+): CD90, CD105, CD106, CD133, CD166, HLA-ABC, S100, P75, GFAP (−): CD34, CD45, HLA-DR Multilineage differentiation capacity: chondrocytes, osteocytes, and neural-like cell types; without adipogenic differentiation potential. |
| 2 | do Amaral et al., 2011 [15] | Collagenase type I | α-MEM, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin | (+): CD105, CD73, CD44 (−): CD146 Multilineage differentiation capacity:
|
| 3 | Baptista et al., 2013 [6] | Collagenase type I | α-MEM, 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin | Satisfactory cartilage formation in growth-factor-free 2D medium and pellet 3D culture. |
| 4 | Shafiee et al., 2014 [20] | Collagenase type I and II; Colony culture | DMEM-low glucose, 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin | (+): CD73, CD90, CD105, CD106, CD166, HLA-ABC (−): CD34, CD45, CD133, HLA-DR Multilineage differentiation capacity: chondrocytes, osteocytes; without adipogenic differentiation potential; Ploy (L-lactide) (PLLA)/Polycaprolactone (PCL) nanofibrous scaffolds fabricated via electrospinning maintained NCPCs proliferation and differentiation, and the aligned scaffolds could significantly enhance chondrogenic differentiation. |
| 5 | Shafiee et al., 2015 [21] | Collagenase type I and II; Colony culture | DMEM-low glucose, 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin | (+): CD73, CD90, CD105, CD106, CD133, CD166 (−): CD34, CD45, HLA-DR Retained a normal karyotype, and no chromosomal abnormalities were envisaged during long-term culture (passage 10). Higher chondrogenic potential, proliferation rate, and level of ECM production compared to ACPCs, BMSCs, and ADSCs. |
| 6 | Elsaesser et al., 2016 [11] | Migration | DMEM/Ham’s F12 (1:1), 10% FBS, 1% PS | (+): CD9, CD29, CD44, CD49d, CD49e, CD49f, CD54, CD 73, CD90, CD105, CD106, CD146, CD166 (−): CD31, CD34, CD45, CD133/1, CD133/2 Higher basal migratory activity than BMSCs and chondrocytes and could be significantly stimulated by PDGF-BB. Multilineage differentiation capacity: adipogenesis, osteogenesis and chondrogenesis. Stronger MMP-9 activation than chondrocytes. |
| 7 | Stuart et al., 2017 [22] | Collagenase type I | DMEM-low glucose, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin | (+): CD44 Formed CPC spheroids by micromolded nonadhesive hydrogel and achieved scaffold-free cartilage engineering without chondrogenic stimulus. |
| 8 | Kim et al., 2018 [23] | Collagenase; Colony culture | DMEM-low glucose, 10% FBS | (+): CD44, CD73, CD90, CD105, CD106, CD166, HLA-ABC (−): CD14, C19, CD34, HLA-DR Multilineage differentiation capacity: adipogenesis, osteogenesis, and chondrogenesis. The characteristics were not influenced by prolonged cultivation (Passage 10). |
| 9 | Jessop et al., 2020 [24] | Fibronectin adhesion assay | DMEM, 10% FBS, 1% PS, 1 mM d-glucose, 0.1% MEM-non-essential amino acids | (+): CD29, CD44, CD56, CD73, CD90 (−): CD34, CD45 Multilineage differentiation capacity: adipogenesis, osteogenesis, and chondrogenesis. Increased expression of CCND1, CCND2, NCAM1, and CDH2 compared to differentiated chondrocytes. Maintains the phenotypic stability of chondrocytes through influence on dedifferentiation. |
| No. | Reference | Species | Background | Results |
|---|---|---|---|---|
| 1 | Koelling et al., 2009 [49] | Human knee joint | OA | CPCs with high migratory and chondrogenic potential were harbored in the late-stage OA articular cartilage. |
| 2 | Koelling et al., 2010 [50] | Human knee joint | OA | Concentrations of testosterone and estrogen influenced the expression of receptor genes and had a positive effect on the chondrogenic potential of CPCs by regulating the gene expression of Sox9, Runx2, type II collagen, and type I collagen. |
| 3 | Seol et al., 2012 [26] | Bovine stifle joint and human knee joint | Healthy/ Non-OA | Blunt impact injury caused local chondrocyte death and induced homing of CPCs via HMGB-1 and RAGE-mediated chemotaxis. |
| 4 | Joos et al., 2013 [38] | Human knee joint | OA | Traumatized cartilage released chemoattractive factors like PDGF-BB and IGF-1 for CPCs, but IL-1β and TNF-α inhibited their migratory activity, which might contribute to the low regenerative potential of cartilage in vivo. |
| 5 | Jang et al., 2014 [51] | Bovine stifle joint | Healthy | Low-intensity pulsed ultrasound stimulated the migration of CPCs toward the injured area of cartilage through focal adhesion kinase activation. |
| 6 | Zhou et al., 2014 [52] | Bovine stifle joint | Healthy | CPCs overexpressed chemokines IL-8 and CCL2 and were phenotypically more similar to synoviocytes and synovial fluid-derived cells than chondrocytes. |
| 7 | Matta et al., 2015 [53] | Human knee joint | OA | CPCs expressed IP3R, STIMI, and Orai1 proteins and were negative for RYR, and Ca2+ signaling played a role in CPCs differentiation. |
| 8 | Yu et al., 2015 [37] | Bovine stifle joint | Healthy | The use of rhSDF-1α improved the recruitment of CPCs and achieved functional repair of full-thickness articular cartilage. |
| 9 | Jiang et al., 2015 [37] | Human knee joint | OA | CPCs were activated in OA via interleukin-1β/nerve growth factor signaling. |
| 10 | Schminke et al., 2015 [54] | Human knee joint | OA and RA | IL-17 upregulated RUNX2, IL-6 and MMP-3, reducing the chondrogenic potential of RA-CPCs, while antagonizing IL-17 activity could enhance the anti-inflammatory IL-10 secretion and restore the chondrogenic potential. |
| 11 | Elsaeaaer et al., 2016 [11] | Human nasal septum | Healthy | CPCs showed higher migratory capacity compared to BMSCs and chondrocytes and similar ECM secretion to chondrocytes. |
| 12 | Zhou et al., 2016 [41] | Bovine stifle joint | Healthy | CPCs played a macrophage-like role regarding injured cartilage and showed time/cathepsin B-dependent clearance of cell debris, which was much more efficient than chondrocytes. |
| 13 | Seol et al., 2016 [55] | Bovine stifle joint-meniscus | Healthy | Injuries to the meniscus could mobilize CPCs with chondrogenic potential and the capacity for the repair of the cartilaginous white zone. |
| 14 | Wang et al., 2017 [56] | Bovine stifle joint | Healthy | The VEGF expression of CPCs could be stimulated by SDF-1 via p38 MAPK activation, which could self-sustain with the co-expression of SDF-1 and its receptor CXCR4. |
| 15 | Batschkus et al., 2017 [57] | Human knee joint | OA | The joing underwent qualitative and quantitative analysis of the secretome of CPCs by mass spectrometry. |
| 16 | Nguyen et al., 2018 [58] | Human knee joint | Non-OA | Platelet lysate induced the increase in HIF-1α, its nuclear relocation, and the binding to HIF-1 responsive elements, inducing quiescent cartilage cell activation and proliferation, leading to new cartilage formation. |
| 17 | Janssen et al., 2019 [59] | Human knee joint | OA | TGFβ3 and EGF stimulation influenced biglycan, SOX9, and RUNX2, while changes in the expression of their receptors contributed to degenerative/regenerative changes in late OA. |
| 18 | Wagner et al., 2019 [35] | Human knee joint | OA | HMBG1 released by chondrocytes had a migratory effect on CPCs, mediated via RAGE and TLR4. |
| 19 | Wang et al., 2019 [60] | Human knee joint | Non-OA | CPCs displayed superiority over BMSCs, chondrocytes, and PRP alone in PRP-mediated chondral defect regeneration. |
| 20 | Matta et al., 2019 [61] | Human knee joint | OA | A repository of quantitative proteomic data on the surfaceome of MSCs and CPCs relevant to cartilage biology and OA was established. |
| 21 | Wang et al., 2020 [46] | Human knee joint | OA | More OA grade 3–4 CPCs migrated to injured cartilage than did grade 1–2 CPCs, but with enhanced osteo-adipogenic and decreased chondrogenic capacity, which might explain the pathological changes in the CPCs during the progression of OA from early to late stages. |
| 22 | Carluccio et al., 2020 [62] | Human hip joint | — | Platelet lysate-recruited CPCs were able to migrate in response to inflammatory stimuli, showed paracrine activity in attracting other cells toward injured sites, and displayed high chondrogenic potential and resistance to hypertrophy. |
| 23 | Morgan et al., 2020 [63] | Bovine metacarpophalangeal joint | Healthy | As a potent chondrogenic factor for CPCs, BMP-9 was capable of inducing morphogenesis of adult-like cartilage, a highly desirable attribute for in vitro tissue-engineered cartilage. |
| 24 | Schminke et al., 2020 [64] | Human knee joint | OA | SMURF1 and SMURF2 were regulatory players for the expression of the major regulator transcription factors RUNX2 and SOX9 in CPCs from articular cartilage and meniscus. |
| 25 | Ding et al., 2021 [40] | Bovine stifle joint | Healthy | Compared with non-CPCs, CPCs expressed significantly more baseline mRNAs of MMP-13, CXCL12, and IL-6, and were more sensitive than non-CPCs in response to DAMPs, especially MTDs. |
| 26 | Vinod et al., 2021 [34] | Human knee joint | OA | Migratory CPCs expressed higher levels of CD146 and CD49b and retained superior intrinsic chondrogenic potential as compared to fibronectin-derived CPCs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, G.; An, Y. Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells. Biomolecules 2023, 13, 1302. https://doi.org/10.3390/biom13091302
Zhang C, Wang G, An Y. Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells. Biomolecules. 2023; 13(9):1302. https://doi.org/10.3390/biom13091302
Chicago/Turabian StyleZhang, Chong, Guanhuier Wang, and Yang An. 2023. "Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells" Biomolecules 13, no. 9: 1302. https://doi.org/10.3390/biom13091302
APA StyleZhang, C., Wang, G., & An, Y. (2023). Achieving Nasal Septal Cartilage In Situ Regeneration: Focus on Cartilage Progenitor Cells. Biomolecules, 13(9), 1302. https://doi.org/10.3390/biom13091302





