Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Laboratory Procedures
2.3. Metabolites Profiling by Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Statistics
3. Results
3.1. Clinical Characteristics, Chronic Medication, and Standard Laboratory Parameters
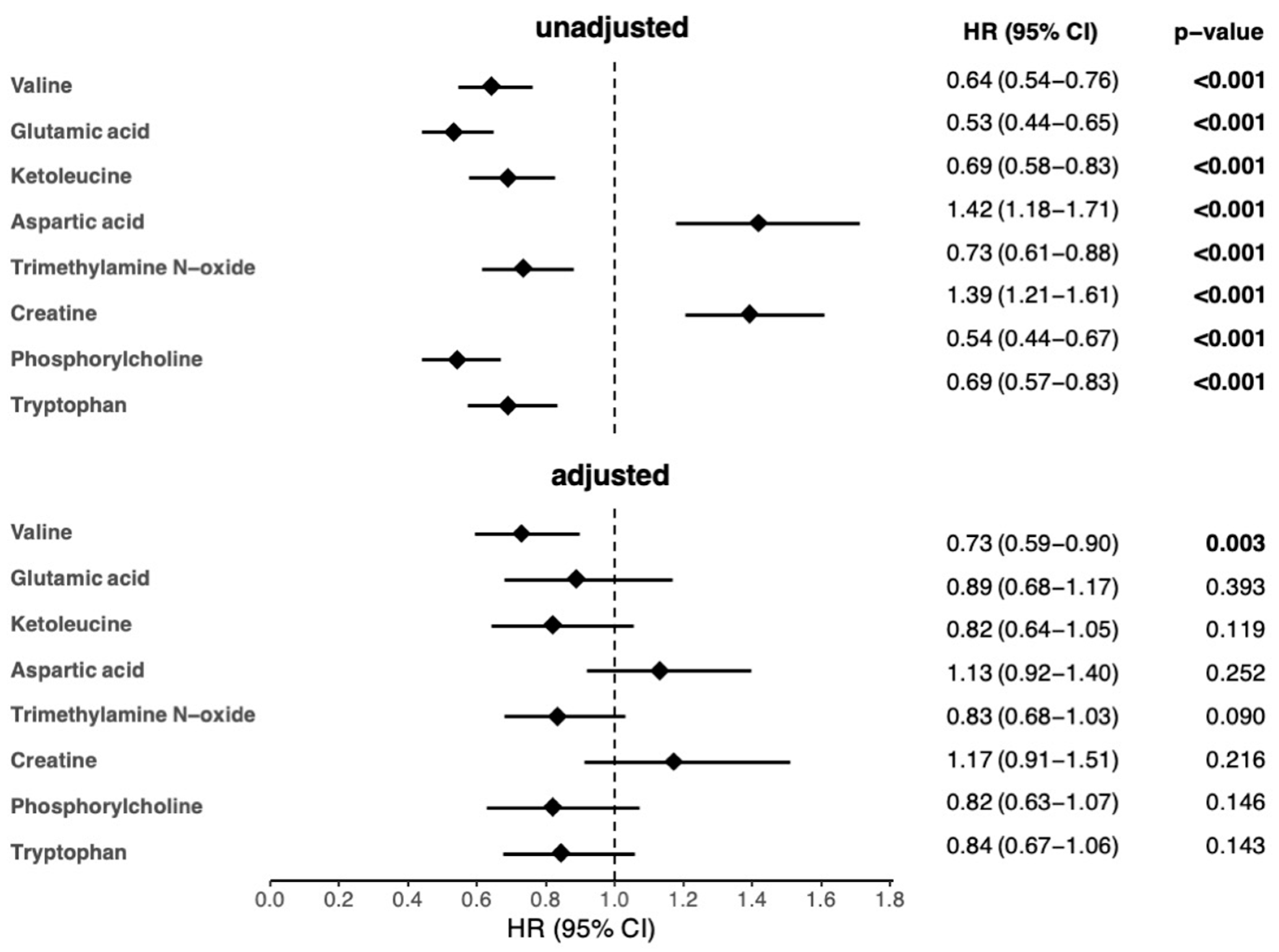
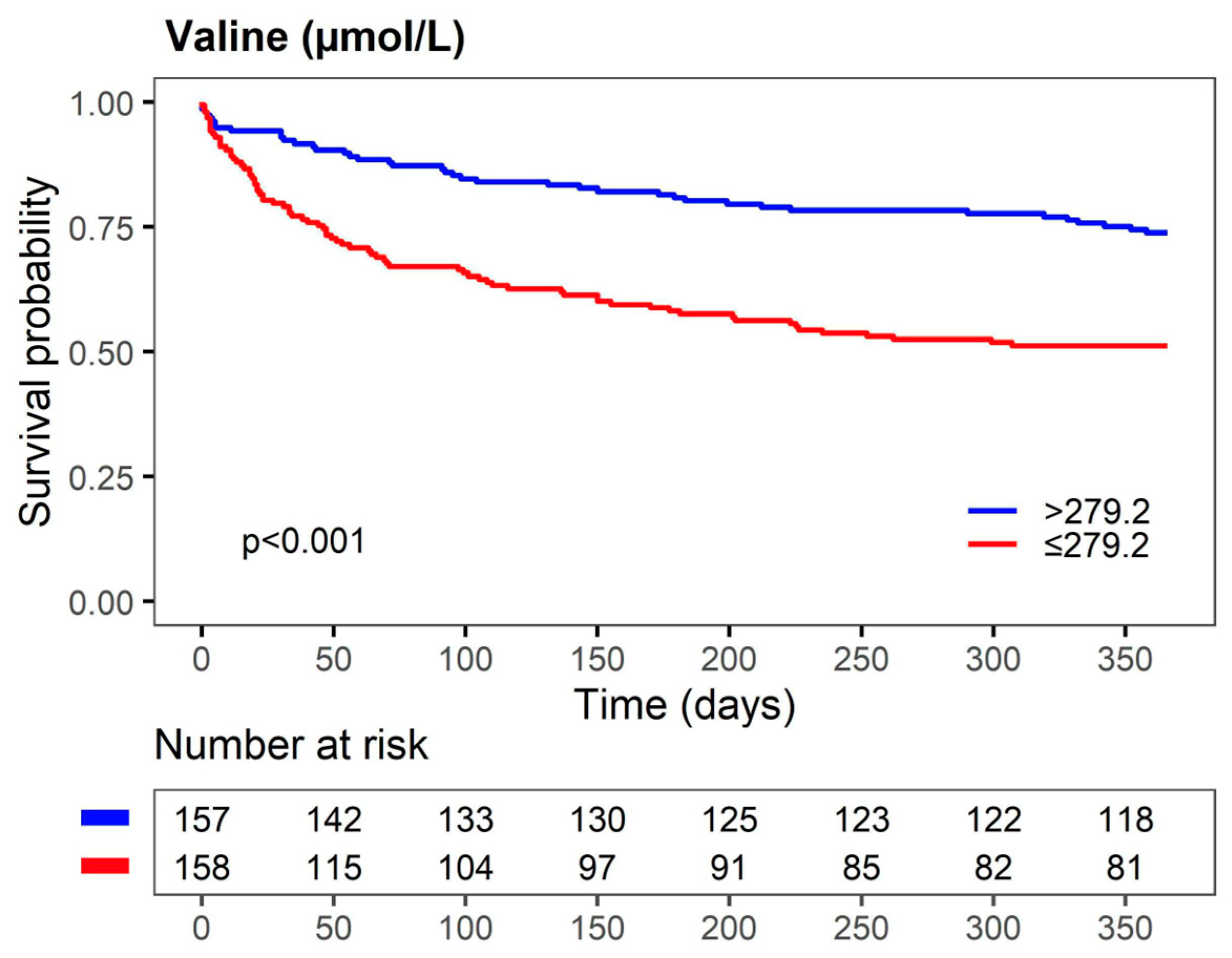
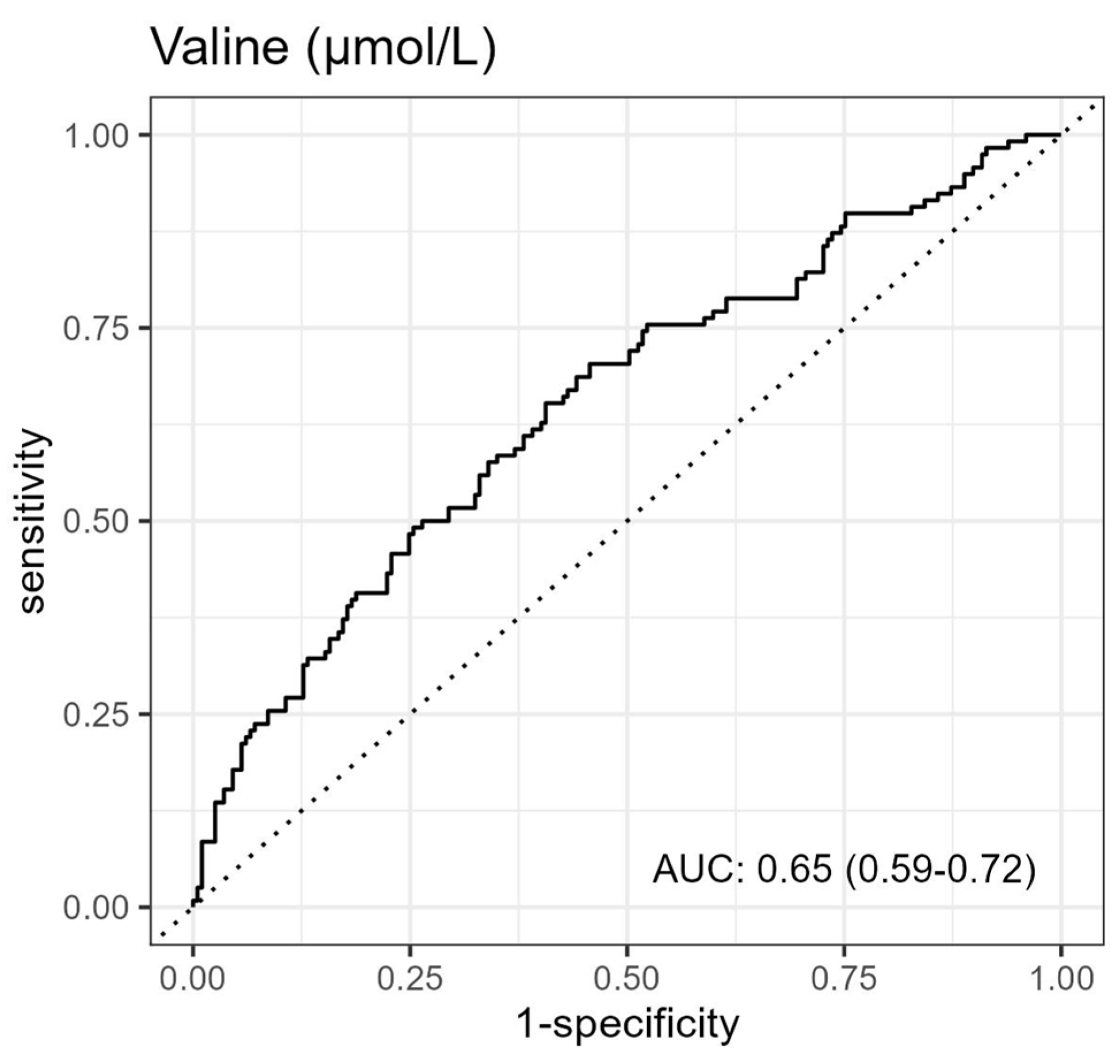
3.2. Association between Metabolites and 1-Year Mortality in AHF Patients
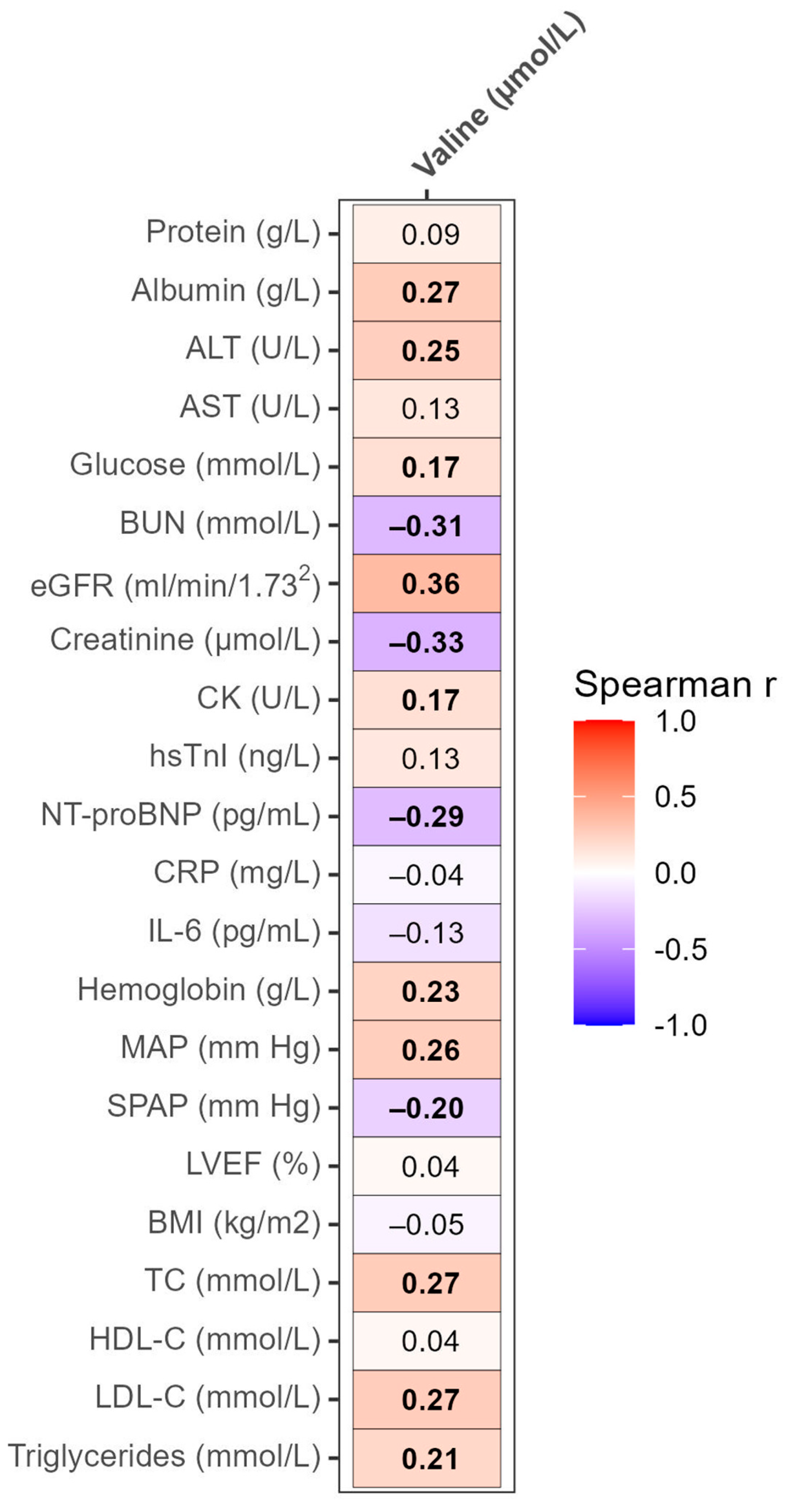
3.3. Correlation Analyses of Valine with Clinical and Laboratory Parameters
3.4. Differences in Valine Serum Levels in Various Groups of AHF Patients
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; Younis, A.; Dai, H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef]
- Wende, A.R.; Brahma, M.K.; McGinnis, G.R.; Young, M.E. Metabolic Origins of Heart Failure. JACC Basic. Transl. Sci. 2017, 2, 297–310. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients with Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Mamic, P.; Chaikijurajai, T.; Tang, W.H.W. Gut microbiome–A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell. Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., III; Kraus, W.E.; Shah, S.H. Metabolic Dysfunction in Heart Failure: Diagnostic, Prognostic, and Pathophysiologic Insights from Metabolomic Profiling. Curr. Heart Fail. Rep. 2016, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 1167. [Google Scholar] [CrossRef] [PubMed]
- Loungani, R.S.; Teerlink, J.R.; Metra, M.; Allen, L.A.; Butler, J.; Carson, P.E.; Chen, C.W.; Cotter, G.; Davison, B.A.; Eapen, Z.J.; et al. Cause of Death in Patients with Acute Heart Failure: Insights from RELAX-AHF-2. JACC Heart Fail. 2020, 8, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Klobucar, I.; Degoricija, V.; Potocnjak, I.; Trbusic, M.; Pregartner, G.; Berghold, A.; Fritz-Petrin, E.; Habisch, H.; Madl, T.; Frank, S. HDL-apoA-II Is Strongly Associated with 1-Year Mortality in Acute Heart Failure Patients. Biomedicines 2022, 10, 1668. [Google Scholar] [CrossRef]
- Degoricija, V.; Klobucar, I.; Potocnjak, I.; Dokoza Teresak, S.; Vidovic, L.; Pregartner, G.; Berghold, A.; Habisch, H.; Madl, T.; Frank, S. Cholesterol Content of Very-Low-Density Lipoproteins Is Associated with 1-Year Mortality in Acute Heart Failure Patients. Biomolecules 2022, 12, 1542. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Reisinger, A.C.; Posch, F.; Hackl, G.; Marsche, G.; Sourij, H.; Bourgeois, B.; Eller, K.; Madl, T.; Eller, P. Branched-Chain Amino Acids Can Predict Mortality in ICU Sepsis Patients. Nutrients 2021, 13, 3106. [Google Scholar] [CrossRef]
- Kouzu, H.; Katano, S.; Yano, T.; Ohori, K.; Nagaoka, R.; Inoue, T.; Takamura, Y.; Ishigo, T.; Watanabe, A.; Koyama, M.; et al. Plasma amino acid profiling improves predictive accuracy of adverse events in patients with heart failure. ESC Heart Fail. 2021, 8, 5045–5056. [Google Scholar] [CrossRef]
- Conners, K.M.; Shearer, J.J.; Joo, J.; Park, H.; Manemann, S.M.; Remaley, A.T.; Otvos, J.D.; Connelly, M.A.; Sampson, M.; Bielinski, S.J.; et al. The Metabolic Vulnerability Index: A Novel Marker for Mortality Prediction in Heart Failure. JACC Heart Fail. 2023; in press. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Okumura, T.; Kondo, T.; Kato, T.; Kazama, S.; Ishihara, T.; Iwata, E.; Shimojo, M.; Kondo, S.; Aoki, S.; et al. Usefulness of the plasma branched-chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J. Cardiol. 2020, 75, 689–696. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Okumura, T.; Kondo, T.; Kato, T.; Kazama, S.; Kimura, Y.; Ishihara, T.; Iwata, E.; Shimojo, M.; Kondo, S.; et al. Prognostic value of leucine/phenylalanine ratio as an amino acid profile of heart failure. Heart Vessel. 2021, 36, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Voors, A.A.; Ouwerkerk, W.; Zannad, F.; van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Ng, L.L.; Metra, M.; Ter Maaten, J.M.; Lang, C.C.; et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur. J. Heart Fail. 2017, 19, 627–634. [Google Scholar] [CrossRef]
- Lin, Y.; Pang, L.; Huang, S.; Shen, J.; Wu, W.; Tang, F.; Su, W.; Zhu, X.; Sun, J.; Quan, R.; et al. The prevalence and survival of pulmonary hypertension due to left heart failure: A retrospective analysis of a multicenter prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 908215. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; Horwich, T.; Fonarow, G.C. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; La Rovere, M.T.; Febo, O.; Baiardi, P.; Boschi, F.; Iadarola, P.; Viglio, S.; Dossena, M.; Bongiorno, A.I.; Pastoris, O.; et al. Lung anabolic activity in patients with chronic heart failure: Potential implications for clinical practice. Nutrition 2012, 28, 1002–1007. [Google Scholar] [CrossRef]
- Flahault, A.; Metzger, M.; Chasse, J.F.; Haymann, J.P.; Boffa, J.J.; Flamant, M.; Vrtovsnik, F.; Houillier, P.; Stengel, B.; Thervet, E.; et al. Low Serum Creatine Kinase Level Predicts Mortality in Patients with a Chronic Kidney Disease. PLoS ONE 2016, 11, e0156433. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, S11–S15. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Okumura, T.; Murohara, T. Amino acid profiling to predict prognosis in patients with heart failure: An expert review. ESC Heart Fail. 2023, 10, 32–43. [Google Scholar] [CrossRef]
- Holecek, M.; Sprongl, L.; Skopec, F.; Andrys, C.; Pecka, M. Leucine metabolism in TNF-alpha- and endotoxin-treated rats: Contribution of hepatic tissue. Am. J. Physiol. 1997, 273, E1052–E1058. [Google Scholar] [CrossRef]
- Hasper, D.; Hummel, M.; Kleber, F.X.; Reindl, I.; Volk, H.D. Systemic inflammation in patients with heart failure. Eur. Heart J. 1998, 19, 761–765. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef]
- Kuzuya, T.; Katano, Y.; Nakano, I.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Hayashi, K.; Honda, T.; Goto, H.; Fujita, Y.; et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2008, 373, 94–98. [Google Scholar] [CrossRef]
- Garibotto, G.; Paoletti, E.; Fiorini, F.; Russo, R.; Robaudo, C.; Deferrari, G.; Tizianello, A. Peripheral metabolism of branched-chain keto acids in patients with chronic renal failure. Miner. Electrolyte Metab. 1993, 19, 25–31. [Google Scholar]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef]
- Sun, H.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.; Wang, Z.; Jeyaraj, D.; Youn, J.Y.; Ren, S.; Liu, Y.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Xia, Y.; Zhao, S.; Yan, W.; Wang, H.; Lee, Y.; Li, C.; Zhang, L.; Lian, K.; et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1160–H1169. [Google Scholar] [CrossRef]
- Tanada, Y.; Shioi, T.; Kato, T.; Kawamoto, A.; Okuda, J.; Kimura, T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci. 2015, 137, 20–27. [Google Scholar] [CrossRef]
- Uchino, Y.; Watanabe, M.; Takata, M.; Amiya, E.; Tsushima, K.; Adachi, T.; Hiroi, Y.; Funazaki, T.; Komuro, I. Effect of Oral Branched-Chain Amino Acids on Serum Albumin Concentration in Heart Failure Patients with Hypoalbuminemia: Results of a Preliminary Study. Am. J. Cardiovasc. Drugs 2018, 18, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okumura, T.; Kazama, S.; Shibata, N.; Oishi, H.; Arao, Y.; Kuwayama, T.; Kato, H.; Yamaguchi, S.; Hiraiwa, H.; et al. Usefulness of Plasma Branched-Chain Amino Acid Analysis in Predicting Outcomes of Patients with Nonischemic Dilated Cardiomyopathy. Int. Heart J. 2020, 61, 739–747. [Google Scholar] [CrossRef]

| Alive (N = 197) | Deceased (N = 118) | All (N = 315) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 72.5 (10.4) | 77.0 (10.1) | 74.2 (10.5) | <0.001 |
| Sex, Female | 85 (43.1%) | 51 (43.2%) | 136 (43.2%) | 1.000 |
| Comorbidities | ||||
| Hypertension | 186 (94.4%) | 108 (91.5%) | 294 (93.3%) | 0.355 |
| T2DM | 76 (38.6%) | 56 (47.5%) | 132 (41.9%) | 0.127 |
| CAD | 100 (50.8%) | 56 (47.5%) | 156 (49.5%) | 0.642 |
| CMP | 173 (87.8%) | 115 (97.5%) | 288 (91.4%) | 0.003 |
| AF | 98 (49.7%) | 72 (61.0%) | 170 (54.0%) | 0.062 |
| CKD | 72 (36.5%) | 71 (60.2%) | 143 (45.4%) | <0.001 |
| MetS | 130 (66.0%) | 87 (73.7%) | 217 (68.9%) | 0.168 |
| Physical measures at admission | ||||
| MAP (mmHg) | 108.1 (24.2) | 96.0 (19.5) | 103.6 (23.3) | <0.001 |
| Heart rate (beats/min) | 103.8 (25.8) | 95.3 (27.5) | 100.6 (26.7) | 0.006 |
| Respiratory rate (breaths/min) | 29.3 (6.9) | 28.8 (6.0) | 29.1 (6.5) | 0.474 |
| BMI (kg/m2) | 27.4 (24.9, 30.7) | 29.1 (25.3, 32.8) | 28.0 (25.0, 31.6) | 0.067 |
| Signs and symptoms | ||||
| Symptom duration (days) | 5.0 (3.0–5.0) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 0.022 |
| Rales or crackles | 193 (98.0%) | 118 (100.0%) | 311 (98.7%) | 0.301 |
| JVD | 97 (49.2%) | 77 (65.3%) | 174 (55.2%) | 0.007 |
| Enlarged liver | 95 (48.2%) | 81 (68.6%) | 176 (55.9%) | <0.001 |
| Ascites | 20 (10.2%) | 29 (24.6%) | 49 (15.6%) | 0.001 |
| Peripheral edema | 114 (57.9%) | 90 (76.3%) | 204 (64.8%) | <0.001 |
| NYHA class | 0.305 | |||
| 3 | 13 (6.6%) | 4 (3.4%) | 17 (5.4%) | |
| 4 | 184 (93.4%) | 114 (96.6%) | 298 (94.6%) | |
| AHF type | 0.003 | |||
| New onset AHF | 24 (12.2%) | 3 (2.5)% | 27 (8.6%) | |
| AHF following CHF | 173 (87.8%) | 115 (97.5%) | 288 (91.4%) | |
| Echocardiography | ||||
| LVEDd/BSA (mm/m2) | 29.1 (4.9) | 28.5 (5.2) | 28.8 (5.0) | 0.346 |
| LVEF (%) | 40.1 (11.9) | 39.1 (12.6) | 39.8 (12.1) | 0.455 |
| SPAP (mmHg) | 47.0 (42.0–55.0) | 50.0 (45.0–60.0) | 50.0 (45.0–60.0) | 0.005 |
| AHF class | 0.575 | |||
| HFrEF, EF < 40% | 88 (44.9%) | 55 (51.4%) | 143 (47.2%) | |
| HFmrEF, EF 41–49% | 55 (28.1%) | 26 (24.3%) | 81 (26.7%) | |
| HFpEF, EF ≥ 50% | 53 (27.3%) | 26 (24.3%) | 79 (26.1%) | |
| Laboratory test results at admission | ||||
| TC (mmol/L) | 3.8 (3.1, 4.9) | 3.3 (2.7, 4.1) | 3.5 (2.9, 4.5) | <0.001 |
| HDL-C (mmol/L) | 1.1 (0.9, 1.4) | 1.1 (0.8, 1.3) | 1.1 (0.9, 1.3) | 0.022 |
| LDL-C (mmol/L) | 2.0 (1.5–2.8) | 1.7 (1.3, 2.4) | 1.9 (1.4, 2.7) | <0.001 |
| Triglycerides (mmol/L) | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.3) | 0.099 |
| Albumin (g/L) | 38.2 (35.5, 42.0) | 36.7 (33.8, 39.7) | 37.8 (34.8, 41.3) | 0.009 |
| Total proteins (g/L) | 67.0 (62.0, 72.0) | 65.5 (61.0, 70.0) | 67.0 (61.0, 72.0) | 0.214 |
| Bilirubin (µmol/L) | 17.4 (11.0, 28.5) | 17.2 (11.9, 29.2) | 17.3 (11.1, 28.7) | 0.336 |
| AST (U/L) | 28.0 (22.0, 42.0) | 27.0 (18.2, 52.5) | 28.0 (20.0, 44.5) | 0.542 |
| ALT (U/L) | 25.0 (16.0, 41.0) | 21.0 (14.0, 46.5) | 25.0 (15.0, 42.0) | 0.226 |
| Glucose (mmol/L) | 7.7 (6.0, 10.8) | 8.1 (6.3, 11.6) | 7.9 (6.1, 11.2) | 0.267 |
| Sodium (mmol/L) | 140.0 (138.0, 142.0) | 138.0 (135.0, 141.0) | 140.0 (136.5, 142.0) | <0.001 |
| Potassium (mmol/L) | 4.5 (4.1, 4.8) | 4.5 (4.1, 5.0) | 4.5 (4.1, 4.8) | 0.194 |
| Chloride (mmol/L) | 104.0 (101.0, 107.0) | 100.0 (97.0, 104.0) | 103.0 (99.0, 106.0) | <0.001 |
| BUN (mmol/L) | 8.3 (6.3, 12.3) | 12.3 (8.9, 16.8) | 9.6 (6.9, 14.4) | <0.001 |
| Creatinine (µmol/L) | 107.0 (86.0, 144.0) | 131.5 (107.0, 164.0) | 117.0 (90.5, 152.5) | <0.001 |
| eGFR (ml/min/1.73 m2) | 54.0 (36.1, 70.5) | 38.4 (29.1, 52.1) | 46.6 (32.3, 65.0) | <0.001 |
| CK (U/L) | 105.0 (65.0, 174.0) | 78.0 (50.2, 147.5) | 93.0 (58.0, 165.5) | 0.007 |
| LDH (U/L) | 252.0 (217.0, 316.0) | 283.0 (230.8, 372.2) | 265.0 (218.5, 332.0) | 0.029 |
| hsTnI (ng/L) | 39.0 (17.5, 136.5) | 61.0 (30.0, 149.0) | 46.0 (20.0, 143.2) | 0.039 |
| NT-proBNP (pg/mL) | 5350.0 (3151.0, 10,691.0) | 10,733.0 (5486.5, 18,385.5) | 6692.0 (3531.0, 14,395.5) | <0.001 |
| CRP (mg/L) | 10.3 (4.9, 21.9) | 24.9 (6.4, 47.3) | 12.2 (5.5, 33.1) | <0.001 |
| IL-6 (pg/mL) | 22.1 (11.3, 44.8) | 40.6 (17.1, 79.6) | 25.1 (12.9, 60.1) | <0.001 |
| Fibrinogen (g/L) | 4.0 (3.4, 4.7) | 4.0 (3.1, 4.9) | 4.0 (3.4, 4.8) | 0.469 |
| Erythrocytes (×1012/L) | 4.7 (4.4, 5.1) | 4.4 (3.8, 4.9) | 4.6 (4.2, 5.1) | <0.001 |
| Hemoglobin (g/L) | 138.0 (124.0, 150.0) | 126.0 (111.0, 141.0) | 134.0 (119.0, 148.0) | <0.001 |
| pH | 7.4 (7.3, 7.5) | 7.4 (7.3, 7.4) | 7.4 (7.3, 7.5) | 0.709 |
| pO2 (kPa) | 8.8 (7.2, 10.4) | 8.8 (7.3, 10.4) | 8.8 (7.2, 10.4) | 0.803 |
| pCO2 (kPa) | 5.2 (4.4, 6.3) | 5.2 (4.5, 7.1) | 5.2 (4.5, 6.4) | 0.386 |
| HCO3 (mmol/L) | 23.9 (21.2, 27.0) | 24.4 (21.3, 28.9) | 23.9 (21.3, 27.4) | 0.368 |
| Valine (µmol/L) | p-Value | ||
|---|---|---|---|
| T2D | No (N = 183) | 275.4 (225.8, 312.1) | 0.045 |
| Yes (N = 132) | 292.1 (249.6, 322.7) | ||
| CAD | No (N = 159) | 267.3 (218.0, 302.6) | <0.001 |
| Yes (N = 156) | 293.3 (254.4, 332.1) | ||
| CKD | No (N = 172) | 295.0 (255.5, 331.5) | <0.001 |
| Yes (N = 143) | 257.4 (212.3, 295.9) | ||
| MetS | No (N = 98) | 284.7 (229.0, 329.0) | 0.616 |
| Yes (N = 217) | 277.8 (242.4, 313.9) | ||
| AF | No (N = 145) | 287.8 (251.5, 322.7) | 0.033 |
| Yes (N = 170) | 276.6 (224.8, 314.5) | ||
| Sign(s) * | No (N = 66) | 303.0 (278.0, 340.5) | <0.001 |
| Yes (N = 249) | 270.0 (228.8, 308.1) | ||
| AHF type | New onset AHF (N = 27) | 292.6 (258.1, 316.4) | 0.288 |
| AHF following CHF (N = 288) | 278.8 (234.1, 318.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klobučar, I.; Vidović, L.; Arih, I.; Lechleitner, M.; Pregartner, G.; Berghold, A.; Habisch, H.; Madl, T.; Frank, S.; Degoricija, V. Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients. Biomolecules 2023, 13, 1323. https://doi.org/10.3390/biom13091323
Klobučar I, Vidović L, Arih I, Lechleitner M, Pregartner G, Berghold A, Habisch H, Madl T, Frank S, Degoricija V. Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients. Biomolecules. 2023; 13(9):1323. https://doi.org/10.3390/biom13091323
Chicago/Turabian StyleKlobučar, Iva, Luka Vidović, Ilona Arih, Margarete Lechleitner, Gudrun Pregartner, Andrea Berghold, Hansjörg Habisch, Tobias Madl, Saša Frank, and Vesna Degoricija. 2023. "Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients" Biomolecules 13, no. 9: 1323. https://doi.org/10.3390/biom13091323
APA StyleKlobučar, I., Vidović, L., Arih, I., Lechleitner, M., Pregartner, G., Berghold, A., Habisch, H., Madl, T., Frank, S., & Degoricija, V. (2023). Low Valine Serum Levels Predict Increased 1-Year Mortality in Acute Heart Failure Patients. Biomolecules, 13(9), 1323. https://doi.org/10.3390/biom13091323








