Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
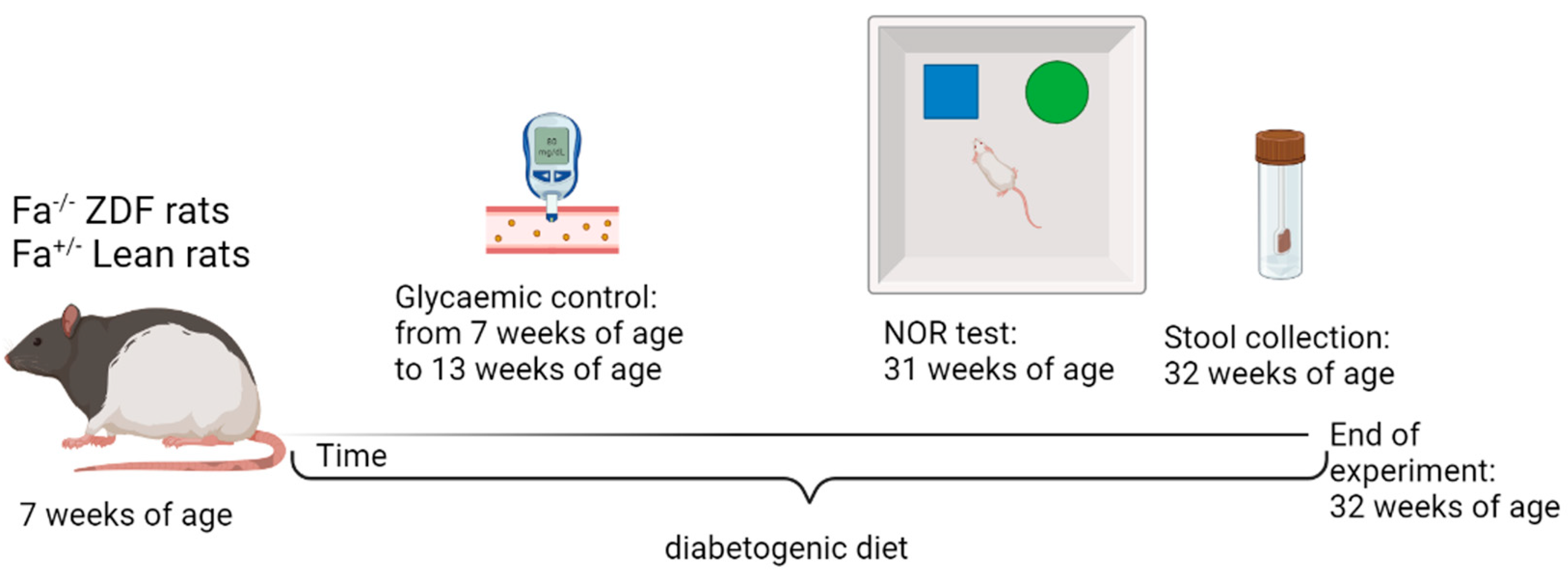
2.2. Experimental Design
2.3. Novel Object Recognition (NOR) Test
2.4. Corticosterone ELISA Kit
2.5. Liquid Chromatography–Tandem Mass Spectrometry Analysis (LC–MS/MS)
2.6. RNA and Protein Extraction
2.7. Real-Time Polymerase Chain Reaction
2.8. Western Blotting
2.9. Thiobarbituric Acid-Reactive Substance
2.10. 16S Next-Generation Sequencing
2.11. Statistical Analysis
3. Results
3.1. Body Weight and Blood Glucose Levels in ZDF and Lean Rats
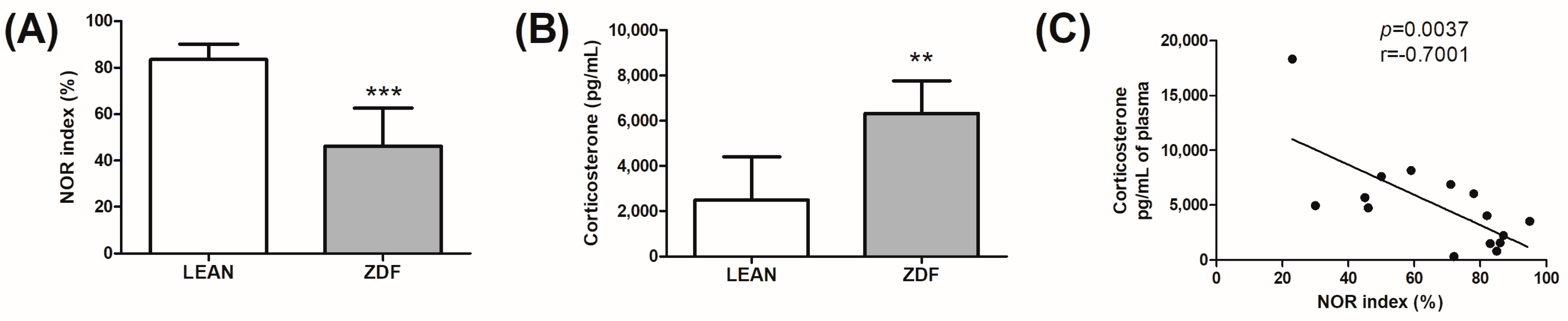
3.2. Memory Assessment and Glucocorticoid Evaluation
3.3. Neuroactive Steroid Levels in ZDF and Lean Rats
3.4. Correlation Analysis among Neuroactive Steroid Levels and NOR Index in ZDF and Lean Rats
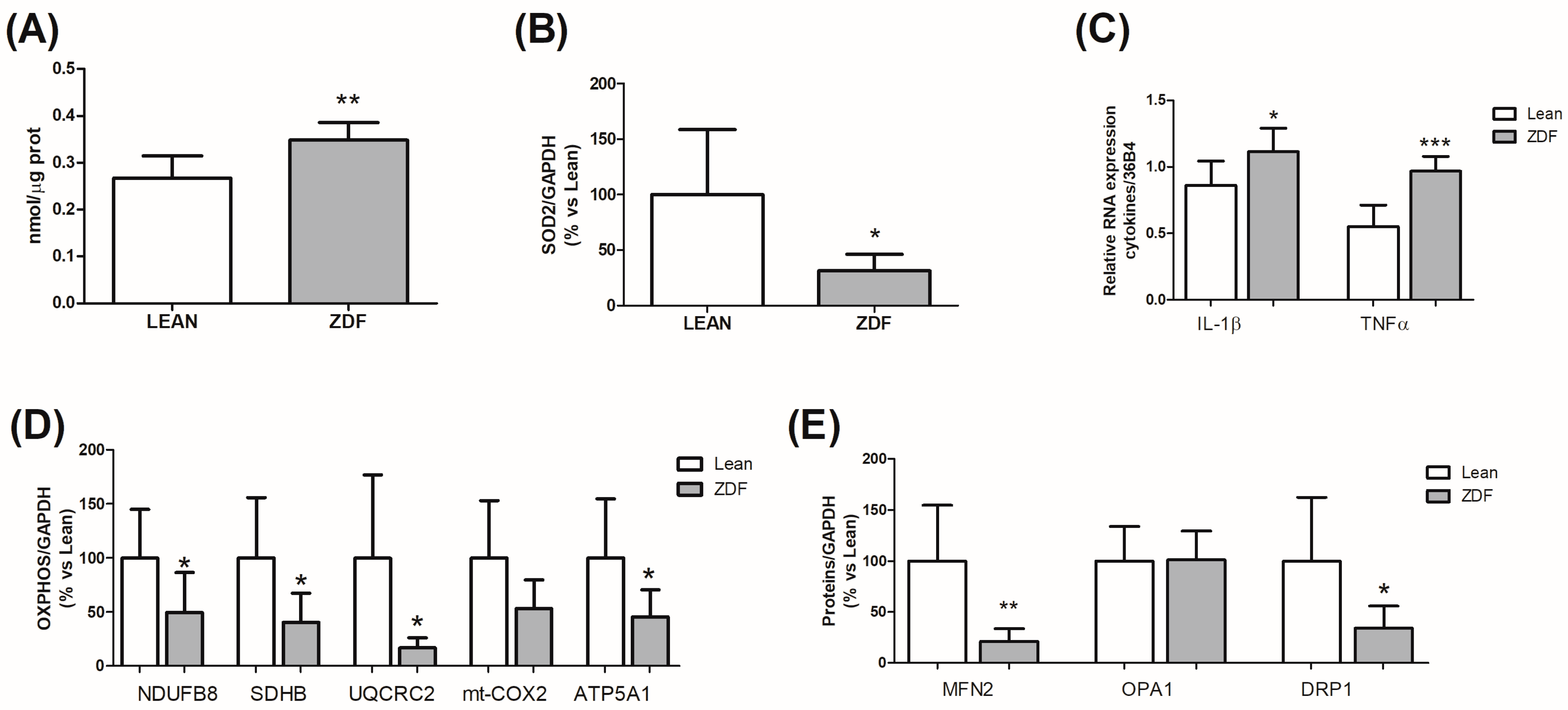
3.5. Hippocampal Alterations Induced by T2DM
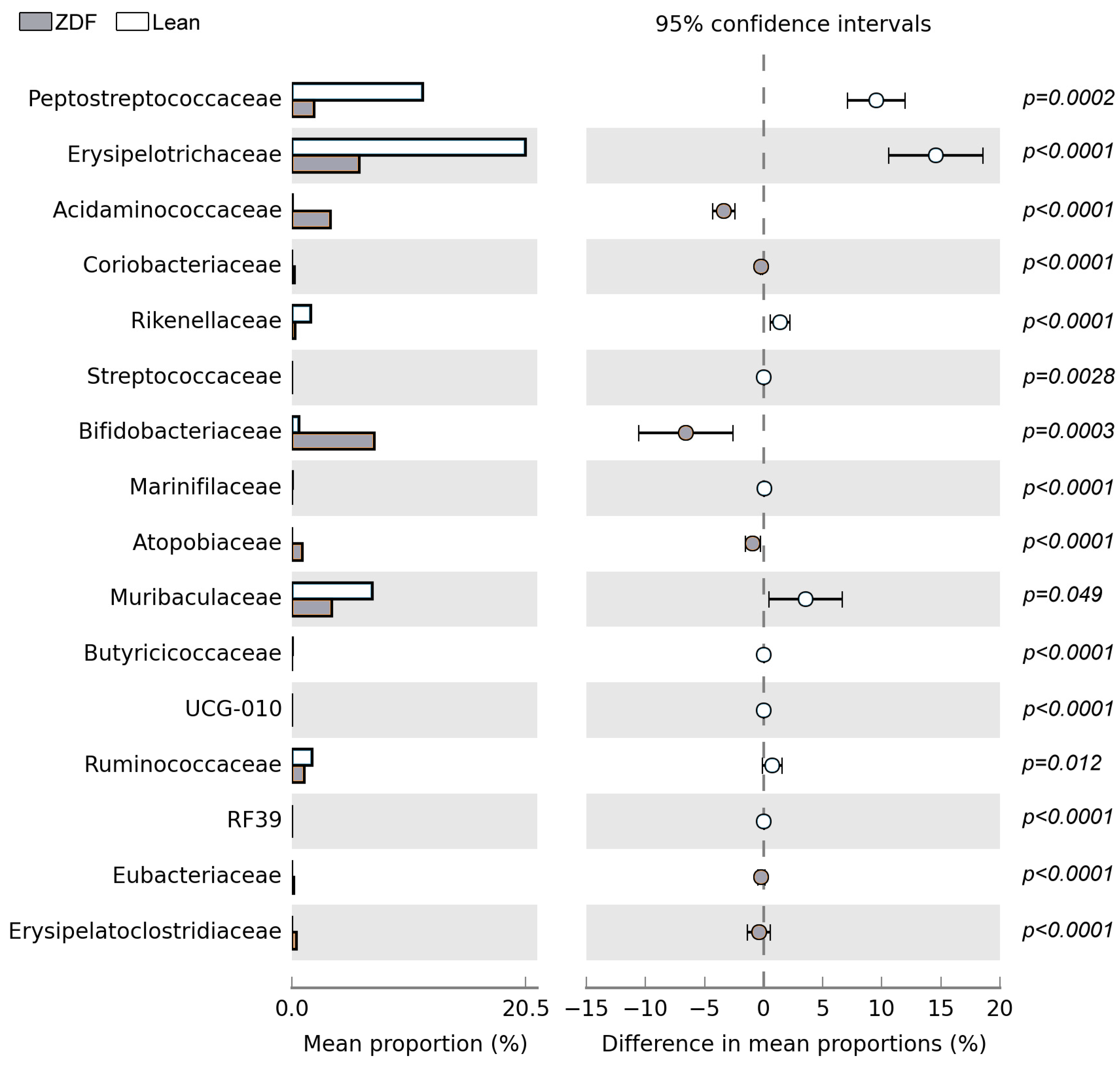
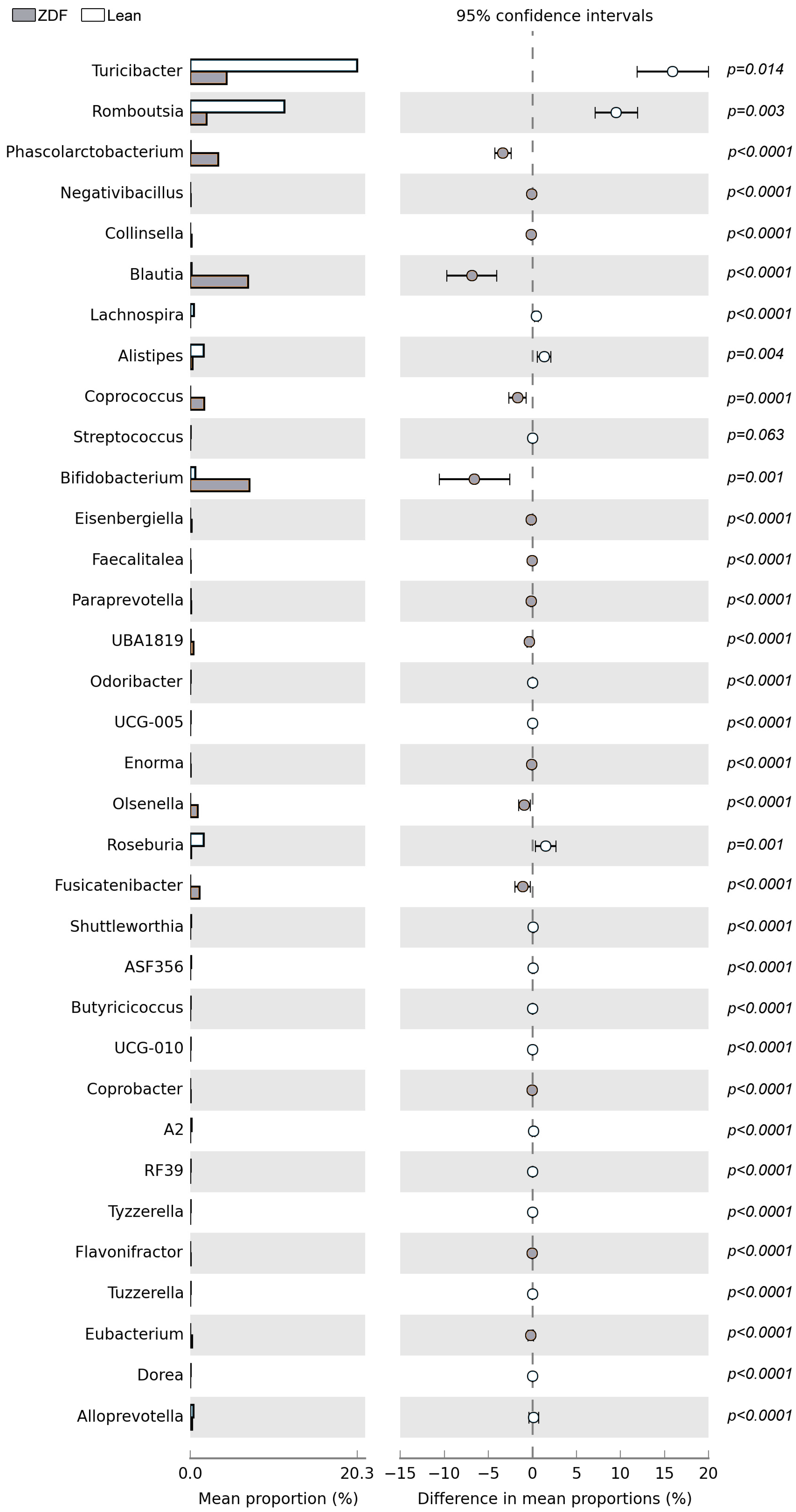
3.6. Diversity and Composition of Gut Microbiota Are Affected by T2DM
3.7. Correlation between Plasma Corticosterone and Hippocampal Allopregnanolone Levels in Genera Affected by T2DM and Correlated to NOR Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groeneveld, O.; Reijmer, Y.; Heinen, R.; Kuijf, H.; Koekkoek, P.; Janssen, J.; Rutten, G.; Kappelle, L.; Biessels, G. Brain imaging correlates of mild cognitive impairment and early dementia in patients with type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1253–1260. [Google Scholar] [CrossRef]
- Hughes, T.M.; Sink, K.M.; Williamson, J.D.; Hugenschmidt, C.E.; Wagner, B.C.; Whitlow, C.T.; Xu, J.; Smith, S.C.; Launer, L.J.; Barzilay, J.I.; et al. Relationships between cerebral structure and cognitive function in African Americans with type 2 diabetes. J. Diabetes Complicat. 2018, 32, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Reijmer, Y.D.; van den Berg, E.; Ruis, C.; Kappelle, L.J.; Biessels, G.J. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2010, 26, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J.; Miller, M.E.; Williamson, J.D.; Lazar, R.M.; Gerstein, H.C.; Murray, A.M.; Sullivan, M.; Horowitz, K.R.; Ding, J.; Marcovina, S.; et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): A randomised open-label substudy. Lancet Neurol. 2011, 10, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Hillson, R.; Bayer, A.J. Diabetes and dementia in older people: A Best Clinical Practice Statement by a multidisciplinary National Expert Working Group. Diabet. Med. 2014, 31, 1024–1031. [Google Scholar] [CrossRef]
- de Galan, B.E.; Zoungas, S.; Chalmers, J.; Anderson, C.; Dufouil, C.; Pillai, A.; Cooper, M.; Grobbee, D.E.; Hackett, M.; Hamet, P.; et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 2009, 52, 2328–2336. [Google Scholar] [CrossRef]
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Grant, M.D.; Hofer, S.M.; Pedersen, N.L.; Nilsson, S.E.; Berg, S.; McClearn, G.; Johansson, B. Type 2 diabetes mellitus contributes to cognitive decline in old age: A longitudinal population-based study. J. Int. Neuropsychol. Soc. 2004, 10, 599–607. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.; Reijmer, Y.D.; de Bresser, J.; Kessels, R.P.; Kappelle, L.J.; Biessels, G.J. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 58–65. [Google Scholar] [CrossRef]
- Moran, C.; Phan, T.G.; Chen, J.; Blizzard, L.; Beare, R.; Venn, A.; Münch, G.; Wood, A.G.; Forbes, J.; Greenaway, T.M.; et al. Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care 2013, 36, 4036–4042. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The interplay among oxidative stress, brain insulin resistance, and AMPK dysfunction contributes to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef]
- Lederbogen, F.; Hummel, J.; Fademrecht, C.; Krumm, B.; Kühner, C.; Deuschle, M.; Ladwig, K.H.; Meisinger, C.; Wichmann, H.E.; Lutz, H.; et al. Flattened circadian cortisol rhythm in type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2011, 119, 573–575. [Google Scholar] [CrossRef]
- Joseph, J.J.; Wang, X.; Spanakis, E.; Seeman, T.; Wand, G.; Needham, B.; Golden, S.H. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 2015, 62, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef]
- Daniel, C.; Oliver, T.W.; Spyros, K.; Benno, R.; Adrian, F.; Dominique, J.F.D.Q. Glucocorticoid Therapy-Induced Memory Deficits: Acute versus Chronic Effects. J. Neurosci. 2008, 28, 3474. [Google Scholar]
- Brunner, R.; Schaefer, D.; Hess, K.; Parzer, P.; Resch, F.; Schwab, S. Effect of corticosteroids on short-term and long-term memory. Neurology 2005, 64, 335. [Google Scholar] [CrossRef]
- Romano, S.; Mitro, N.; Diviccaro, S.; Spezzano, R.; Audano, M.; Garcia-Segura, L.M.; Caruso, D.; Melcangi, R.C. Short-term effects of diabetes on neurosteroidogenesis in the rat hippocampus. J. Steroid Biochem. Mol. Biol. 2017, 167, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Giatti, S.; Diviccaro, S.; Cioffi, L.; Herian, M.; Brivio, P.; Calabrese, F.; Caruso, D.; Melcangi, R.C. Diabetic Encephalopathy in a Preclinical Experimental Model of Type 1 Diabetes Mellitus: Observations in Adult Female Rat. Int. J. Mol. Sci. 2023, 24, 1196. [Google Scholar] [CrossRef]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Front. Neuroendocrinol. 2020, 56, 100804. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Caruso, D.; Pesaresi, M.; Romano, S.; Diviccaro, S.; Giatti, S. Neuroactive steroid levels in healthy and diseased. Eur. Neuropsychopharmacol. 2016, 26, S122. [Google Scholar] [CrossRef]
- Napoli, E.; Schneider, A.; Wang, J.Y.; Trivedi, A.; Carrillo, N.R.; Tassone, F.; Rogawski, M.; Hagerman, R.J.; Giulivi, C. Allopregnanolone Treatment Improves Plasma Metabolomic Profile Associated with GABA Metabolism in Fragile X-Associated Tremor/Ataxia Syndrome: A Pilot Study. Mol. Neurobiol. 2019, 56, 3702–3713. [Google Scholar] [CrossRef]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef]
- Giatti, S.; Boraso, M.; Melcangi, R.C.; Viviani, B. Neuroactive steroids, their metabolites, and neuroinflammation. J. Mol. Endocrinol. 2012, 49, R125–R134. [Google Scholar] [CrossRef]
- Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. New steps forward in the neuroactive steroid field. J. Steroid Biochem. Mol. Biol. 2015, 153, 127–134. [Google Scholar] [CrossRef]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Romano, S.; Mitro, N.; Giatti, S.; Diviccaro, S.; Pesaresi, M.; Spezzano, R.; Audano, M.; Garcia-Segura, L.M.; Caruso, D.; Melcangi, R.C. Diabetes induces mitochondrial dysfunction and alters cholesterol homeostasis and neurosteroidogenesis in the rat cerebral cortex. J. Steroid Biochem. Mol. Biol. 2018, 178, 108–116. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Diviccaro, S.; Caputi, V.; Cioffi, L.; Giatti, S.; Lyte, J.M.; Caruso, D.; O’Mahony, S.M.; Melcangi, R.C. Exploring the Impact of the Microbiome on Neuroactive Steroid Levels in Germ-Free Animals. Int. J. Mol. Sci. 2021, 22, 12551. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 2014, 817, 357–371. [Google Scholar]
- Diviccaro, S.; Falvo, E.; Piazza, R.; Cioffi, L.; Herian, M.; Brivio, P.; Calabrese, F.; Giatti, S.; Caruso, D.; Melcangi, R.C. Gut microbiota composition is altered in a preclinical model of type 1 diabetes mellitus: Influence on gut steroids, permeability, and cognitive abilities. Neuropharmacology 2023, 226, 109405. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.L.; Kibble, S.; Dubester, K.; Bell, A.; Swartzwelder, H.S. Adolescent intermittent ethanol exposure enhances adult stress effects in male rats. Pharmacol. Biochem. Behav. 2023, 223, 173513. [Google Scholar] [CrossRef]
- Caruso, D.; Pesaresi, M.; Maschi, O.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J. Neuroendocrinol. 2010, 22, 1137–1147. [Google Scholar] [CrossRef]
- Caruso, D.; Abbiati, F.; Giatti, S.; Romano, S.; Fusco, L.; Cavaletti, G.; Melcangi, R.C. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J. Steroid Biochem. Mol. Biol. 2015, 146, 74–79. [Google Scholar] [CrossRef]
- Giatti, S.; Diviccaro, S.; Cioffi, L.; Falvo, E.; Caruso, D.; Melcangi, R.C. Effects of paroxetine treatment and its withdrawal on neurosteroidogenesis. Psychoneuroendocrinology 2021, 132, 105364. [Google Scholar] [CrossRef] [PubMed]
- Hummon, A.B.; Lim, S.R.; Difilippantonio, M.J.; Ried, T. Isolation and solubilization of proteins after TRIzol extraction of RNA and DNA from patient material following prolonged storage. Biotechniques 2007, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Garcia-Segura, L.M.; Melcangi, R.C. Sex differences in the brain expression of steroidogenic molecules under basal conditions and after gonadectomy. J. Neuroendocrinol. 2019, 31, e12736. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, M.; Giatti, S.; Spezzano, R.; Romano, S.; Diviccaro, S.; Borsello, T.; Mitro, N.; Caruso, D.; Garcia-Segura, L.M.; Melcangi, R.C. Axonal transport in a peripheral diabetic neuropathy model: Sex-dimorphic features. Biol. Sex Differ. 2018, 9, 6. [Google Scholar] [CrossRef]
- Giatti, S.; Rigolio, R.; Romano, S.; Mitro, N.; Viviani, B.; Cavaletti, G.; Caruso, D.; Garcia-Segura, L.M.; Melcangi, R.C. Dihydrotestosterone as a Protective Agent in Chronic Experimental Autoimmune Encephalomyelitis. Neuroendocrinology 2015, 101, 296–308. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- GalSeq Srl. Available online: www.galseq.com (accessed on 11 July 2023).
- FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 11 July 2023).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T. Animal models of type 2 diabetes: Clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006, 47, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Dvorakova, M.C. Future Perspective of Diabetic Animal Models. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, E.; Schönberger, T.; Sumalla, M.; Stierstorfer, B.; Solà, R.; Doods, H.; Serra, J.; Gorodetskaya, N. Behavioural, morphological and electrophysiological assessment of the effects of type 2 diabetes mellitus on large and small nerve fibres in Zucker diabetic fatty, Zucker lean and Wistar rats. Eur. J. Pain 2018, 22, 1457–1472. [Google Scholar] [CrossRef]
- Kamal, A.; Ramakers, G.M.; Gispen, W.H.; Biessels, G.J. Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms. Exp. Brain Res. 2013, 226, 45–51. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Aghanoori, M.R.; Navarro-Diaz, M.C.; Han, M.M.; Sanchez, G.; Guernsey, L.; Quach, D.; Johe, K.; Fernyhough, P.; Calcutt, N.A. Enhancement of Mitochondrial Function by the Neurogenic Molecule NSI-189 Accompanies Reversal of Peripheral Neuropathy and Memory Impairment in a Rat Model of Type 2 Diabetes. J. Diabetes Res. 2022, 2022, 8566970. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Type 2 diabetes as a risk factor for cognitive impairment: Current insights. Clin. Interv. Aging 2014, 9, 1011–1019. [Google Scholar] [CrossRef]
- Chan, O.; Inouye, K.; Riddell, M.C.; Vranic, M.; Matthews, S.G. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 2003, 28, 87–102. [Google Scholar]
- Jöhren, O.; Dendorfer, A.; Dominiak, P.; Raasch, W. Gene expression of mineralocorticoid and glucocorticoid receptors in the limbic system is related to type-2 like diabetes in leptin-resistant rats. Brain Res. 2007, 1184, 160–167. [Google Scholar] [CrossRef]
- Bruehl, H.; Wolf, O.T.; Convit, A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 2009, 34, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Sapolsky, R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007, 21, 259–272. [Google Scholar] [CrossRef]
- Dey, A.; Hao, S.; Erion, J.R.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J. Neuroimmunol. 2014, 269, 20–27. [Google Scholar] [CrossRef][Green Version]
- Pesaresi, M.; Maschi, O.; Giatti, S.; Garcia-Segura, L.M.; Caruso, D.; Melcangi, R.C. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm. Behav. 2010, 57, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Talaei, F.; Van Praag, V.M.; Shishavan, M.H.; Landheer, S.W.; Buikema, H.; Henning, R.H. Increased protein aggregation in Zucker diabetic fatty rat brain: Identification of key mechanistic targets and the therapeutic application of hydrogen sulfide. BMC Cell Biol. 2014, 15, 1. [Google Scholar] [CrossRef]
- Qin, L.; Chong, T.; Rodriguez, R.; Pugazhenthi, S. Glucagon-Like Peptide-1-Mediated Modulation of Inflammatory Pathways in the Diabetic Brain: Relevance to Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Choi, J.H.; Nam, S.M.; Park, O.K.; Yoo, D.Y.; Kim, W.; Yi, S.S.; Won, M.H.; Seong, J.K.; Yoon, Y.S. Activation of microglia and induction of pro-inflammatory cytokines in the hippocampus of type 2 diabetic rats. Neurol. Res. 2014, 36, 824–832. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Howarth, F.C. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell. Physiol. Biochem. 2015, 35, 1241–1251. [Google Scholar] [CrossRef]
- Rajab, E.; Keogh, M.B.; Laiva, A.L.; Al Hannan, F.; Fateel, M.; Abdulwahab, F.; Al Madhoob, F.; AlHamer, B.; Ghazzal, S.Y.; Dawaishan, A.; et al. Gum Arabic supplementation prevents loss of learning and memory through stimulation of mitochondrial function in the hippocampus of type 2 diabetic rats. J. Funct. Foods 2021, 87, 104757. [Google Scholar] [CrossRef]
- Zampieri, S.; Mellon, S.H.; Butters, T.D.; Nevyjel, M.; Covey, D.F.; Bembi, B.; Dardis, A. Oxidative stress in NPC1 deficient cells: Protective effect of allopregnanolone. J. Cell. Mol. Med. 2009, 13, 3786–3796. [Google Scholar] [CrossRef]
- Qian, X.; Cao, H.; Ma, Q.; Wang, Q.; He, W.; Qin, P.; Ji, B.; Yuan, K.; Yang, F.; Liu, X.; et al. Allopregnanolone attenuates Aβ25-35-induced neurotoxicity in PC12 cells by reducing oxidative stress. Int. J. Clin. Exp. Med. 2015, 8, 13610–13615. [Google Scholar] [PubMed]
- Lejri, I.; Grimm, A.; Miesch, M.; Geoffroy, P.; Eckert, A.; Mensah-Nyagan, A.G. Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 631–642. [Google Scholar] [CrossRef]
- Lejri, I.; Agapouda, A.; Grimm, A.; Eckert, A. Mitochondria- and Oxidative Stress-Targeting Substances in Cognitive Decline-Related Disorders: From Molecular Mechanisms to Clinical Evidence. Oxid. Med. Cell. Longev. 2019, 2019, 9695412. [Google Scholar] [CrossRef] [PubMed]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.J.; Kang, J.; Han, N.N.; Ma, H.T. Effect of dehydroepiandrosterone treatment on hormone levels and antioxidant parameters in aged rats. Genet. Mol. Res. 2015, 14, 11300–11311. [Google Scholar] [CrossRef]
- Racchi, M.; Balduzzi, C.; Corsini, E. Dehydroepiandrosterone (DHEA) and the aging brain: Flipping a coin in the “fountain of youth”. CNS Drug Rev. 2003, 9, 21–40. [Google Scholar] [CrossRef]
- Giatti, S.; Caruso, D.; Boraso, M.; Abbiati, F.; Ballarini, E.; Calabrese, D.; Pesaresi, M.; Rigolio, R.; Santos-Galindo, M.; Viviani, B.; et al. Neuroprotective Effects of Progesterone in Chronic Experimental Autoimmune Encephalomyelitis. J. Neuroendocrinol. 2012, 24, 851–861. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Panzica, G. Neuroactive steroids: An update of their roles in central and peripheral nervous system. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Cosimo, M.R.; Miguel, G.-S.L. Sex-Specific therapeutic strategies based on neuroactive steroids: In search for innovative tools for neuroprotection. Horm. Behav. 2010, 57, 2–11. [Google Scholar] [CrossRef]
- Russo, V.; Chen, R.; Armamento-Villareal, R. Hypogonadism, Type-2 Diabetes Mellitus, and Bone Health: A Narrative Review. Front. Endocrinol. 2021, 11, 607240. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, M.; Gao, X.; Wang, S.; Fu, C.; Zeng, J.; He, X. Phocea, Pseudoflavonifractor and Lactobacillus intestinalis: Three Potential Biomarkers of Gut Microbiota That Affect Progression and Complications of Obesity-Induced Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, H.; Zhan, L.; Lu, X.; Zhang, L. Dynamic Development of Fecal Microbiome During the Progression of Diabetes Mellitus in Zucker Diabetic Fatty Rats. Front. Microbiol. 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Bi, T.; Zhang, L.; Zhan, L.; Feng, R.; Zhao, T.; Ren, W.; Hang, T.; Zhou, W.; Lu, X. Integrated Analyses of Microbiomics and Metabolomics Explore the Effect of Gut Microbiota Transplantation on Diabetes-Associated Cognitive Decline in Zucker Diabetic Fatty Rats. Front. Aging Neurosci. 2022, 14, 913002. [Google Scholar] [CrossRef]
- Goldsmith, F.; Guice, J.; Page, R.; Welsh, D.A.; Taylor, C.M.; Blanchard, E.E.; Luo, M.; Raggio, A.M.; Stout, R.W.; Carvajal-Aldaz, D.; et al. Obese ZDF rats fermented resistant starch with effects on gut microbiota but no reduction in abdominal fat. Mol. Nutr. Food Res. 2017, 61, 1501025. [Google Scholar] [CrossRef]
- Gu, C.; Yang, Y.; Xiang, H.; Li, S.; Liang, L.; Sui, H.; Zhan, L.; Lu, X. Deciphering bacterial community changes in zucker diabetic fatty rats based on 16S rRNA gene sequences analysis. Oncotarget 2016, 7, 48941–48952. [Google Scholar] [CrossRef]
- So, S.Y.; Savidge, T.C. Gut feelings: The microbiota-gut-brain axis on steroids. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G1–G20. [Google Scholar] [CrossRef]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocrinol. 2020, 56, 100815. [Google Scholar] [CrossRef]
- Diviccaro, S.; FitzGerald, J.A.; Cioffi, L.; Falvo, E.; Crispie, F.; Cotter, P.D.; O’Mahony, S.M.; Giatti, S.; Caruso, D.; Melcangi, R.C. Gut Steroids and Microbiota: Effect of Gonadectomy and Sex. Biomolecules 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Giagulli, V.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Vitale, E.; Triggiani, V. Chronic Stress as a Risk Factor for Type 2 Diabetes: Endocrine, Metabolic, and Immune Implications. Endocr. Metab. Immune Disord. Drug Targets 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J.; Shah, V.O. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 1. [Google Scholar] [PubMed]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Sun, W.H.; Chen, T.H.; Tsai, P.C.; Chen, C.C.; Huang, S.L. Gut Mucosal Microbiome Is Perturbed in Rheumatoid Arthritis Mice and Partly Restored after TDAG8 Deficiency or Suppression by Salicylanilide Derivative. Int. J. Mol. Sci. 2022, 23, 3527. [Google Scholar] [CrossRef]
- Shin, J.; Noh, J.R.; Choe, D.; Lee, N.; Song, Y.; Cho, S.; Kang, E.J.; Go, M.J.; Ha, S.K.; Chang, D.H.; et al. Ageing and rejuvenation models reveal changes in key microbial communities associated with healthy ageing. Microbiome 2021, 9, 240. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 580001. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jiang, Q.; Ji, H.; Ning, J.; Li, C.; Zheng, H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165541. [Google Scholar] [CrossRef]



| Lean | ZDF | |
|---|---|---|
| Weight (g)—7 weeks | 169.3 ± 17.2 | 207.9 ± 8.8 *** |
| Weight (g)—32 weeks | 426.7 ± 32.4 | 377.4 ± 28.2 ** |
| Glycemia (mg/dL)—32 weeks | 114.5 ± 10.6 | 524.8 ± 126.2 *** |
| Plasma | Hippocampus | |||
|---|---|---|---|---|
| Lean | ZDF | Lean | ZDF | |
| PREG | 0.34 ± 0.27 | 2.1 ± 1.07 ** | 4.39 ± 1.80 | 7.23 ± 1.83 * |
| PROG | 1.16 ± 0.78 | 7.62 ± 2.85 ** | 1.04 ± 0.48 | 4.14 ± 2.16 * |
| DHP | 0.33 ± 0.13 | 1.19 ± 0.53 ** | 0.86 ± 0.63 | 1.55 ± 0.62 |
| ALLO | 0.37 ± 0.30 | 0.4 ± 0.15 | 0.75 ± 0.42 | 0.16 ± 0.11 ** |
| ISOALLO | 0.12 ± 0.06 | 0.14 ± 0.06 | 0.37 ± 0.27 | <0.1 |
| DHEA | <0.05 | <0.05 | 0.06 ± 0.02 | 0.23 ± 0.11 * |
| T | 1.62 ± 0.72 | 0.5 ± 0.34 ** | 0.99 ± 0.19 | 0.48 ± 0.23 ** |
| DHT | 0.06 ± 0.02 | <0.05 | 0.37 ± 0.30 | 0.27 ± 0.18 |
| 3α-DIOL | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.36 ± 0.34 | 0.44 ± 0.15 |
| 17β-E | 0.03 ± 0.02 | <0.02 | 0.05 ± 0.02 | 0.03 ± 0.02 |
| Genus | NOR Index |
|---|---|
| Turicibacter | r(13) = 0.883; p = <0.000; F(1,13) = 45.98 |
| UBA1819 | r(13) = −0.832; p = 0.000; F(1,13) = 28.19 |
| Negativibacillus | r(13) = −0.818; p = 0.000; F(1,13) = 26.2 |
| Collinsella | r(13) = −0.816; p = 0.000; F(1,13) = 25.96 |
| Bifidobacterium | r(13) = −0.813; p = 0.000; F(1,13) = 25.39 |
| Romboutsia | r(13) = 0.798; p = 0.000; F(1,13) = 22.72 |
| Paraprevotella | r(13) = −0.768; p = 0.001; F(1,13) = 18.71 |
| Blautia | r(13) = −0.707; p = 0.003; F(1,13) = 13.03 |
| Faecalitalea | r(13) = −0.703; p = 0.004; F(1,13) = 12.69 |
| Phascolarctobacterium | r(13) = −0.700; p = 0.004; F(1,13) = 12.46 |
| Flavonifractor | r(13) = −0.691; p = 0.004; F(1,13) = 11.9 |
| Alistipes | r(13) = 0.664; p = 0.007; F(1,13) = 10.23 |
| Enorma | r(13) = −0.662; p = 0.007; F(1,13) = 10.12 |
| Eisenbergiella | r(13) = −0.631; p = 0.012; F(1,13) = 8.62 |
| Lachnospira | r(13) = 0.610; p = 0.016; F(1,13) = 7.71 |
| Coprococcus | r(13) = −0.578; p = 0.024; F(1,13) = 6.51 |
| Streptococcus | r(13) = 0.577; p = 0.024; F(1,13) = 6.50 |
| Odoribacter | r(13) = 0.572; p = 0.026; F(1,13) = 6.31 |
| Eubacterium | r(13) = −0.567; p = 0.028; F(1,13) = 6.16 |
| UCG-010 | r(13) = 0.548; p = 0.035; F(1,13) = 5.58 |
| Fusicatenibacter | r(13) = −0.536; p = 0.039; F(1,13) = 5.25 |
| RF39 | r(13) = 0.517; p = 0.049; F(1,13) = 4.74 |
| Genus | Corticosterone | Allopregnanolone |
|---|---|---|
| Collinsella (+) | r(12) = 0.633; p = 0.015; F(1,12) = 8.026 | r(10) = −0.586; p = 0.045; F(1,10) = 5.226 |
| Paraprevotella (+) | r(12) = 0.667; p = 0.009; F(1,12) = 9.633 | r(10) = −0.620 p = 0.031; F(1,10) = 6.258 |
| Phascolarctobacterium (+) | r(12) = 0.723; p = 0.004; F(1,12) = 13.15 | r(10) = −0.708; p = 0.010; F(1,10) = 10.04 |
| Turicibacter (−) | r(12) = −0.687; p = 0.007; F(1,12) = 10.72 | r(10) = 0.831; p = 0.001; F(1,10) = 22.22 |
| Romboutsia (−) | r(12) = −0.544; p = 0.045; F(1,12) = 5.032 | r(10) = 0.816; p = 0.001; F(1,10) = 19.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diviccaro, S.; Cioffi, L.; Piazza, R.; Caruso, D.; Melcangi, R.C.; Giatti, S. Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy. Biomolecules 2023, 13, 1325. https://doi.org/10.3390/biom13091325
Diviccaro S, Cioffi L, Piazza R, Caruso D, Melcangi RC, Giatti S. Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy. Biomolecules. 2023; 13(9):1325. https://doi.org/10.3390/biom13091325
Chicago/Turabian StyleDiviccaro, Silvia, Lucia Cioffi, Rocco Piazza, Donatella Caruso, Roberto Cosimo Melcangi, and Silvia Giatti. 2023. "Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy" Biomolecules 13, no. 9: 1325. https://doi.org/10.3390/biom13091325
APA StyleDiviccaro, S., Cioffi, L., Piazza, R., Caruso, D., Melcangi, R. C., & Giatti, S. (2023). Neuroactive Steroid–Gut Microbiota Interaction in T2DM Diabetic Encephalopathy. Biomolecules, 13(9), 1325. https://doi.org/10.3390/biom13091325






