Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia
Abstract
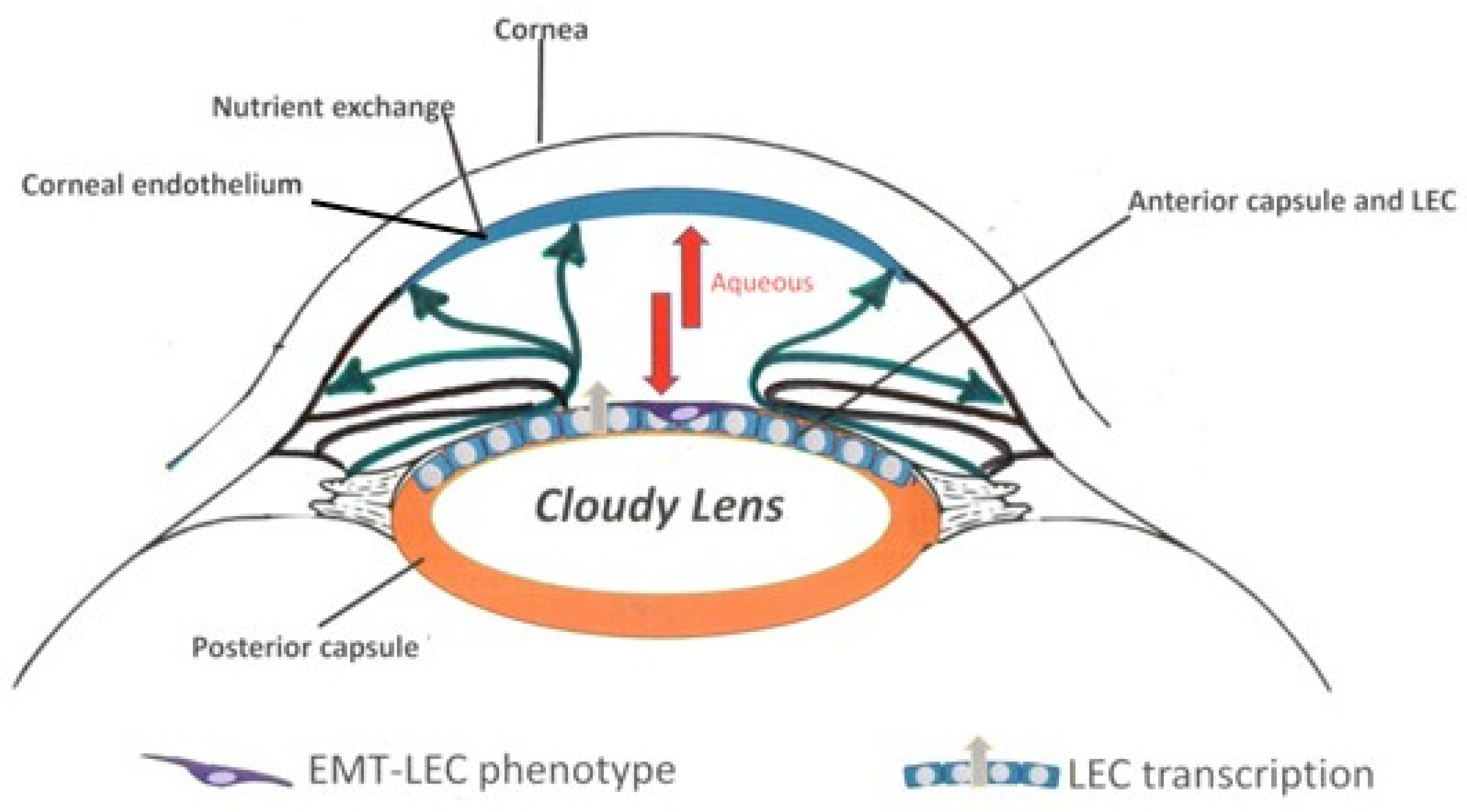
:1. Introduction
2. Materials and Methods
2.1. Study Population: Clinical Assessment and Sampling
2.2. Pre-Analytical Procedures
2.3. Analytical Procedures
2.3.1. Ella™ and ELISA Analysis
2.3.2. Molecular Analysis: 2-Step Real-Time RT-PCR Analysis
2.3.3. Microscopy, Digital Imaging, and Densitometry
2.4. Statistical Analysis
3. Results
3.1. AH Expression of IL-6, IL-8, VEGF-A, and ANG-2
3.2. LECs Express the αSMA-Phenotype (Protein and Transcript) That Correlates with AL Elongation
3.3. LECs Express Transcripts Specific for TGFβR and TGFβ Isoforms
3.4. LECs might Contribute and Respond to AH Signature
3.5. IL-6R/IL-6 and VEGF-R1/VEGF-A Transcripts Correlate with α-SMA Transcripts
3.6. Correlation Analyses for AH Signature and Eye Elongation: Ocular Fluid Circulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential Lost Productivity Resulting from the Global Burden of Myopia: Systematic Review, Meta-analysis, and Modeling. Ophthalmology 2019, 126, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Goh, D.X.; Koh, J.H.Z.; Chan, X.; Brennan, N.A.; Barathi, V.A.; Hoang, Q.V. Applications of Genomics and Transcriptomics in Precision Medicine for Myopia Control or Prevention. Biomolecules 2023, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Zhang, L.; Cai, Y.; Teng, H.; Liang, J.; Yue, Y.; Li, Y.; Huang, Y.; Liu, M.; Zhang, Y.; et al. Identification and characterization of extrachromosomal circular DNA in patients with high myopia and cataract. Epigenetics 2023, 18, 2192324. [Google Scholar] [CrossRef]
- Tedja, M.S.; Haarman, A.E.; Meester-Smoor, M.A.; Kaprio, J.; Mackey, D.A.; Guggenheim, J.A.; Hammond, C.J.; Verhoeven, V.J.; Klaver, C.C.; CREAM Consortium. Myopia Genetics Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M89–M105. [Google Scholar] [CrossRef]
- Du, R.; Xie, S.; Igarashi-Yokoi, T.; Watanabe, T.; Uramoto, K.; Takahashi, H.; Nakao, N.; Yoshida, T.; Fang, Y.; Ohno-Matsui, K. Continued Increase of Axial Length and Its Risk Factors in Adults With High Myopia. JAMA Ophthalmol. 2021, 139, 1096–1103. [Google Scholar] [CrossRef]
- Chamberlain, P.; Lazon de la Jara, P.; Arumugam, B.; Bullimore, M.A. Axial length targets for myopia control. Ophthalmic Physiol. Opt. 2021, 41, 523–531. [Google Scholar] [CrossRef]
- Khan, M.H.; Lam, A.; Armitage, J.A.; Hanna, L.; To, C.H.; Gentle, A. Impact of Axial Eye Size on Retinal Microvasculature Density in the Macular Region. J. Clin. Med. 2020, 9, 2539. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, S.; Wang, Y.; Pan, S.; Wang, P.; Cheng, L. Inflammatory cytokines in highly myopic eyes. Sci. Rep. 2019, 9, 3517. [Google Scholar] [CrossRef]
- Xue, M.; Ke, Y.; Ren, X.; Zhou, L.; Liu, J.; Zhang, X.; Shao, X.; Li, X. Proteomic analysis of aqueous humor in patients with pathologic myopia. J. Proteom. 2021, 234, 104088. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, B.; Dai, L.; Wang, Z.; Feng, L.; Zhao, J. Association between TGF-β gene polymorphism and myopia: A systematic review and meta-analysis. Medicine 2022, 101, e29961. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chawla, R.; Kumawat, D.; Pillay, G. Insight into high myopia and the macula. Indian J. Ophthalmol. 2017, 65, 85–91. [Google Scholar] [CrossRef]
- Zhu, X.J.; Chen, M.J.; Zhang, K.K.; Yang, J.; Lu, Y. Elevated TGF-β2 level in aqueous humor of cataract patients with high myopia: Potential risk factor for capsule contraction syndrome. J. Cataract. Refract. Surg. 2016, 42, 232–238. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp. Eye Res. 2013, 114, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hu, D.N.; Zhu, D.; Zhang, L.; Gu, P.; Fan, X.; Zhou, J. MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels in human aqueous humor: Relationship with axial length. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3922–8928. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.R.; Jody, A. The Dynamic Sclera: Extracellular Matrix Remodeling in Normal Ocular Growth and Myopia Development. Exp. Eye Res. 2015, 133, 100–111. [Google Scholar] [CrossRef]
- Hsiao, Y.; Cao, Y.; Yue, Y.; Zhou, J. Relationship between Axial Length and Levels of TGF-β in the Aqueous Humor and Plasma of Myopic Patients. BioMed Res. Int. 2021, 9, 8863637. [Google Scholar] [CrossRef]
- Xia, Y.F.; Wei, J. Study on Factors Associated with High Myopia CNV in Aqueous Humor and Serum. BioMed Res. Int. 2022, 2022, 8592729. [Google Scholar] [CrossRef]
- Yue, Y.; Che, D.; Hsiao, Y.W.; Zhou, J.; Zhao, K. Association between transforming growth factors-β and matrix metalloproteinases in the aqueous humor and plasma in myopic patients. J. Français d’Ophtalmol. 2022, 45, 1177–1183. [Google Scholar] [CrossRef]
- Ji, Y.; Rao, J.; Rong, X.; Lou, S.; Zheng, Z.; Lu, Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp. Eye Res. 2017, 159, 147–155. [Google Scholar] [CrossRef]
- Shin, Y.J.; Nam, W.H.; Park, S.E.; Kim, J.H.; Kim, H.K. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol. Vis. 2012, 18, 2265–2270. [Google Scholar] [PubMed]
- Lai, T.Y.; Cheung, C.M. Myopic choroidal neovascularization: Diagnosis and Treatment. Retina 2016, 36, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Tao, Y.; Li, X.X. Inflammatory cytokines in aqueous humor of patients with choroidal neovascularization. Mol. Vis. 2012, 18, 574–580. [Google Scholar] [PubMed]
- Ku, H.; Chen, J.J.; Hu, M.; Tien, P.T.; Lin, H.J.; Xu, G.; Wan, L.; Gan, D. Myopia Development in Tree Shrew Is Associated with Chronic Inflammatory Reactions. Curr. Issues Mol. Biol. 2022, 44, 4303–4313. [Google Scholar] [CrossRef]
- Wei, Q.; Zhuang, X.; Fan, J.; Jiang, R.; Chang, Q.; Xu, G.; Yu, Z. Proinflammatory and angiogenesis-related cytokines in vitreous samples of highly myopic patients. Cytokine 2021, 137, 155308. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, J.; Chen, N.; Fang, Y.; Chen, Y.; Zheng, Z.; Chen, Y.; Shen, L. Difference in aqueous concentration and vitreous mass of cytokines in high myopias with and without choroidal neovascularization. Front. Med. 2022, 9, 1029425. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, W.; Fan, Y.; Zhang, L.; Liu, S.; Song, S.; Li, H. Effect of Interleukin 6 on Scleral Fibroblast Proliferation, Differentiation, and Apoptosis Involved in Myopic Scleral Remodeling. Ophthalmic Res. 2022, 65, 529–539. [Google Scholar] [CrossRef]
- Shchuko, A.G.; Zaytseva, N.V.; Yur’eva, T.N.; Chernykh, E.R.; Mikhalevich, I.M.; Grigor’eva, A.V. Role of immunological factors in the development of myopic choroidal neovascularization. Vestn. Oftalmol. 2016, 132, 5–14. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and Disease Burden of Pathologic Myopia and Myopic Choroidal Neovascularization: An Evidence-Based Systematic Review. Am. J. Ophthalmol. 2014, 157, 9–25. [Google Scholar] [CrossRef]
- Shchuko, A.G.; Zaitseva, N.V.; Yurieva, T.N.; Chernykh, E.R.; Mikhalevich, I.M.; Shevela, E.Y.; Grigorieva, A.V. Intraocular Cytokines and Their Correlations with Clinical Parameters in Patients with Myopic Choroidal Neovascularization. Ophthalmologica 2017, 237, 96–104. [Google Scholar] [CrossRef]
- Foxton, R.H.; Uhles, S.; Grüner, S.; Relevant, F.; Ullmer, C. Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol. Med. 2019, 11, e10204. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhao, X.; Li, S.; Sun, L.; Xin, W.; Wang, Z.; Zhang, A.; Zhang, J.; Ding, X. Risk factors for subretinal fibrosis after anti-VEGF treatment of myopic choroidal neovascularisation. Br. J. Ophthalmol. 2021, 105, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Niu, T.; Xing, X.; Wang, H.; Shi, X.; Liu, Y.; Liu, X.; Chen, C.; Liu, K. Serum Ang-1/Ang-2 ratio may be a promising biomarker for evaluating severity of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Langmann, T. Anti-VEGF-A/ANG2 combotherapy limits pathological angiogenesis in the eye: A replication study. EMBO Mol. Med. 2019, 11, e10362. [Google Scholar] [CrossRef]
- Lovicu, F.J.; Shin, E.H.; McAvoy, J.W. Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract. Exp. Eye Res. 2016, 142, 92–101. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, T.; Singh, D.P.; Sasaki, H. Roles of TGFβ and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int. J. Mol. Sci. 2018, 19, 3093. [Google Scholar] [CrossRef]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- He, W.; Cheng, K.; Zhao, L.; Liu, S.; Huang, Z.; Zhang, K.; Du, Y.; Zhou, X.; Lu, Y.; Zhu, X. Long-Term Outcomes of Posterior Capsular Opacification in Highly Myopic Eyes and Its Influencing Factors. Ophthalmol. Ther. 2023, 12, 1881–1891. [Google Scholar] [CrossRef]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. eBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef]
- Sawada, O.; Miyake, T.; Kakinoki, M.; Sawada, T.; Kawamura, H.; Ohji, M. Negative correlation between aqueous vascular endothelial growth factor levels and axial length. Jpn. J. Ophthalmol. 2011, 55, 401–404. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Wang, Y.Y.; Chen, L.S.; Li, W.D.; Shen, Y.; Jin, Y.; Yang, J.; Wang, Y.; Yuan, J.; Cheng, L. Implication of inflammatory cytokines in the aqueous humour for management of macular diseases. Acta Ophthalmol. 2020, 98, e309–e315. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Yanagi, Y.; Tsai, A.S.H.; Shihabuddeen, W.A.; Cheung, N.; Lee, S.Y.; Jonas, J.B.; Cheung, C.M.G. Correlation of axial length and myopic macular degeneration to levels of molecular factors in the aqueous. Sci. Rep. 2019, 9, 15708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Zheng, R.; Cui, T.; Qin, J.; Su, Z.; Shang, M.; Bao, Y. High irisin and low BDNF levels in aqueous humor of high myopia. Adv. Clin. Exp. Med. 2021, 30, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rong, X.; Ye, H.; Zhang, K.; Lu, Y. Proteomic analysis of aqueous humor proteins associated with cataract development. Clin. Biochem. 2015, 48, 1304–1309. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Enhanced EGF receptor-signaling potentiates TGFβ-induced lens epithelial-mesenchymal transition. Exp. Eye Res. 2019, 185, 107693. [Google Scholar] [CrossRef]
- Ghinelli, E.; Aloe, L.; Cortes, M.; Micera, A.; Lambiase, A.; Bonini, S. Nerve growth factor (NGF) and lenses: Effects of NGF in an in vitro rat model of cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 845–851. [Google Scholar] [CrossRef]
- Flokis, M.; Lovicu, F.J. FGF-2 Differentially Regulates Lens Epithelial Cell Behaviour during TGF-β-Induced EMT. Cells 2023, 12, 827. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, X.; Li, Q.; Wang, H.; Zhang, Z.; Han, Z.; Ke, M.; Chen, X. Identification of proteins in the aqueous humor associated with cataract development using iTRAQ methodology. Mol. Med. Rep. 2017, 15, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- McAvoy, J.W.; Chamberlain, C.G.; de Iongh, R.U.; Hales, A.M.; Lovicu, F.J. Peter Bishop Lecture: Growth factors in lens development and cataract: Key roles for fibroblast growth factor and TGF-beta. Clin. Exp. Ophthalmol. 2000, 28, 133–139. [Google Scholar] [CrossRef]
- Gao, C.; Lin, X.; Fan, F.; Liu, X.; Wan, H.; Yuan, T.; Zhao, X.; Luo, Y. Status of higher TGF-β1 and TGF-β2 levels in the aqueous humour of patients with diabetes and cataracts. BMC Ophthalmol. 2022, 22, 156. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Comparison of Optical Coherence Tomography Structural Parameters for Diagnosis of Glaucoma in High Myopia. JAMA Ophthalmol. 2023, 141, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Y.; Hu, G.Y.; Yu, S.Q.; Qian, T.W.; Xu, X. Comprehensive assessment of growth factors, inflammatory mediators, and cytokines in vitreous from patients with proliferative diabetic retinopathy. Int. J. Ophthalmol. 2022, 15, 1736–1742. [Google Scholar] [CrossRef]
- Iacono, P.; Parodi, M.B.; Papayannis, A.; Kontadakis, S.; Sheth, S.; Bandello, F. Intravitreal bevacizumab therapy on an as-per-needed basis in subfoveal choroidal neovascularization secondary to pathological myopia: 2-year outcomes of a prospective case series. Retina 2011, 31, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.B.; Shao, Y.R.; Yu, J.F.; Deng, X.Y.; Liu, C.Y.; Chen, Y.Q.; Zhang, Y.; Xu, Z.K.; Wu, S.L.; Shen, L.J. Macular density alterations in myopic choroidal neovascularization and the effect of anti-VEGF on it. Int. J. Ophthalmol. 2021, 14, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, N.; Ishiko, S.; Abiko, T.; Mori, F.; Kagokawa, H.; Kojima, M.; Saito, K.; Yoshida, A. Changes in blood-retinal barrier permeability in form deprivation myopia in tree shrews. Vis. Res. 2000, 40, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.F.; Chen, M.S.; Huang, J.K.; Lin, L.L.; Hung, P.T.; Hou, P.K. The blood-aqueous barrier in anisometropia and high myopia. Ophthalmic Res. 1996, 28, 137–140. [Google Scholar] [CrossRef]
- McCarty, C.A. Cataract in the 21st Century: Lessons from previous epidemiological research. Clin. Exp. Optom. 2002, 85, 91–96. [Google Scholar] [CrossRef]
- Bhandary, S.K.; Dhakal, R.; Sanghavi, V.; Verkicharla, P.K. Ambient light level varies with different locations and environmental conditions: Potential to impact myopia. PLoS ONE 2021, 16, e0254027. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional Strategies to Prevent Lens Cataract: Current Status and Future Strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W.; Mitchell, P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.; Kondo, S.; Miyauchi, M.; et al. Violet light exposure can be a preventive strategy against myopia progression. eBiomedicine 2017, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.A.; Bahmani, H. A review of the current state of research on artificial blue light safety as it applies to digital devices. Heliyon 2022, 8, e10282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, H.; Yu, X.; Wang, L.; Wen, Y.; Zhang, L.; Tong, J.; Shen, Y. Blue light attenuates TGF-β2-induced epithelial-mesenchymal transition in human lens epithelial cells via autophagy impairment. BMC Ophthalmol. 2022, 22, 456. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Wong, C.W.; Hoang, Q.V.; Cheung, G.C.M.; Lee, S.Y.; Chia, A.; Saw, S.M.; Ohno-Matsui, K.; Schmetterer, L. Imaging in myopia: Potential biomarkers, current challenges and future developments. Br. J. Ophthalmol. 2019, 103, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, Y.; Cao, J.; Yan, H. TGF-β2 levels in the aqueous humor are elevated in the second eye of high myopia within two weeks after sequential cataract surgery. Sci. Rep. 2022, 12, 17974. [Google Scholar] [CrossRef]
- Su, L.; Ji, Y.S.; Tong, N.; Sarraf, D.; He, X.; Sun, X.; Xu, X.; Sadda, S.R. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1173–1180. [Google Scholar] [CrossRef]
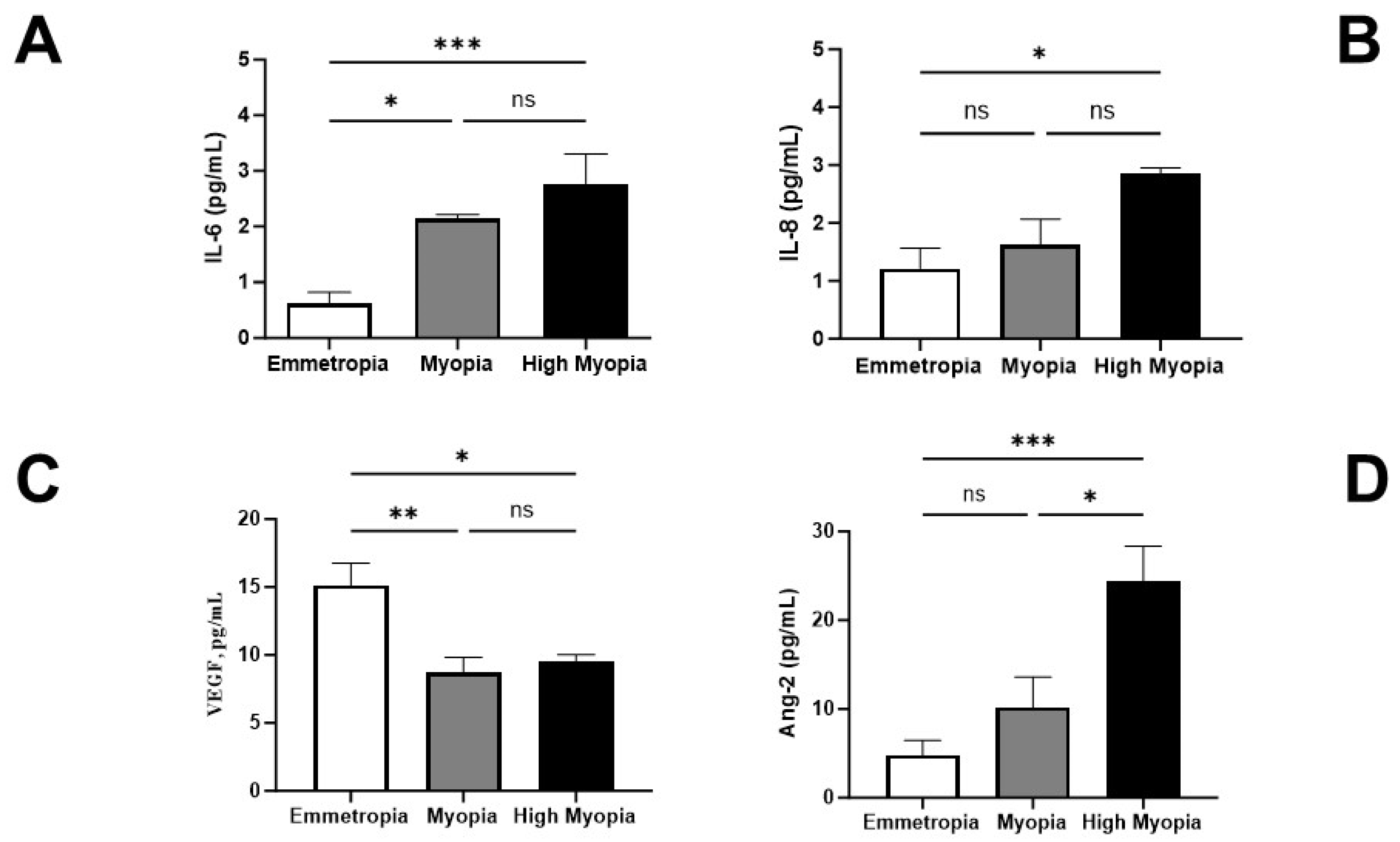

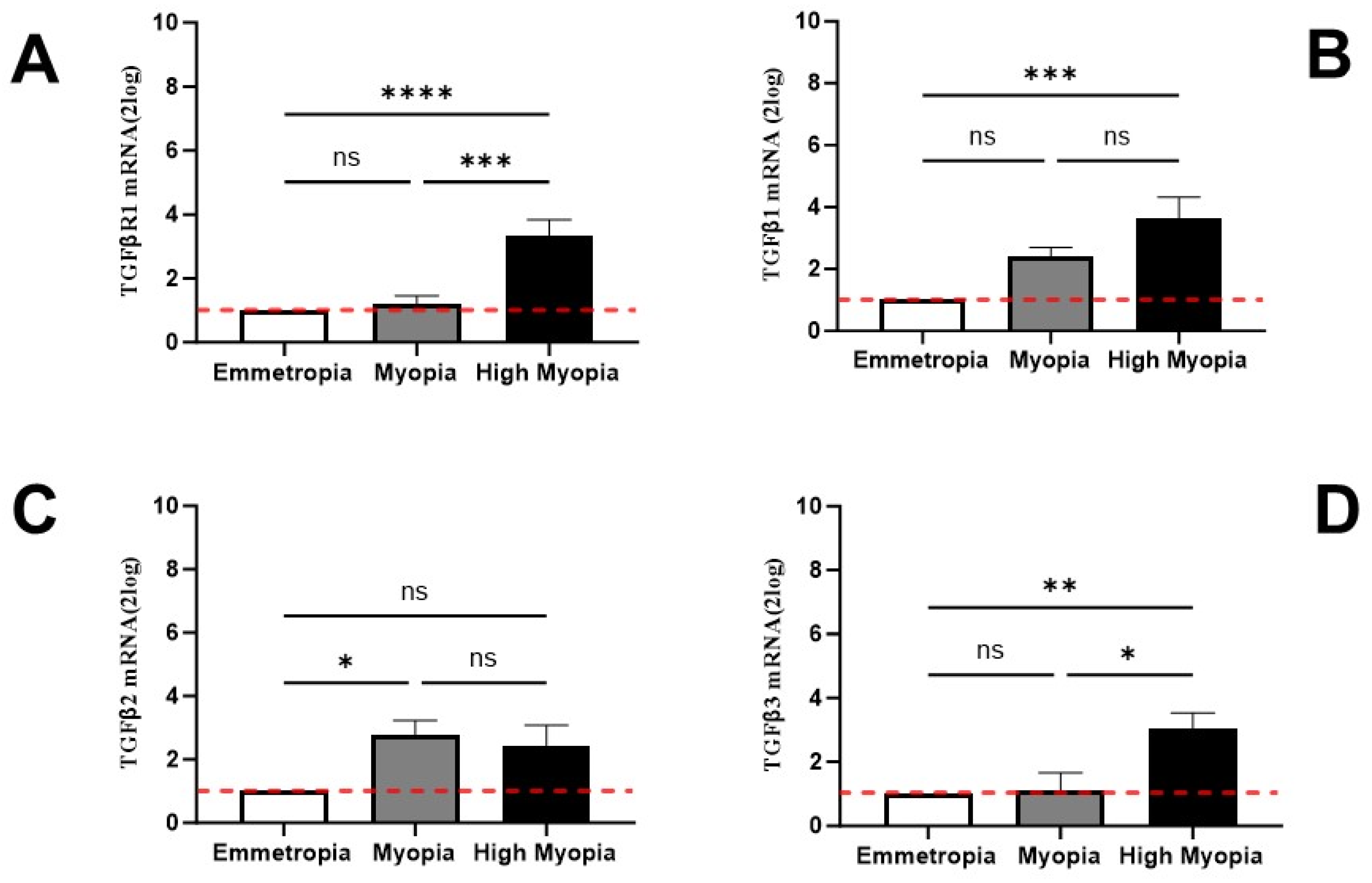

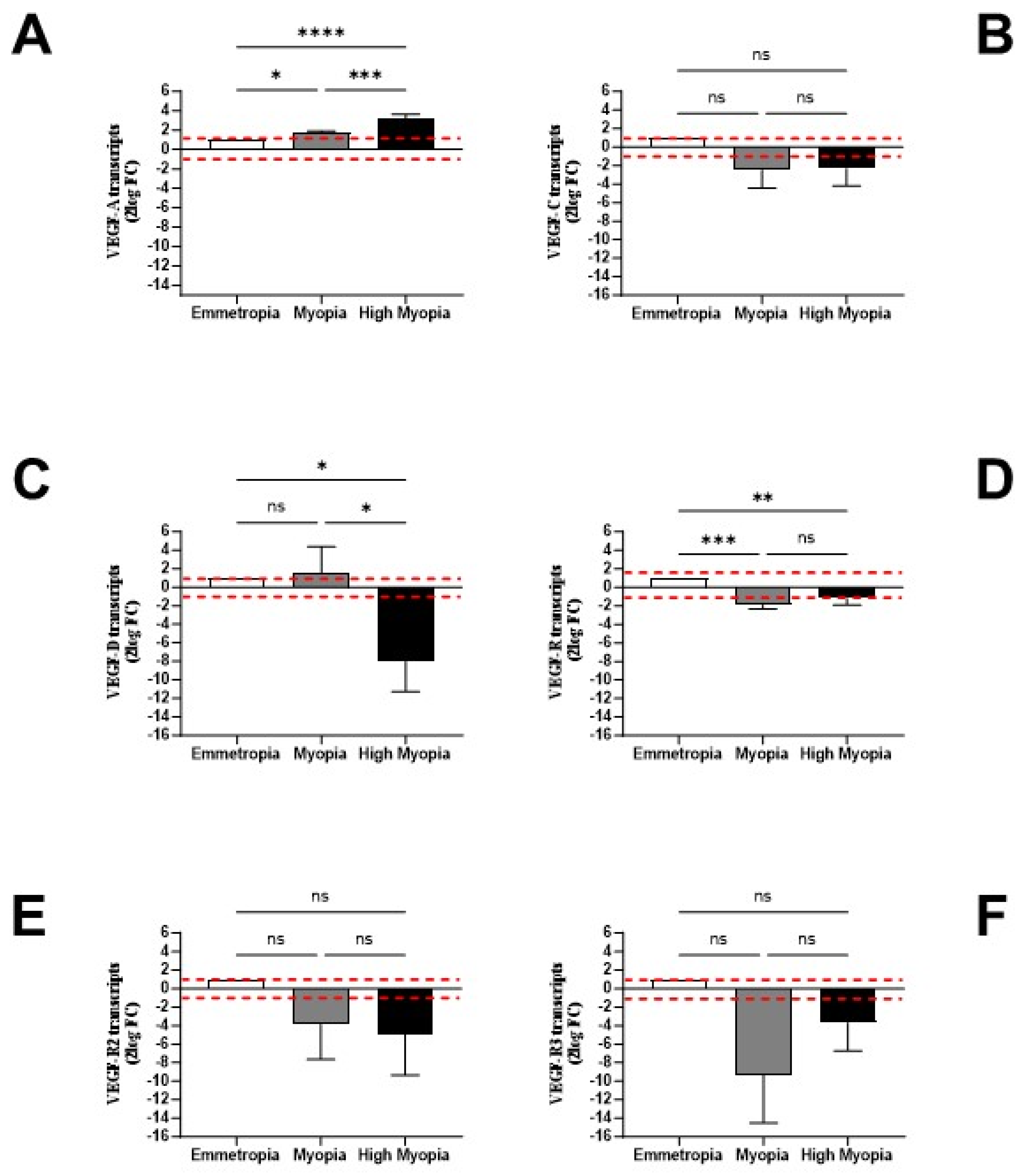
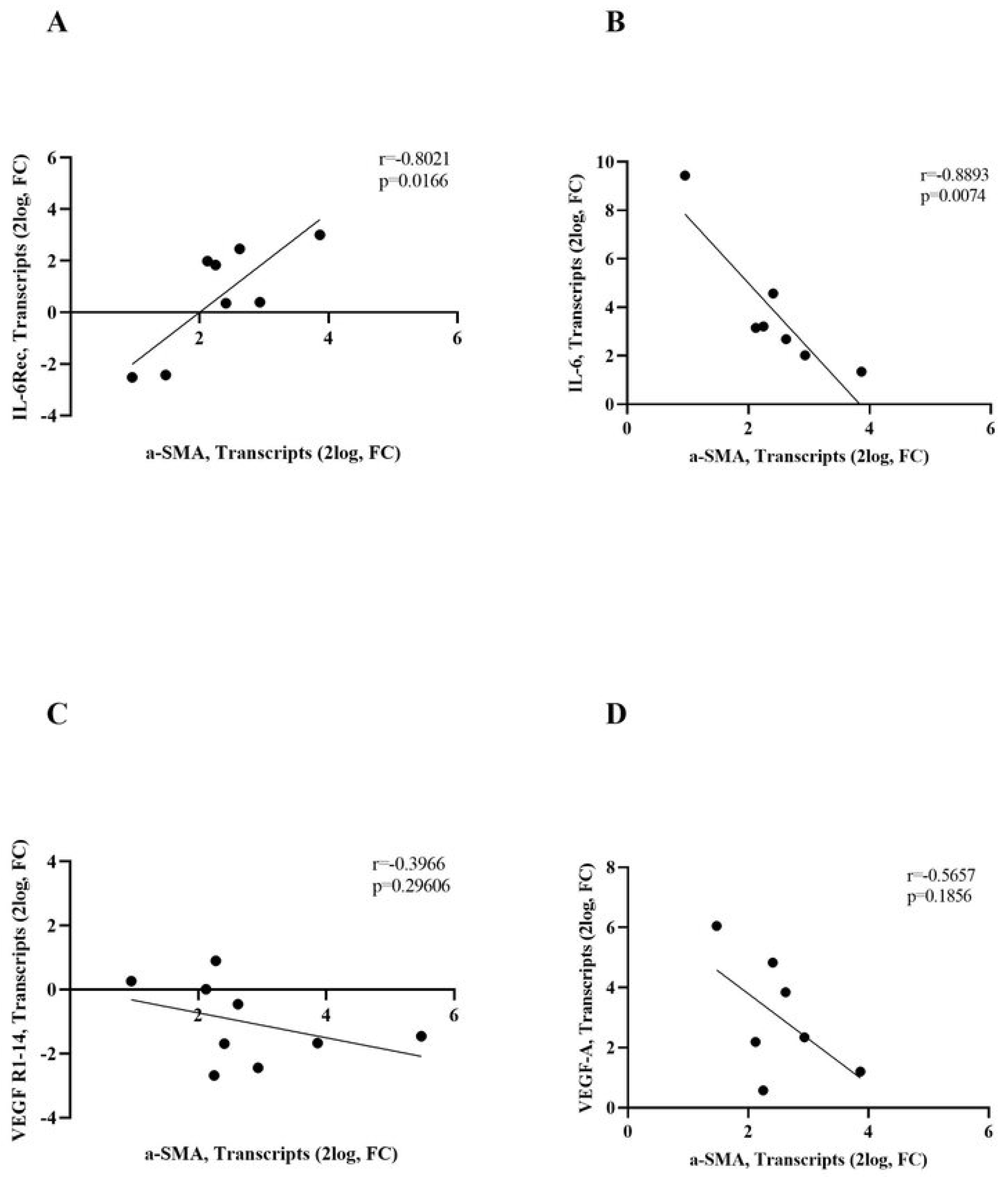
| Study Population | Emmetropia | Myopia | High Myopia | p Value | |
|---|---|---|---|---|---|
| Subjects | 102 | 38 | 32 | 32 | p > 0.05 |
| Age | 74.87 ± 9.47 | 78.22 ± 7.43 | 77.7 ± 7.80 | 67.71 ± 9.78 | p > 0.05 |
| Sex F/M | 58/44 | 23/15 | 13/19 | 22/10 | p > 0.05 |
| Axial Length | 24.98 ± 2.77 | 23.04 ± 0.51 | 24.92 ± 0.41 | 29.57 ± 0.66 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Piano, M.; Cacciamani, A.; Balzamino, B.O.; Scarinci, F.; Cosimi, P.; Cafiero, C.; Ripandelli, G.; Micera, A. Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia. Biomolecules 2023, 13, 1328. https://doi.org/10.3390/biom13091328
De Piano M, Cacciamani A, Balzamino BO, Scarinci F, Cosimi P, Cafiero C, Ripandelli G, Micera A. Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia. Biomolecules. 2023; 13(9):1328. https://doi.org/10.3390/biom13091328
Chicago/Turabian StyleDe Piano, Maria, Andrea Cacciamani, Bijorn Omar Balzamino, Fabio Scarinci, Pamela Cosimi, Concetta Cafiero, Guido Ripandelli, and Alessandra Micera. 2023. "Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia" Biomolecules 13, no. 9: 1328. https://doi.org/10.3390/biom13091328









